Abstract
Systemic administration of Salmonella to tumor-bearing mice leads to its preferential accumulation in tumor sites, the enhancement of host immunity, and the inhibition of tumor growth. However, the underlying mechanism for Salmonella-induced antitumor immune response via oral delivery remained uncertain. Herein, we used mouse colorectal cancer (CT26) as tumor model to study the therapeutic effects after oral delivery of Salmonella. When orally administered into tumor-bearing mice, Salmonella significantly accumulated in the tumor sites, inhibited tumor growth and extended the survival of mice. No obvious toxicity was observed during orally administered Salmonella by examining body weight and inflammatory cytokines. As indoleamine 2, 3-dioxygenase 1 (IDO) is a crucial mediator for tumor-mediated immune tolerance, we examined the expression of IDO. We demonstrated that Salmonella inhibited IDO expression in mouse cancer cells. Furthermore, immunohistochemical studies of the tumors revealed the infiltration of neutrophils and T cells in mice treated with Salmonella. In conclusion, our results indicate that Salmonella exerts its tumoricidal effects and stimulates T cell activities by inhibiting IDO expression. Oral delivery of Salmonella may, represent a potential strategy for the treatment of tumor.
Keywords: Colorectal cancer, tumor-targeting, Salmonella, oral delivery, indoleamine 2, 3-dioxygenase 1, T cell
Introduction
The use of Salmonella as an oncolytic agent is one of the innovative approaches for the treatment of cancer. This is based on the observation that Salmonella is capable of multiplying selectively in tumors and inhibiting their growth. Several factors significantly influenced the tumor colonization of Salmonella. It was noticed that different administration routes could affect Salmonella colonization. Intraperitoneal injection of Salmonella resulted in less tumor colonization compared to intravenous injection [1]. Previously, we also found that Salmonella could target the untreated tumor when injected intratumorally into one of the bilaterally implanted tumors. These results indicated that Salmonella, administered via either intratumoral or systemic route, were able to accumulate in the tumors at distant sites [2]. In addition, oral administration of Salmonella still had antitumor activity and reduced toxicity [3]. Salmonella has been developed as oral vaccine vector for a number of infectious disease and several types of cancer [4]. To develop oral administration route for tumor-targeting Salmonella, we tested whether Salmonella could be orally as a therapeutic antitumor agent. The results suggest that oral administration of Salmonella not only colonized within tumors, but also led to significant antitumor immunoresponses. In this study, we want to elucidate the potential mechanism of antitumor effects by oral delivery Salmonella.
Material and methods
Cells, bacteria and mice
Mouse colorectal cancer (CT26) was cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 50 μg/ml gentamicin, 2 mM L-glutamine, and 10% heat-inactivated fetal bovine serum (FBS) at 37°C in 5% CO2. A vaccine strain of S. Choleraesuis (ATCC 15480) was obtained from Bioresources Collection and Research Center (Hsinchu, Taiwan). This rough variant of S. Choleraesuis, designated vaccine 51, was obtained by spreading an 18-h broth culture of the virulent strain 188 of S. Choleraesuis strain Dublin over the surface of a dried nutrient agar plate and placing a drop of a suspension of salmonella anti-O phage no. 1, and selecting for a phage-resistant colony after incubation at 37°C for 24 h [5]. Male BABL/c mice at the age of 6 to 8 weeks were obtained from National Laboratory Animal Center of Taiwan. The animals were maintained in a specific pathogen-free animal care facility under isothermal conditions with regular photoperiods. The experimental protocol adhered to the rules of the Animal Protection Act of Taiwan, and was approved by the Laboratory Animal Care and Use Committee of the China Medical University.
Animal studies
The mice were inoculated subcutaneously (s.c.) with 106 tumor cells. When the tumors had grown to 50 mm3 to 100 mm3, the mice were oral administered with Salmonella (2 × 106 colony-forming units; cfu) at day 7 for continuous 5 days. At various time points post infection, groups of mice were sacrificed, and the numbers of Salmonella in the tumors, livers, and spleens were determined on LB agar plates and expressed as cfu per gram of tissues. In a separate experiment, palpable tumors were measured every 5 days in two perpendicular axes with a tissue caliper and the tumor volume was calculated as: (length of tumor) × (width of tumor)2 × 0.45, and body weight, the survival of the mice in the treated and control groups was monitored daily.
Assessment of cytokines and immunofluorescence staining
To determine the expression of inflammation cytokines (tumor necrosis factor-α, TNF-α and interleukin-1β, IL-1β) after oral administration Salmonella, mice were inoculated with CT26 cells (106) at day 0. Then, the groups of mice were treated with Salmonella (2 × 106 cfu) by oral administration at day 7 for continuous 5 days. To detect the protein and cytokine expressions, the organs were collected at day 16. Levels of inflammation cytokines in the tissue homogenates or sera were determined by ELISA (R & D, Minneapolis, MN). The protein content in each sample was determined by bicinchoninic acid (BCA) protein assay (Pierce Biotechnology, Rockford, IL). To analyze cell infiltrates in the tumors, groups of 4 mice that had been inoculated s.c. with 106 CT26 cells at day 0 were oral delivery with 2 × 106 cfu of Salmonella at day 7 for continuous 5 days. Control mice received PBS. The tumors were excised and snap frozen at time point. Cryostat sections (5 μm) were also prepared, fixed, and incubated with rat anti-mouse Ly-6G (Gr-1) (RB6-8C5, BD Biosciences, San Diego, CA), rat anti-mouse CD4 (L3T4) (GK1.5, BD Biosciences), or rat anti-mouse CD8a (Ly-2) (53-6.7, BD Biosciences) antibody. After sequential incubation with appropriate fluorescein isothiocyanate (FITC)-labeled secondary antibody, the slides were counterstained with 4’,6-diamidino-2-phenylindole (DAPI). The infiltrating cells were quantified by averaging the number of each cell type in three areas of highest cell density at × 400 magnification in each section [6]. Terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) assay was used to detect cell apoptosis within tumors and was performed according to the manufacturer’s instructions (Promega, Madison, WI). Nuclei were stained with 50 μg/ml of DAPI. TUNEL-positive cells were counted under the microscope. We counted three high-power (× 400) fields that showed highest density of positive-stained cells per field to determine the average percentage of apoptotic (TUNEL positive) cells in each section [7].
Immunoblot analysis
The protein content in each sample was determined by BCA protein assay (Pierce Biotechnology). Proteins were fractionated on SDS-PAGE, transferred onto Hybond enhanced chemiluminescence nitrocellulose membranes (Amersham, Little Chalfont, UK), and probed with Indoleamine-pyrrole 2,3-dioxygenase (IDO) (Thermo Scientific, Rockford, IL) or monoclonal antibodies against β-actin (AC-15, Sigma Aldrich). Horseradish peroxidase-conjugated goat anti-mouse IgG or anti-rabbit IgG (Jackson, West Grove, PA) was used as the secondary antibody and protein-antibody complexes were visualized by enhanced chemiluminescence system (Amersham) [8-10].
Statistical analysis
The unpaired, two-tailed Student’s t test was used to determine differences between groups for the comparisons of body weight, tumor volume, cytokine expression, the numbers of CD4+, CD8+ and neutrophils and the number of apoptotic cells. The survival analysis was performed using the Kaplan-Meier survival curve and log-rank test. Any P value less than 0.05 is regarded statistically significant.
Results
Preferential accumulation in tumors via oral administration Salmonella
To determine the localization of oral administration Salmonella, we treated mice bearing syngeneic tumors with Salmonella, and monitored bacterial burdens in the tumors, livers, spleens, and blood at various time points. As shown in Figure 1, five days after Salmonella inoculation, the amount of Salmonella (cfu/g tissue) in the tumors was approximately four to five orders of magnitude higher than that found in the livers or spleens in tumor-bearing mice. Even at day 7, Salmonella could still be detected in the tumor. Collectively, these results demonstrate that Salmonella, when oral administration to mice bearing established tumors, preferentially accumulated and retained in large amounts in the tumors for at least one week.
Figure 1.
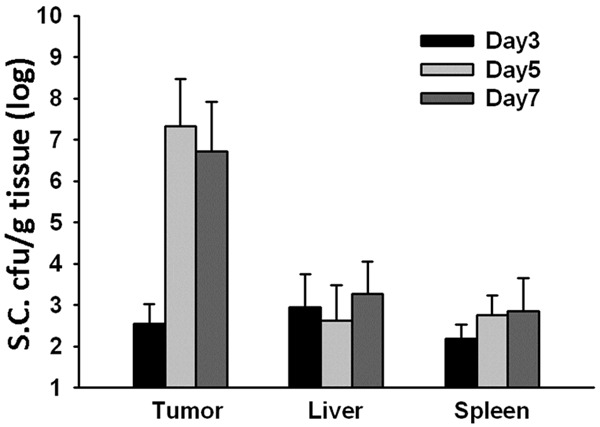
Preferential accumulation of Salmonella (S.C.) in the tumor microenvironment of mice administered orally with Salmonella. Mice bearing CT26 tumors were oral administered with 2 × 106 cfu of Salmonella for continuous 5 days. The amounts of accumulated Salmonella in tumors, livers, and spleens were determined on 3, 5, and 7 day post infection (p.i.). Each value represents mean ± SD from 4 mice.
Effects of oral administration Salmonella on cytokine induction in mice
Because significant proinflammatory cytokine induction occurs with Salmonella infection, the administration of agents such as antibiotics may reduce the biological activity of these cytokines and their side effects. To examine the host inflammation response induced by Salmonella, tumor-bearing mice were orally treated with Salmonella, and body weight was measured. As indicated in Figure 2A, the body weights of mice treated with Salmonella were not significantly decreased compared with control group. After oral Salmonella treatment, the levels of inflammatory cytokines including TNF-α (Figure 2B) and IL-1β (Figure 2C) were measured in the livers, spleen. Regardless of whether the mice were naïve or immunized, the induction of inflammation cytokines (i.e., IL-1β and TNF-α) in mice treated with Salmonella was not significantly different compared with the induction by PBS treatment (Figure 2B and 2C). Taken together, these results suggest that oral administration Salmonella had a greater tumor-targeting efficiency with potentially lower side effects in the host.
Figure 2.
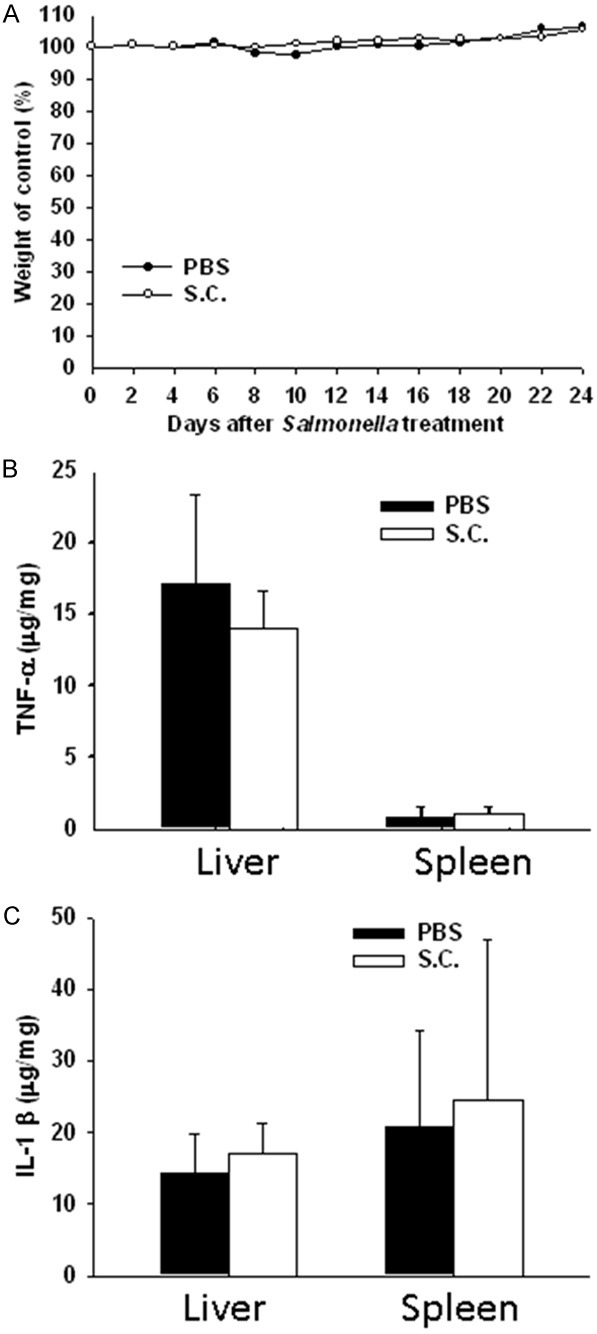
Susceptibility of mice to infection with Salmonella (S.C.). Mice were injected orally with Salmonella (2 × 106 cfu) for continuous 5 days; (A) The body weights and (B) TNF-α and (C) IL-1β levels were determined. The data are reported as means ± SD (n = 4).
Inhibition of tumor growth by oral administration Salmonella
We hypothesize that Salmonella breaks tumor immune tolerance via suppressing IDO as a crucial mediator of tumor-mediated immune tolerance by causing T cell suppression via tryptophan starvation in a tumor environment. Furthermore, to examine the effect of Salmonella on IDO levels in CT26 cells, CT26 cells were incubated with different multiplicity of infection (MOI) of Salmonella, and then analyzed by immunoblot analyses. Treatment of CT26 cells with MOI 0, 1, 10 or 100 of Salmonella caused a dose-dependent decrease in IDO levels compared to controls (Figure 3A). Antitumor effects of Salmonella were evaluated in terms of tumor growth and survival in mice bearing CT26 tumors. Figure 3B shows that tumor growth was significantly retarded in mice treated with Salmonella compared with that in PBS-treated control mice. The mean tumor volume in Salmonella-treated group was lowered by 98.60% compared with that in PBS-treated groups. Figure 3C shows that survival of the mice treated with Salmonella was significantly prolonged compared with that treated with PBS. Salmonella completely inhibited tumor growth in the four mice of Salmonella-treated group. The host memory immunresponses were observed by challenging high dose tumor cells (5 × 106) and the mice did not develop the tumor. Taken together, Salmonella exerted antitumor effects on CT26 tumor models. Salmonella accumulated in tumor microenvironment and induced strong host immune cells infiltrating into tumor. Infiltrates of T-cells and neutrophils within tumors from CT26-tumor-bearing mice inoculated with Salmonella were analyzed by immunohistochemistry. The results of immunohistochemical staining are shown in Figure 4A. A notable increase of neutrophils and T-cells infiltrates in the tumors was observed in Salmonella -treated mice. As shown in Figure 4B, the number of neutrophils and T-cells infiltrates in Salmonella -treated mice was obviously increased compared with that in the control groups. We then measured the numbers of apoptotic cells in TUNEL (Figure 4A). The number of apoptotic cells in the Salmonella-treated group was significantly higher than that in the PBS-treated (Figure 4C). Taken together, these results reveal that oral administration Salmonella was capable of inhibiting tumor growth, enhancing cellular infiltration into tumor regions and increasing the death of tumor cells.
Figure 3.
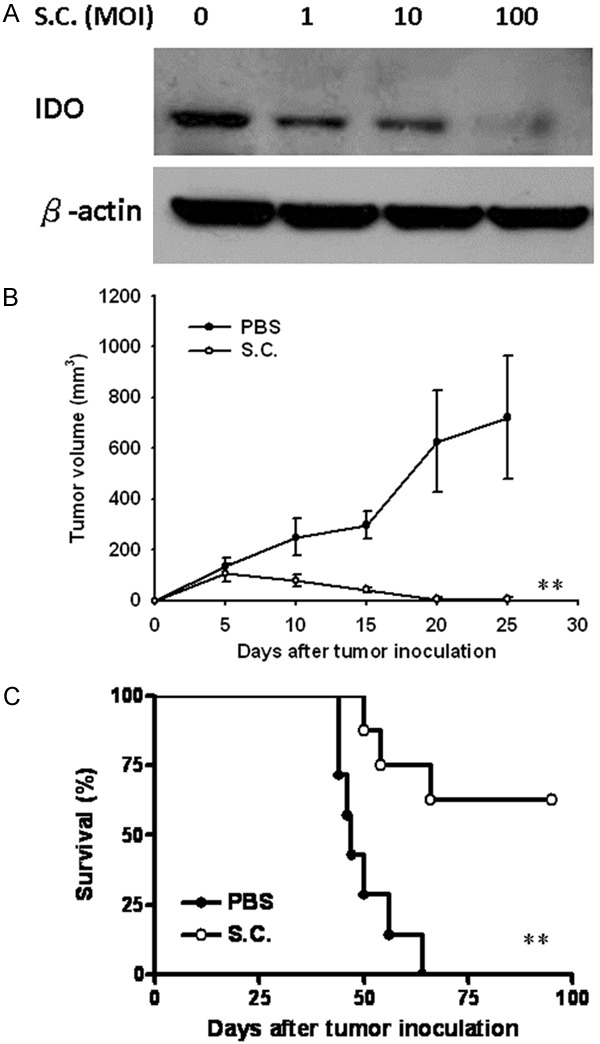
Antitumor effects of Salmonella on mice bearing CT26 tumors. (A) Salmonella reduced IDO protein expression in CT26 cells in a dose-dependent manner. After exposure to Salmonella (MOI: 0-100) for 4 h, the expression of IDO levels in CT26 cells were determined by immunoblot analysis. Groups of 7-8 BALB/c mice that had been inoculated subcutaneously with CT26 cells (106 ) on day 0, were injected orally with 2 × 106 cfu of Salmonella on day 7 for continuous 5 days. Control mice only received PBS. Tumor volumes (mean ± SEM, n = 7-8) among different groups were compared on mice bearing (B) CT26 tumors. Kaplan-Meier survival curves of mice bearing (C) CT26 tumors with different treatments are shown. Data were analyzed by the log-rank test. **, P < 0.01.
Figure 4.
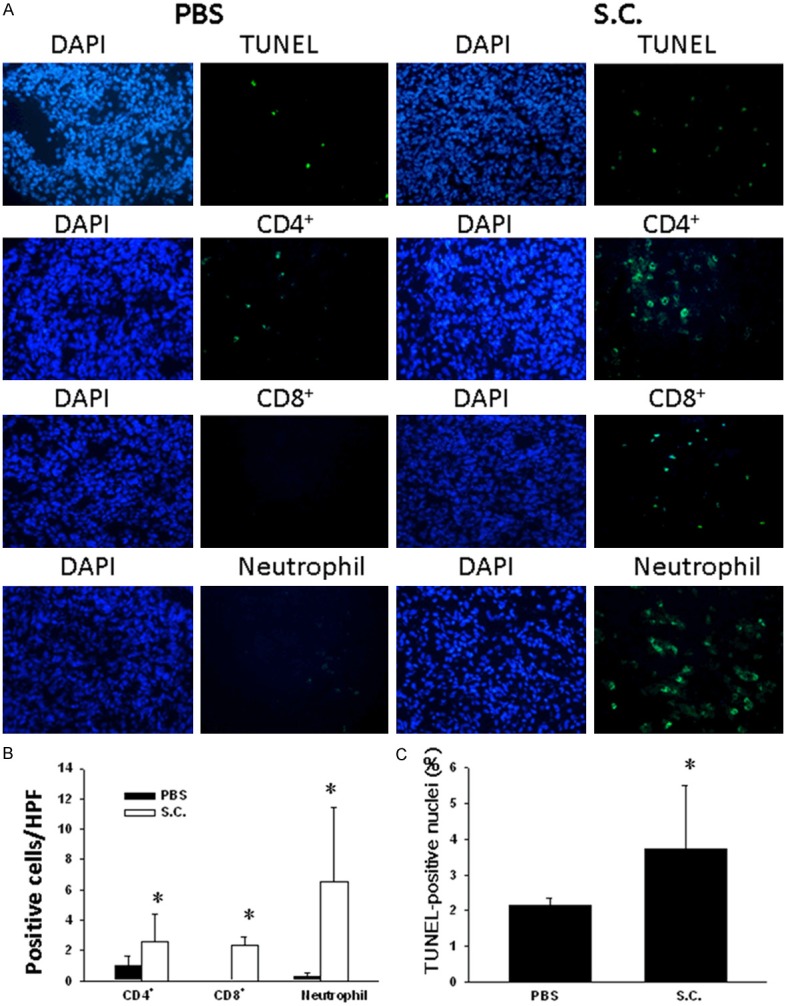
Increase of cellular infiltrates and apoptosis in the tumors from mice treated with Salmonella. Groups of 4 BALB/c mice that had been inoculated subcutaneously with CT26 cells (106) on day 0, were injected orally with 2 × 106 cfu of Salmonella on day 7 for continuous 5 days. Control mice only received PBS. A. Tumors were excised on day 16 and immunostained with TUNEL assay to analyze apoptotic cell (× 400) and with antibodies against Gr-1, CD4+ or CD8+ (× 400). B. Neutrophils CD4+ and CD8+ T-cell infiltrates were determined by averaging the cell numbers from three independent fields at × 400 magnification in each section (mean ± SEM n = 4). C. TUNEL-positive cells (mean ± SEM n = 4) were counted from three random fields in each section to determine the apoptotic index. *, P < 0.05.
Discussion
In the experiments described above, we prove that Salmonella is capable of targeting and multiplying in primary tumors via oral administration. Using murine colorectal tumor models, we demonstrated that orally administered Salmonella preferentially accumulated and amplified within implanted tumors in mice, achieving the ratio of the tumor to normal tissue 1000-10000 to 1. Salmonella preferentially targets tumors, with numbers far exceeding those in the liver, which is a normal site for Salmonella replication in non-tumor-bearing mice. The propensity of Salmonella to target primary tumors may also contribute to enhance host immunoresponses, as well as prolonging the survival of tumor-bearing mice. Our results also suggested that oral delivery Salmonella induced a strong additive effect on delaying tumor growth and enhancing survival of the mice.
Salmonella can induce multiple signaling pathways in tumors [11]. Salmonella has been widely studies; especially with respect to its immunopotentiating properties its ability to enhance host immunoresponse [12,13]. However, the mechanism by which Salmonella inhibited tumor and the issue of whether that mechanism involved the reversal of IDO is not completely clear. IDO is an inducible enzyme that catalyzes the rate-limiting first step in tryptophan catabolism. IDO causes immunosuppression through breakdown of tryptophan in the tumor microenvironment. The depletion of tryptophan and toxic catabolites renders effector T cells inactive and dendritic cells immunosuppressive [14]. The present studies described an investigation of the effects of Salmonella on downregulation of IDO expression. The successful induction of immunity against poorly immunogenic malignancies is a major challenge for cancer therapy. Previously, we have demonstrated that host immune responses cooperate with Salmonella-mediated tumor destruction [2,11]. Herein, we show that oral delivery of Salmonella results in substantial control of CT26 tumors. The reduction of IDO expression with tumor colonization by Salmonella results in the intratumoral recruitment of neutrophil and T cells, which may promote the induction of apoptosis of tumor cells. Strong neutrophils activation can cause tissue damage and this represents the basis for tumor destruction [15].
Salmonella is a powerful antitumor agent due to its tumor-targeting potential, antitumor capability, and ability to deliver therapeutic gene [16]. Host factors including innate and adaptive immune responses play roles in Salmonella-induced antitumor activity. Oral delivery is a relatively novel method for Salmonella cancer therapy. Previously, mice treated with Salmonella had a 9% lower average body weight compared with naïve mice treated with PBS during systemic injection [17]. In contrast, the body weights of mice treated with oral delivery-Salmonella were not significantly decreased. It was also reported that oral administration may provide an alternative route for low toxic delivery of Salmonella for effective antitumor therapy [18].
In summary, oral delivery-Salmonella displayed lower toxicity and improved efficacy and safety. Oral-delivery Salmonella also can provide a useful platform for oral, gastric or colorectal tumor, perhaps allowing other chemotherapeutic drugs to combine with tumor-targeting Salmonella. By taking advantage of the tumor-targeting activity and the stimulating host immunity activities of Salmonella, this system appears to hold promise for tumor treatment. These results yield insight into the complex interactions between Salmonella and host immunity, maximizing the possibility of therapeutic success. Therefore, oral administration Salmonella has promising potential for further clinical studies.
Acknowledgements
This work was supported by grants from Ministry of Science and Technology, Taiwan (NSC 101-2320-B-039-012-MY3) (MOST 103-2221-E-035-003-MY3) and China Medical University (CMU103-S-09).
Disclosure of conflict of interest
None.
References
- 1.Mei S, Theys J, Landuyt W, Anne J, Lambin P. Optimization of tumor-targeted gene delivery by engineered attenuated Salmonella typhimurium. Anticancer Res. 2002;22:3261–3266. [PubMed] [Google Scholar]
- 2.Lee CH, Wu CL, Tai YS, Shiau AL. Systemic administration of attenuated Salmonella choleraesuis in combination with cisplatin for cancer therapy. Mol Ther. 2005;11:707–716. doi: 10.1016/j.ymthe.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Chen G, Wei DP, Jia LJ, Tang B, Shu L, Zhang K, Xu Y, Gao J, Huang XF, Jiang WH, Hu QG, Huang Y, Wu Q, Sun ZH, Zhang JF, Hua ZC. Oral delivery of tumor-targeting Salmonella exhibits promising therapeutic efficacy and low toxicity. Cancer Sci. 2009;100:2437–2443. doi: 10.1111/j.1349-7006.2009.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiau AL, Chen CC, Yo YT, Chu CY, Wang SY, Wu CL. Enhancement of humoral and cellular immune responses by an oral Salmonella choleraesuis vaccine expressing porcine prothymosin α. Vaccine. 2005;23:5563–5571. doi: 10.1016/j.vaccine.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Lee CH, Lin ST, Liu JJ, Chang WW, Hsieh JL, Wang WK. Salmonella induce autophagy in melanoma by the downregulation of AKT/mTOR pathway. Gene Ther. 2014;21:309–316. doi: 10.1038/gt.2013.86. [DOI] [PubMed] [Google Scholar]
- 6.Lee CH, Hsieh JL, Wu CL, Hsu PY, Shiau AL. T cell augments the antitumor activity of tumor-targeting Salmonella. Appl Microbiol Biotechnol. 2011;90:1381–1388. doi: 10.1007/s00253-011-3180-z. [DOI] [PubMed] [Google Scholar]
- 7.Chang WW, Lai CH, Chen MC, Liu CF, Kuan YD, Lin ST, Lee CH. Salmonella enhance chemosensitivity in tumor through connexin 43 upregulation. Int J Cancer. 2013;133:1926–1935. doi: 10.1002/ijc.28155. [DOI] [PubMed] [Google Scholar]
- 8.Liu WS, Kuan YD, Chiu KH, Wang WK, Chang FH, Liu CH, Lee CH. The extract of Rhodobacter sphaeroides inhibits melanogenesis through the MEK/ERK signaling pathway. Mar Drugs. 2013;11:1899–1908. doi: 10.3390/md11061899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee CH, Lee SD, Ou HC, Lai SC, Cheng YJ. Eicosapentaenoic acid protects against palmitic acid-induced endothelial dysfunction via activation of the AMPK/eNOS pathway. Int J Mol Sci. 2014;15:10334–10349. doi: 10.3390/ijms150610334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang WW, Liu JJ, Liu CF, Liu WS, Lim YP, Cheng YJ, Lee CH. An extract of Rhodobacter sphaeroides reduces cisplatin-induced nephrotoxicity in mice. Toxins. 2013;5:2353–2365. doi: 10.3390/toxins5122353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang WW, Lee CH. Salmonella as an innovative therapeutic antitumor agent. Int J Mol Sci. 2014;15:14546–14554. doi: 10.3390/ijms150814546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Miwa S, Zhang N, Hoffman RM, Zhao M. Tumor-targeting Salmonella typhimurium A1-R arrests growth of breast-cancer brain metastasis. Oncotarget. 2015;6:2615–262. doi: 10.18632/oncotarget.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miwa S, Zhang Y, Baek KE, Uehara F, Yano S, Yamamoto M, Hiroshima Y, Matsumoto Y, Kimura H, Hayashi K, Yamamoto N, Bouvet M, Tsuchiya H, Hoffman RM, Zhao M. Inhibition of spontaneous and experimental lung metastasis of soft-tissue sarcoma by tumor-targeting Salmonella typhimurium A1-R. Oncotarget. 2014;5:12849–12861. doi: 10.18632/oncotarget.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soliman H, Mediavilla-Varela M, Antonia S. Indoleamine 2,3-dioxygenase: is it an immune suppressor ? Cancer J. 2010;16:354–359. doi: 10.1097/PPO.0b013e3181eb3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee CH. Engineering bacteria toward tumor targeting for cancer treatment: current state and perspectives. Appl Microbiol Biotechnol. 2012;93:517–523. doi: 10.1007/s00253-011-3695-3. [DOI] [PubMed] [Google Scholar]
- 16.Blache CA, Manuel ER, Kaltcheva TI, Wong AN, Ellenhorn JD, Blazar BR, Diamond DJ. Systemic delivery of Salmonella typhimurium transformed with IDO shRNA enhances intratumoral vector colonization and suppresses tumor growth. Cancer Res. 2012;72:6447–6456. doi: 10.1158/0008-5472.CAN-12-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee CH, Lin YH, Hsieh JL, Chen MC, Kuo WL. A polymer coating applied to Salmonella prevents the binding of Salmonella-specific antibodies. Int J Cancer. 2013;132:717–725. doi: 10.1002/ijc.27700. [DOI] [PubMed] [Google Scholar]
- 18.Jia LJ, Wei DP, Sun QM, Huang Y, Wu Q, Hua ZC. Oral delivery of tumor-targeting Salmonella for cancer therapy in murine tumor models. Cancer Sci. 2007;98:1107–1112. doi: 10.1111/j.1349-7006.2007.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


