Abstract
Erlotinib, bevacizumab, and pemetrexed improved survival of metastatic non-small cell lung cancer (mNSCLC) in clinical trials, but their benefits are restricted to non-squamous histology. We studied recent survival trends in mNSCLC subpopulations defined by histology and associated clinical factors correlating with adenocarcinoma or endothelial growth factor receptor mutations. Using the Surveillance, Epidemiology and End Results database, we calculated relative survival at 1 year from diagnosis for mNSCLC cases diagnosed in 2000-2011. Trends by histology, age, sex, race, prevalence of smoking or poverty, expressed as annual percent change (APC) using joinpoint regression, were compared by test of slope parallelism (Ppar). Among 226,446 cases, 47% had adenocarcinoma, 20% squamous carcinoma, 6% other, and 27% unspecified histology. The proportion of cases designated as adenocarcinoma significantly increased after 2005. One-year survival increased from 23.5% in 2000 to 30.5% in 2010, significantly more for adenocarcinoma (APC, 3.3%) than squamous carcinoma (APC, 2.1%, Ppar=0.0018). For patients with adenocarcinoma, these trends were significantly better for Asians than Whites (Ppar=0.012) and for areas with fewer smokers (Ppar=0.014). Such differences were not observed for squamous carcinoma (Ppar=0.87 and 0.14, respectively). The absolute disparity in one-year survival between adenocarcinoma and squamous carcinoma increased from 1.6% in 2000 to 5.5% in 2010. The disparity between Asians and Whites increased from 5.2% to 13.1%, respectively. These data demonstrate that improvement in survival of mNSCLC since 2000 is now evident on a population scale. The superior increment for patients with adenocarcinoma, particularly among Asians and in communities with fewer smokers, suggests impact of the newly introduced, histology-specific agents, rather than better supportive care alone. Growing disparities between adenocarcinoma and squamous carcinoma highlight the needs to intensify research on treatment for subgroups that did not benefit from recent advances.
Keywords: Epidemiology, non-small cell lung cancer, relative survival, lung adenocarcinoma, squamous cell carcinoma
Introduction
The past decade was marked by significant advances in therapy of metastatic non-small cell lung cancer (mNSCLC). Increased attention to efficacious and timely delivery of palliative care was shown to improve both quality of life and survival in this incurable disease. Additionally, three novel agents approved by the United States (US) Food and Drug Administration (FDA) between 2000 and 2010 demonstrated overall survival (OS) advantage over standard platinum-based doublet chemotherapy, primarily for non-squamous histologies. The epidermal growth factor receptor (EGFR) inhibitor erlotinib was initially approved in 2004 thanks to a modest OS benefit in second-line therapy for unselected mNSCLC, but dramatic benefits were subsequently demonstrated in the subset of 15-20% of tumors harboring EGFR mutations [1,2]. These mutations correlate with certain clinical features: adenocarcinoma (ADE) histology, female sex, Asian race and non-smoking status [3-5]. Bevacizumab, a monoclonal antibody targeting the vascular endothelial growth factor (VEGF), was approved in 2006 in combination with carboplatin and paclitaxel, specifically excluding patients with squamous carcinomas (SQC) because of an unacceptably high rate of severe hemoptysis [6]. Similarly, the survival benefit of pemetrexed, initially approved in 2004 for second-line treatment, proved to be restricted to non-squamous histology [7].
The differential impact of these agents on OS in histologic subtypes of mNSCLC has not been evaluated outside of the clinical trial setting. We hypothesized that a difference in population-derived survival trends between ADE and SQC, and within populations with higher prevalence of ADE histology or EGFR mutations, would become detectable throughout the decade. Demonstration of such differential trends would strongly indicate that novel systemic therapies acting through their histology-specific mechanisms significantly contributed to survival in some subsets of mNSCLC, beyond the effects of stage migration or enhanced supportive care.
Methods—patients
Data source
We conducted a retrospective cohort analysis using data from the US Surveillance, Epidemiology and End Results (SEER) program [8]. The study used a de-identified public dataset and was exempt from oversight of the institutional review committee for human subject protection. The SEER program collects cancer incidence and survival data from 18 US geographic areas, currently representing 28% of the total population. The program mandates a 98% case ascertainment rate and conducts quality assurance programs for completeness and consistency of coding. We queried the data base for records of all mNSCLC patients diagnosed between 2000 and 2011, with survival follow-up until December 31st, 2011. Patients had to have microscopically confirmed histology and active follow-up with recorded survival time (i.e. cases diagnosed by autopsy or death certificate were excluded). mNSCLC was defined according to the 2000 World Health Organization (WHO) International Classification of Diseases for Oncology, 3rd edition, using a combination of topography (“lung and bronchus”, C340-C349) and histology codes, grouped into adenocarcinoma (ADE), squamous cell carcinoma (SQC), other specified and unspecified (NOS) histology (Table 1) [9]. Metastatic disease was defined by the SEER “summary stage” variable indicating a “distant site involvement”. The summary stage is the most basic way of categorizing the spread of a cancer across evolving staging systems using all available clinical and pathologic information. It has been consistently applied by the US cancer registries since 2001 and used for epidemiologic research [10,11].
Table 1.
Histologic subtypes of metastatic non-small cell carcinoma in the SEER data base according to the World Health Organization classification. Small cell lung cancer, pulmonary carcinoid and non-carcinoma histologies were excluded
| Histology grouping | N | ICD-O-3 code | Description |
|---|---|---|---|
| Adenocarcinoma (ADE) | 96,047 | 8140/3 | Adenocarcinoma, NOS |
| 17 | 8230/3 | Solid carcinoma, NOS | |
| 3,472 | 8250/3 | Bronchiolo-alveolar adenocarcinoma | |
| 213 | 8252/3 | Bronchiolo-alveolar carcinoma, non-mucinous | |
| 414 | 8253/3 | Bronchiolo-alveolar carcinoma, mucinous | |
| 27 | 8254/3 | Bronchiolo-alveolar carcinoma, mucinous and non-mucinous | |
| 526 | 8255/3 | Adenocarcinoma with mixed subtypes | |
| 1,240 | 8260/3 | Papillary adenocarcinoma, NOS | |
| 255 | 8310/3 | Clear cell adenocarcinoma, NOS | |
| 1,601 | 8480/3 | Mucinous adenocarcinoma | |
| 1,586 | 8481/3 | Mucin-producing adenocarcinoma | |
| 518 | 8490/3 | Signet ring cell carcinoma | |
| 188 | 8550/3 | Acinar cell carcinoma | |
| Squamous carcinoma (SQC) | 17 | 8052/3 | Papillary squamous cell carcinoma |
| 41,359 | 8070/3 | Squamous cell carcinoma, NOS | |
| 2,750 | 8071/3 | Squamous cell carcinoma, keratinizing, NOS | |
| 980 | 8072/3 | Squamous cell carcinoma, large cell, nonkeratinizing | |
| 67 | 8073/3 | Squamous cell carcinoma, small cell, nonkeratinizing | |
| 76 | 8083/3 | Basaloid squamous cell carcinoma | |
| 17 | 8084/3 | Squamous cell carcinoma, clear cell type | |
| Other histologies (OTH), including: Large cell | 8,681 | 8012/3 | Large cell carcinoma, NOS |
| 1,347 | 8013/3 | Large cell neuroendocrine carcinoma | |
| 17 | 8014/3 | Large cell carcinoma with rhabdoid phenotype | |
| 8 | 8082/3 | Lymphoepithelial carcinoma | |
| 20 | 8123/3 | Basaloid carcinoma | |
| Adenosquamous Sarcomatoid | 2,250 | 8560/3 | Adenosquamous carcinoma |
| 170 | 8022/3 | Pleomorphic carcinoma | |
| 306 | 8031/3 | Giant cell carcinoma | |
| 301 | 8032/3 | Spindle cell carcinoma | |
| 544 | 8033/3 | Pseudosarcomatous carcinoma | |
| 12 | 8972/3 | Pulmonary blastoma | |
| 119 | 8980/3 | Carcinosarcoma, NOS | |
| Unspecified (NOS) | 13,019 | 8010/3 | Carcinoma, NOS |
| 48,282 | 8046/3 | Non-small cell carcinoma |
ICD-O-3: International Classification of Diseases in Oncology, 3rd edition; NOS, not otherwise specified.
Variables and endpoints
The study cohort included all patients with mNSCLC, regardless of whether they received any cancer-directed treatment. Information about systemic chemotherapy was not recorded. Subgroups for trend analysis were defined by: age at diagnosis (dichotomized at rounded median as <70 or ≥70 years), sex, race (specifically White, Black, or Asian), prevalence of current smokers (dichotomized at rounded median as <19% or ≥19%) and prevalence of persons living below the poverty level (dichotomized at rounded median of <15% or ≥15%) in the patient’s county of residence. The latter two attributes were derived from the 2007-2011 Census American Community Survey, and two National Cancer Institute surveys (Behavioral Risk Factor Surveillance System and National Health Interview Survey), as previously described [12].
We used relative survival (RS) at 1 year from diagnosis as the primary endpoint. RS is a population survival measure, defined as the ratio of survival observed in the population under study to survival expected in the general population, calculated from the national life tables and stratified by calendar year, age, sex, and race. Although almost all patients with mNSCLC die as a consequence of their cancer, using RS for trend analysis has the advantage of automatic adjustment for survival trends occurring throughout the entire population [13]. Except for comparisons between age groups, all rates were age-standardized to also account for varying age distribution in the compared subpopulations [14]. RS rates in each year were calculated using the period survival method, which provides more updated measures of survival trends than cohort-based methods [15]. For each calendar year, survival was calculated using only data from the two preceding calendar years. Because of incomplete mortality records in the last year of each annual SEER data submission, RS survival was only calculated up to 2010 [16].
Statistical analysis
Tables of frequencies and all survival rates were calculated by SEER*Stat software (http://seer.cancer.gov/seerstat/). Proportions of histologies were compared as relative risk (RR) using log-binomial regression [17]. Linearized trends were analyzed using log-linear joinpoint regression (http://surveillance.cancer.gov/joinpoint/) and expressed as annual percent change (APC) with 95% confidence intervals (CI). APC measures the average relative change in survival rates between two consecutive calendar years. Because of the short timeframe of analysis (11 data points between 2000 and 2010) we did not allow subsegments in joinpoint regression lines. Trends in subpopulations were compared using the test of trend parallelism [18]. We used unweighted regression for these comparisons in order to avoid differential weighting of subpopulations of markedly different sizes (for example, racial groups). As a sensitivity test, all significant results were confirmed using inverse variance-weighted regression. Statistical significance was defined as Pparalellism (Ppar) <0.05. Because of exploratory nature of the analysis, no correction for multiple testing was introduced. All other statistical analyses were performed using Stata/SE 13.1 (StataCorp LP, College Station, TX).
Results
Patient characteristics
We identified 226,446 mNSCLC cases (Table 2) recorded in the SEER data between 2000 and 2011. The median age was 69 years (interquartile range, 60-77), unchanged during the decade. The proportion of women increased from 41.0% in 2000 to 44.7% in 2011. There were 81.1% White, 12.5% Black, and 6.4% Asian patients. The percentages of patients residing in areas with higher prevalence of smoking (54%) or poverty (43%) were relatively constant (±1%) between 2000 and 2011.
Table 2.
Proportions of different metastatic non-small cell carcinoma histologies in subgroups defined by socio-demographic characteristics
| All histologies | Adenocarcinoma | Squamous cell | Other histologya | Unspecified | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Variable | Subgroup | N | N | % | N | % | N | % | N | % |
| Total | 226,446 | 106,104 | (46.9) | 45,266 | (20.0) | 13,775 | (6.1) | 61,301 | (27.1) | |
| Age (years) | <55 | 30,115 | 15,708 | (52.2) | 4,311 | (14.3) | 2,022 | (6.7) | 8,074 | (26.8) |
| 55-64 | 52,817 | 25,152 | (47.6) | 9,903 | (18.7) | 3,342 | (6.3) | 14,420 | (27.3) | |
| 65-69 | 34,514 | 15,725 | (45.6) | 7,366 | (21.3) | 2,126 | (6.2) | 9,297 | (26.9) | |
| 70-74 | 36,335 | 16,152 | (44.5) | 8,153 | (22.4) | 2,156 | (5.9) | 9,874 | (27.2) | |
| 75-79 | 34,378 | 15,408 | (44.8) | 7,591 | (22.1) | 2,030 | (5.9) | 9,349 | (27.2) | |
| ≥80 | 38,287 | 17,959 | (46.9) | 7,942 | (20.7) | 2,099 | (5.5) | 10,287 | (26.9) | |
| Sex | Male | 128,616 | 55,402 | (43.1) | 29,671 | (23.1) | 8,172 | (6.4) | 35,371 | (27.5) |
| Female | 97,830 | 50,702 | (51.8) | 15,595 | (15.9) | 5,603 | (5.7) | 25,930 | (26.5) | |
| Raceb | White | 182,653 | 85,163 | (46.6) | 36,672 | (20.1) | 11,160 | (6.1) | 49,658 | (27.2) |
| Black | 28,031 | 12,243 | (43.7) | 6,257 | (22.3) | 1,932 | (6.9) | 7,599 | (27.1) | |
| Asian | 14,432 | 8,154 | (56.5) | 2,029 | (14.1) | 617 | (4.3) | 3,632 | (25.2) | |
| Smoking prevalenceb,c | <16% | 59,055 | 29,467 | (49.9) | 10,055 | (17.0) | 3,422 | (5.8) | 16,111 | (27.3) |
| 16-18% | 44,444 | 21,991 | (49.5) | 8,413 | (18.9) | 2,593 | (5.8) | 11,447 | (25.8) | |
| 19-24% | 74,140 | 34,174 | (46.1) | 14,830 | (20.0) | 4,255 | (5.7) | 20,881 | (28.2) | |
| ≥25% | 48,792 | 20,461 | (41.9) | 11,967 | (24.5) | 3,505 | (7.2) | 12,859 | (26.4) | |
| Poverty prevalenceb,c | <10% | 51,075 | 25,550 | (50.0) | 9,253 | (18.1) | 2,556 | (5.0) | 13,716 | (26.9) |
| 10-14% | 77,101 | 36,834 | (47.8) | 14,310 | (18.6) | 4,103 | (5.3) | 21,854 | (28.3) | |
| 15-19% | 63,524 | 29,075 | (45.8) | 13,123 | (20.7) | 4,334 | (6.8) | 16,992 | (26.7) | |
| ≥20% | 34,732 | 14,634 | (42.1) | 8,579 | (24.7) | 2,782 | (8.0) | 8,737 | (25.2) | |
All percentages are per row, and may not sum up to 100 due to rounding.
The “other specified” histologies consisted in 73% of large cell carcinoma (N=10,073), 16% of adenosquamous (N=2,250) and 11% of sarcomatoid (N=1,452) subtypes;
Excluding <1% of cases with unknown values;
prevalence in patient’s county of residence.
ADE was the predominant subtype (47%), followed by SQC (20%) and other specified subtypes (6%), but a large proportion of cases (27%) had unspecified histology. The “other specified” group consisted in 73% of large cell carcinoma (N=10,073), with 16% of adenosquamous (N=2,250) and 11% of sarcomatoid (N=1,452) subtypes. The proportion of cases diagnosed as adenocarcinoma was significantly larger among patients younger than 55 years (RR, 1.13; CI, 1.12-1.15, P<0.00001—compared with other age groups combined), women (RR, 1.20; CI, 1.19-1.21, P<0.00001) and Asians (RR, 1.22; CI, 1.20-1.24, P<0.00001—compared with other races combined). Conversely, the proportion of SQC was significantly higher in patients living in areas with high prevalence of smoking (RR, 1.22; CI, 1.20-1.24, P<0.00001), or poverty (RR, 1.20; CI, 1.18-1.22, P<0.00001).
The aggregate number of reported mNSCLC cases increased between 2000 (N=17,110) and 2006 (N=19,546, Figure 1), but was stable afterwards. Between 2005 and 2011 the proportion of ADE cases rapidly increased (from 43% to 57%) with a corresponding decrease in cases with unassigned histology (from 33% to 16%). The proportion of SQC cases increased slightly (from 18% to 22%).
Figure 1.
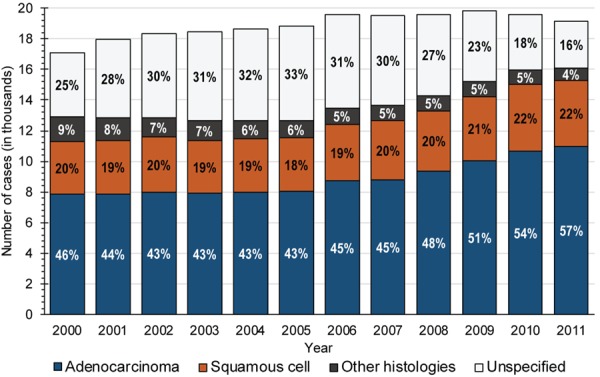
Number of cases diagnosed with histologic subtypes of metastatic non-small cell lung cancer, by year of diagnosis; percentage of each histology is listed.
Survival outcomes and trends
Crude (not age-standardized) RS was 26.6% (CI, 26.4-26.8) at 1 year and 4.0% (CI, 3.9-4.1) at 5 years, with OS estimates of 25.9% and 3.5%, respectively. One-year RS was significantly higher for ADE (30.3%; CI, 30.0-30.6) than for SQC (26.2%; CI, 25.7-26.6), other specified (21.6%; CI, 20.9-22.4) or NOS histology (21.8%; CI, 21.5-22.2). The most common recorded causes of death included: lung cancer (85.9%), “miscellaneous cancer” (3.9%), cardiac/cerebrovascular events (2.7%), and pulmonary disease (1.0%).
Overall, between 2000 and 2010, RS increased from 23.5% (CI, 22.9-24.0) to 30.5% (CI, 30.0-31.0), with an APC of +3.0% (CI, 2.7 to 3.3; Figure 2A). This was significantly larger for ADE (APC, +3.3%; CI, 2.9-3.7, Figure 2B) than for SQC (APC, +2.1%; CI, 1.8-2.4, Ppar=0.0018 versus ADE), other specified (APC, +2.1%; CI, 1.0-3.1, Ppar=0.042 versus ADE) or NOS histology (APC, +2.1%; CI, 1.2-2.9, Ppar=0.05 versus ADE). As a result of this divergence, the absolute discrepancy between 1-year RS of ADE and SQC more than tripled between 2000 and 2010, from 1.6% to 5.5%.
Figure 2.
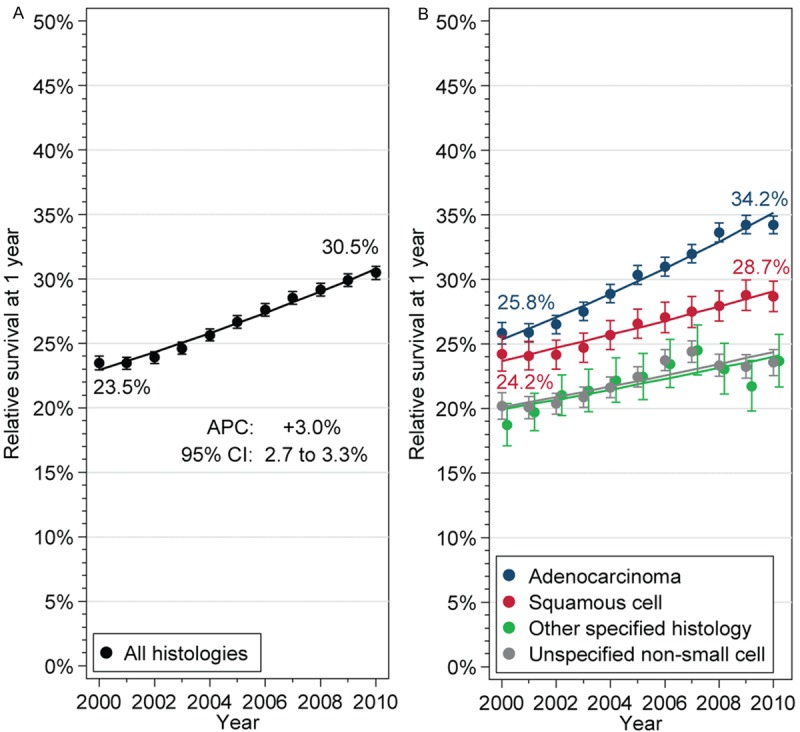
Trends in 1-year relative survival of patients with metastatic non-small cell lung cancer, for the entire cohort (A), and stratified by histologic subgroup (B). Point estimates with 95% confidence intervals (bars), and fitted values from log-linear models (lines) are shown. Selected point estimates in 2000 and 2010 are listed. APC, annual percent change; CI, confidence interval.
Survival trends in socio-demographic subgroups
Although younger patients had consistently better RS than those who were older (Figure 3A), the improvement occurred at a similar rate for both age groups. There was no significant difference between the slopes in any histology except for the “other specified” group. Similarly, women had numerically better RS than men (Figure 3B), and more so for ADE than SQC, but the trends did not differ between genders for either histology.
Figure 3.
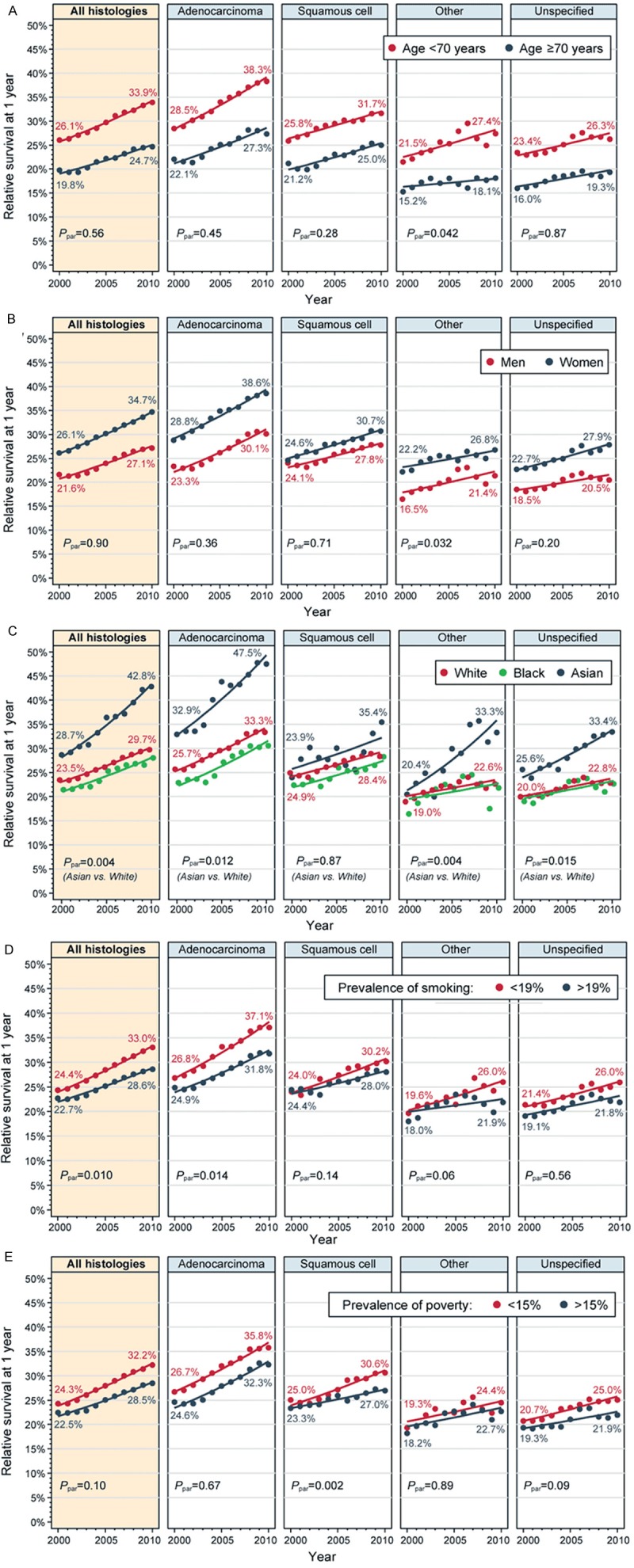
Estimated trends in 1-year relative survival for patients with metastatic non-small cell lung cancer in subpopulations defined by: (A) age at diagnosis, (B) sex, (C) race, (D) prevalent smoking in the patient’s county of residence (dichotomized at median), (E) prevalent poverty in the patient’s county of residence (dichotomized at median). Point estimates, observed values in 2000 and 2010, and fitted trends from log-linear models (lines) are shown. Rates in panels (B-E) are age-standardized. Ppar: P value from the test of slope parallelism.
In contrast, the trends were highly heterogeneous among racial subgroups (Figure 3C). RS increased significantly more for Asians (APC, +4.4%; CI, 3.9-4.9) than for Whites (APC, +2.8%; CI, 2.5-3.0, Ppar=0.004) or Blacks (APC, +3.0%; CI, 2.4-3.6, Ppar=0.02 versus Asians, Ppar=0.17 versus Whites). This inequality in trends between Asians and Whites was statistically significant for ADE (Ppar=0.012), but not for SQC (Ppar=0.87). In consequence, the disparity between Asian and White patients widened from absolute 5.2% in 2000 to 13.1% in 2010. Furthermore, by 2010, Asians with ADE had 1-year RS of 47.5%, contrasting with 33.3% among Whites and 30.5% among Blacks.
Survival among patients living in areas with fewer smokers was better (Figure 3D), and the improvement was more pronounced (APC, +3.4%; CI, 3.1-3.6) than in areas with higher prevalence of smokers (APC, +2.8%; CI, 2.5-3.5, Ppar=0.010). When analyzed by histology subtype, this difference in trends was only significant for ADE (Ppar=0.014). Conversely, the difference between areas with lower or higher prevalence of poverty was significant only for SQC (Ppar=0.002), with SQC patients from poorer areas experiencing little improvement (Figure 3E).
Discussion
In this population-based analysis, we compared updated survival trends among different mNSCLC histologies, for the first time studying the period when erlotinib, bevacizumab and pemetrexed became available. We found a significant increase in the proportion of cases designated as ADE between 2006 and 2011. Our main findings are that while survival improved in every histologic and clinical subgroup over the past decade, the extent of improvement was significantly larger for ADE than for SQC or other histologies. Additional growing disparities by race and smoking prevalence—factors associated with EGFR mutations—were evident in ADE, but absent in SQC. Given that benefits of supportive care should be similar regardless of histology, the trend differences between ADE and SQC can be plausibly explained by a differential effect of novel, histology-specific agents.
EGFR inhibitors (gefitinib and erlotinib), bevacizumab and pemetrexed have all improved survival of mNSCLC patients suitably selected in randomized trials. Representative one-year OS in those studies ranged from 43% in an international trial of cisplatin/pemetrexed, 51% for carboplatin-paclitaxel-bevacizumab in the US Easter Cooperative Oncology Group 4599 trial, to 68% for gefitinib in the East-Asian IPASS Study [1,2,6,7]. Most remarkably, Asian patients with EGFR mutations, which are present nearly exclusively in ADE, can now achieve median survival of 28 months when treated with first-line EGFR inhibitors [19]. Population-derived statistics in our analysis are markedly lower, encompassing patients who never received systemic therapy.
The effects of new treatments on population statistics have been difficult to disentangle from other phenomena concurrently affecting survival trends. Firstly, widespread application of positron emission tomography resulted in a significant stage migration between 1998 and 2003, seemingly improving stage-specific outcomes despite unchanged overall survival in aggregate NSCLC [20-22]. Stage migration explains our observation of the increasing absolute number of mNSCLC cases in 2000-2006, but not afterwards. Secondly, utilization of first- and second-line chemotherapy increased thanks to development of less toxic regimens, prophylactic growth factors, and recognition of the quality-of-life advantage provided by chemotherapy [23,24]. For example, researchers from the British Columbia Cancer Agency reported that the proportion of patients receiving chemotherapy increased from 16% to 34% between 1998 and 2006 (without difference between histologies), and their median OS increased from 9.4 to 11.0 months. In contrast, survival did not change for patients receiving supportive care alone [25]. Although similar evolution was confirmed in the US, the proportion of mNSCLC receiving chemotherapy remains in the 25 to 55% range, depending on population statistics, and is strongly influenced by patients’ age, comorbidities and performance status [26-29]. Thirdly, application of early, evidence-based palliative care and elimination of futile or excessively toxic therapies may also contribute to survival gains [30]. This contribution is difficult to quantify, because unfortunately delivery of palliative care remains suboptimal across the world, although it is not likely to differ for ADE and SQC [31-33].
In a 2008 meta-analysis of 16 randomized trials, standard platinum-based regimens showed the same survival benefit for ADE or SQC [23]. Some population-based studies attempted to interpret survival trends using the cohort approach with cut-points set at years of specific drug approvals, but this “historical control” methodology is confounded by other factors affecting survival. One such study using the SEER data showed improved OS in four cohorts diagnosed between 1990 and 2005, without any difference between ADE and SQC up to 2001, and a borderline disadvantage for SQC in 2002-2005 (hazard ratio, 1.03; CI, 1.00-1.06) [34]. Our period survival methodology is more efficient for evaluation of updated survival trends, which exhibit a substantial divergence between ADE and SQC during the past decade. Although interpretation of this finding is limited by lack of direct chemotherapy records in the SEER data, a differential effect of histology-specific treatments is plausible. The improvement in RS was identical for SQC, other specified and NOS histologies (APC, +2.1%), consistent with uniform effects of stage migration and supportive care, but it was significantly higher for ADE (APC, +3.3%). In consequence, the disparity between ADE and SQC, marginal in 2000, more than tripled by 2010. Additionally, after the approval of erlotinib, bevacizumab and pemetrexed, nearly half of the “unspecified” cases were successfully reclassified as ADE. This may represent clinicians’ greater efforts to determine specific histology using immunohistochemistry or repeat biopsies in an era when treatment decisions depend on explicit histology assignment [35].
Two studies used SEER data to compare survival of older mNSCLC patients receiving pemetrexed- or bevacizumab-containing chemotherapy, which was identified by linked Medicare claims. Owonikoko et al. found that enrollees who received pemetrexed (14%) or bevacizumab (7%) in 2004-2005 had better OS than those who did not, but the very small proportions of cases treated with those agents and rathera extreme hazard ratios (0.38 and 0.33, respectively) suggest an indication bias in this study, with the best-performing patients selectively receiving the novel drugs [36]. In fact, using the same data source, Zhu et al. found no survival difference with or without bevacizumab among 4,168 Medicare beneficiaries treated in 2002-2007 for non-SQC mNSCLC [37]. Two large observational studies reported on outcomes of first-line bevacizumab-containing regimens in the community. In a phase IV SAiL study of 2,212 patients from 40 countries treated with front-line bevacizumab (86% ADE), one-year OS was about 57% [38]. In the US ARIES cohort study of 1,967 patients (median age, 65 years), 1-year OS was about 52%, and was not significantly different between age groups [39]. Population-based statistics on the outcomes of EGFR inhibitor therapy in mNSCLC are limited to one post-marketing study of erlotinib in Japan, in which the outcomes were also similar for all age groups [40]. This corroborates our finding of parallel survival increase in younger and older patients over the past decade. In the US, Ritzwoller et al. presented utilization data on 6,614 mNSCLC cases diagnosed between 2000 and 2007 from four health maintenance organizations, indicating that that by 2007 approximately 12% of all mNSCLC patients received first-line erlotinib and 11% received bevacizumab-containing chemotherapy [27]. Survival outcomes were not available in that report.
EGFR mutations strongly correlate with female sex, ADE histology, Asian race and non-smoking status. In a multi-national screening study, they were detected in 40% of ADE (versus 3% of others subtypes), 51% of non-smokers (versus 10%), 42% of women (versus 14%) and 30% of Asians (versus 8% among other races) [4]. In a predominantly Caucasian Spanish cohort, they were present in 18% of ADE, 30% of women (versus 8%), 37.7% of never-smokers, but only 5.8% of current smokers [3]. We demonstrated a steep improvement in survival for Asians and for patients from areas with fewer smokers, but only within the ADE category. The largest treatment-related impact on survival in mNSCLC might thus be due to the efficacy of EGFR inhibitors in subpopulations enriched with the driver mutations. Interestingly, we additionally found a significant improvement in survival for Asians with other specified and NOS histology, generating a hypothesis that many of those patients may have benefitted from novel therapies as well. By virtue of geographic concentration, some racial minorities are known to be overrepresented in the SEER registries, which cover only 25% of Whites, but over 50% of Asians in the US. Female sex also correlates with EGFR mutation, yet survival trends for women were not different from men in our analysis. This suggests that many ADE cases in American women are tobacco-related rather than driven by specific mutations, thus mitigating the impact of targeted agents. Unfortunately, we did not have records of the actual EGFR mutation prevalence, and the use of surrogate survey-based measure of smoking further limits the interpretation. Moreover, our methodology enabled only univariate comparisons, but the studied socio-demographic factors likely correlate with each other. Nevertheless, the lower rate of improvement among SQC patients from areas with high poverty (APC of only +1.6%) potentially identifies a particularly vulnerable group with impaired access to chemotherapy and/or supportive care. In the US reports by Ritzwoller et al. and Davidoff et al., patients from areas with the highest median income had odds ratio of 1.4-1.5 for receipt of any chemotherapy when compared with the poorest areas [26,27].
In conclusion, the beneficial effects of ADE-specific systemic therapies in mNSCLC are now detectable on a population scale. Growing disparities between ADE and SQC and between subpopulations with high or low estimated prevalence of EGFR mutations underscore the need to improve treatments for groups that did not benefit from the recent advances. In fact, such patients still constitute a majority of mNSCLC cases in the US. Clinical trials using immunotherapy that disrupt the programmed death-1 (PD-1) signaling pathway or agents that target newly identified oncogenic pathways operational in SQC hold the promise of curbing the current growing disparities [41,42].
Acknowledgements
This study used the SEER database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; Information Management Services Inc; and the SEER program registries in the creation and maintenance of the database as a research resource. Preliminary data from this study were presented at the 2014 Chicago Multidisciplinary Symposium in Thoracic Oncology, October 30-November 1, 2014, Chicago, IL.
Disclosure of conflict of interest
None.
References
- 1.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 2.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, Longo F, Moran T, Insa A, De Marinis F, Corre R, Bover I, Illiano A, Dansin E, de Castro J, Milella M, Reguart N, Altavilla G, Jimenez U, Provencio M, Moreno MA, Terrasa J, Munoz-Langa J, Valdivia J, Isla D, Domine M, Molinier O, Mazieres J, Baize N, Garcia-Campelo R, Robinet G, Rodriguez-Abreu D, Lopez-Vivanco G, Gebbia V, Ferrera-Delgado L, Bombaron P, Bernabe R, Bearz A, Artal A, Cortesi E, Rolfo C, Sanchez-Ronco M, Drozdowskyj A, Queralt C, de Aguirre I, Ramirez JL, Sanchez JJ, Molina MA, Taron M, Paz-Ares L Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 3.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, Insa A, Massuti B, Gonzalez-Larriba JL, Paz-Ares L, Bover I, Garcia-Campelo R, Moreno MA, Catot S, Rolfo C, Reguart N, Palmero R, Sanchez JM, Bastus R, Mayo C, Bertran-Alamillo J, Molina MA, Sanchez JJ, Taron M Spanish Lung Cancer Group. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 4.Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, Fujisawa T, Feng Z, Roth JA, Herz J, Minna JD, Gazdar AF. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 5.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 6.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 7.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S, Rolski J, Goksel T, de Marinis F, Simms L, Sugarman KP, Gandara D. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 8.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER-18 Research Data + Hurricane Katrina Impacted Louisiana Cases, Linked To County Attributes - Total US 1969-2012 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014 (updated 5/7/2014), based on the November 2013 submission. 2013
- 9.Travis WD World Health Organization. International Agency for Research on Cancer. International Association for the Study of Lung Cancer and International Academy of Pathology. Pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon, France: IARC Press; 2004. [Google Scholar]
- 10.Young JL Jr, Roffers SD, Ries LAG, Fritz AG, HA , editors. SEER Summary Staging Manual - 2000: Codes and Coding Instructions. Bethesda, MD: National Cancer Institute; 2001. NIH Pub. No. 01-4969. [Google Scholar]
- 11.Bishop KD, Olszewski AJ. Epidemiology and survival outcomes of ocular and mucosal melanomas: a population-based analysis. Int J Cancer. 2014;134:2961–2971. doi: 10.1002/ijc.28625. [DOI] [PubMed] [Google Scholar]
- 12.Raghunathan TE, Xie D, Schenker N, Parsons VL, Davis WW, Dodd KW, Feuer EJ. Combining Information From Two Surveys to Estimate County-Level Prevalence Rates of Cancer Risk Factors and Screening. J Am Stat Assoc. 2007;102:474–486. [Google Scholar]
- 13.Dickman PW, Adami HO. Interpreting trends in cancer patient survival. J Intern Med. 2006;260:103–117. doi: 10.1111/j.1365-2796.2006.01677.x. [DOI] [PubMed] [Google Scholar]
- 14.Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer. 2004;40:2307–2316. doi: 10.1016/j.ejca.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Brenner H, Hakulinen T. Period estimates of cancer patient survival are more up-to-date than complete estimates even at comparable levels of precision. J Clin Epidemiol. 2006;59:570–575. doi: 10.1016/j.jclinepi.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Cronin K, Mariotto A, Scoppa S, Green D, Clegg L, editors. Differences Between Brenner et al. and NCI Methods for Calculating Period Survival. Technical Report # 2003-02-A. Statistical Research and Applications Branch, National Cancer Institute. 2003
- 17.Deddens JA, Petersen MR. Approaches for estimating prevalence ratios. Occup Environ Med. 2008;65:481, 486–501. doi: 10.1136/oem.2007.034777. [DOI] [PubMed] [Google Scholar]
- 18.Kim HJ, Fay MP, Yu B, Barrett MJ, Feuer EJ. Comparability of segmented line regression models. Biometrics. 2004;60:1005–1014. doi: 10.1111/j.0006-341X.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- 19.Inoue A, Kobayashi K, Maemondo M, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Saijo Y, Hagiwara K, Morita S, Nukiwa T North-East Japan Study Group. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002) Ann Oncol. 2013;24:54–59. doi: 10.1093/annonc/mds214. [DOI] [PubMed] [Google Scholar]
- 20.Dinan MA, Curtis LH, Carpenter WR, Biddle AK, Abernethy AP, Patz EF Jr, Schulman KA, Weinberger M. Stage migration, selection bias, and survival associated with the adoption of positron emission tomography among medicare beneficiaries with non-small-cell lung cancer, 1998-2003. J. Clin. Oncol. 2012;30:2725–2730. doi: 10.1200/JCO.2011.40.4392. [DOI] [PubMed] [Google Scholar]
- 21.Morgensztern D, Goodgame B, Baggstrom MQ, Gao F, Govindan R. The effect of FDG-PET on the stage distribution of non-small cell lung cancer. J Thorac Oncol. 2008;3:135–139. doi: 10.1097/JTO.0b013e3181622c2c. [DOI] [PubMed] [Google Scholar]
- 22.Morgensztern D, Ng SH, Gao F, Govindan R. Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J Thorac Oncol. 2010;5:29–33. doi: 10.1097/JTO.0b013e3181c5920c. [DOI] [PubMed] [Google Scholar]
- 23.NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and metaanalysis of individual patient data from 16 randomized controlled trials. J. Clin. Oncol. 2008;26:4617–4625. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dooms CA, Pat KE, Vansteenkiste JF. The effect of chemotherapy on symptom control and quality of life in patients with advanced non-small cell lung cancer. Expert Rev Anticancer Ther. 2006;6:531–544. doi: 10.1586/14737140.6.4.531. [DOI] [PubMed] [Google Scholar]
- 25.Ho C, Ramsden K, Zhai Y, Murray N, Sun S, Melosky B, Laskin J. Less toxic chemotherapy improves uptake of all lines of chemotherapy in advanced non-small-cell lung cancer: a 10-year retrospective population-based review. J Thorac Oncol. 2014;9:1180–1186. doi: 10.1097/JTO.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 26.Davidoff AJ, Tang M, Seal B, Edelman MJ. Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 2010;28:2191–2197. doi: 10.1200/JCO.2009.25.4052. [DOI] [PubMed] [Google Scholar]
- 27.Ritzwoller DP, Carroll NM, Delate T, Hornbrook MC, Kushi L, Aiello Bowles EJ, Freml JM, Huang K, Loggers ET. Patterns and predictors of first-line chemotherapy use among adults with advanced non-small cell lung cancer in the cancer research network. Lung Cancer. 2012;78:245–252. doi: 10.1016/j.lungcan.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasco DW, Yan J, Xie Y, Dowell JE, Gerber DE. Looking beyond surveillance, epidemiology, and end results: patterns of chemotherapy administration for advanced non-small cell lung cancer in a contemporary, diverse population. J Thorac Oncol. 2010;5:1529–1535. doi: 10.1097/JTO.0b013e3181e9a00f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S, Wong ML, Hamilton N, Davoren JB, Jahan TM, Walter LC. Impact of age and comorbidity on non-small-cell lung cancer treatment in older veterans. J. Clin. Oncol. 2012;30:1447–1455. doi: 10.1200/JCO.2011.39.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF, Billings JA, Lynch TJ. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 31.Hui D, Elsayem A, De la Cruz M, Berger A, Zhukovsky DS, Palla S, Evans A, Fadul N, Palmer JL, Bruera E. Availability and integration of palliative care at US cancer centers. JAMA. 2010;303:1054–1061. doi: 10.1001/jama.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith CB, Nelson JE, Berman AR, Powell CA, Fleischman J, Salazar-Schicchi J, Wisnivesky JP. Lung cancer physicians’ referral practices for palliative care consultation. Ann Oncol. 2012;23:382–387. doi: 10.1093/annonc/mdr345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vergnenegre A, Hominal S, Tchalla AE, Berard H, Monnet I, Fraboulet G, Baize N, Audigier-Valette C, Robinet G, Oliviero G, Le Caer H, Thomas P, Geriniere L, Mastroianni B, Chouaid C GFPC team. Assessment of palliative care for advanced non-small-cell lung cancer in France: a prospective observational multicenter study (GFPC 0804 study) Lung Cancer. 2013;82:353–357. doi: 10.1016/j.lungcan.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Morgensztern D, Waqar S, Subramanian J, Gao F, Govindan R. Improving survival for stage IV non-small cell lung cancer: a surveillance, epidemiology, and end results survey from 1990 to 2005. J Thorac Oncol. 2009;4:1524–1529. doi: 10.1097/JTO.0b013e3181ba3634. [DOI] [PubMed] [Google Scholar]
- 35.Langer CJ, Besse B, Gualberto A, Brambilla E, Soria JC. The evolving role of histology in the management of advanced non-small-cell lung cancer. J. Clin. Oncol. 2010;28:5311–5320. doi: 10.1200/JCO.2010.28.8126. [DOI] [PubMed] [Google Scholar]
- 36.Owonikoko TK, Ragin C, Chen Z, Kim S, Behera M, Brandes JC, Saba NF, Pentz R, Ramalingam SS, Khuri FR. Real-world effectiveness of systemic agents approved for advanced nonsmall cell lung cancer: a SEER-Medicare analysis. Oncologist. 2013;18:600–610. doi: 10.1634/theoncologist.2012-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu J, Sharma DB, Gray SW, Chen AB, Weeks JC, Schrag D. Carboplatin and paclitaxel with vs without bevacizumab in older patients with advanced non-small cell lung cancer. JAMA. 2012;307:1593–1601. doi: 10.1001/jama.2012.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crino L, Dansin E, Garrido P, Griesinger F, Laskin J, Pavlakis N, Stroiakovski D, Thatcher N, Tsai CM, Wu YL, Zhou C. Safety and efficacy of first-line bevacizumab-based therapy in advanced non-squamous non-small-cell lung cancer (SAiL, MO19390): a phase 4 study. Lancet Oncol. 2010;11:733–740. doi: 10.1016/S1470-2045(10)70151-0. [DOI] [PubMed] [Google Scholar]
- 39.Lynch TJ Jr, Spigel DR, Brahmer J, Fischbach N, Garst J, Jahanzeb M, Kumar P, Vidaver RM, Wozniak AJ, Fish S, Flick ED, Leon L, Hazard SJ, Kosty MP, Investigators AS. Safety and effectiveness of bevacizumab-containing treatment for non-small-cell lung cancer: final results of the ARIES observational cohort study. J Thorac Oncol. 2014;9:1332–1339. doi: 10.1097/JTO.0000000000000257. [DOI] [PubMed] [Google Scholar]
- 40.Yoshioka H, Komuta K, Imamura F, Kudoh S, Seki A, Fukuoka M. Efficacy and safety of erlotinib in elderly patients in the phase IV POLARSTAR surveillance study of Japanese patients with non-small-cell lung cancer. Lung Cancer. 2014;86:201–206. doi: 10.1016/j.lungcan.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 41.Perez-Moreno P, Brambilla E, Thomas R, Soria JC. Squamous cell carcinoma of the lung: molecular subtypes and therapeutic opportunities. Clin Cancer Res. 2012;18:2443–2451. doi: 10.1158/1078-0432.CCR-11-2370. [DOI] [PubMed] [Google Scholar]
- 42.Champiat S, Ileana E, Giaccone G, Besse B, Mountzios G, Eggermont A, Soria JC. Incorporating immune-checkpoint inhibitors into systemic therapy of NSCLC. J Thorac Oncol. 2014;9:144–153. doi: 10.1097/JTO.0000000000000074. [DOI] [PubMed] [Google Scholar]


