Abstract
The majority of sporadic forms of colorectal carcinomas is characterized by deregulation of Wnt/β-catenin signaling early in colorectal carcinogenesis. As a consequence, ITF-2B protein levels are increased in adenomas of these patients. However, ITF-2B protein levels are strongly reduced with increasing carcinoma stages, suggesting that reduction of ITF-2B protein is required for progression of adenomas to colorectal carcinomas. To find out if ITF-2B protein levels are correlated with the survival of patients with colorectal carcinomas, a tissue microarray containing samples from 213 colorectal carcinomas (T-categories T2 and T3) with corresponding survival information was stained with an ITF-2B antibody. In addition, we analyzed if detection of ITF-2B in microsatellite instable and microsatellite stable carcinomas as well as in colorectal carcinomas with KRAS mutations is correlated with survival. Detection of cytoplasmic ITF-2B protein was associated with better overall and progression free survival of patients with colorectal carcinomas (P=0.033 and 0.024, respectively). Multivariate Cox regression analysis revealed an increased risk to suffer from poor overall survival and recurrent disease if no cytoplasmic ITF-2B was detectable (HR=1.91; P=0.033 and HR=1.75; P=0.033, respectively). Similarly, patients with MSS carcinomas had a better overall survival, if they showed cytoplasmic positivity for ITF-2B (P=0.013). Remarkably, patients with colorectal carcinomas carrying KRAS mutations had a better overall and progression free survival rate if the carcinomas were positive for cytoplasmic ITF-2B (HR=4.71; P=0.002 and HR=2.57; P=0.024, respectively). These data suggest that cytoplasmic protein levels of ITF-2B could be used as a prognostic marker for patients with colorectal carcinomas.
Keywords: ITF2, MSI, MSS, CIN, KRAS, colorectal carcinomas, prognosis
Introduction
Colorectal carcinomas (CRCs) are the third most common form of cancer and are characterized by high cancer-related morbidity. In sporadic forms of CRCs, inactivation of the tumor suppressor gene adenomatous polyposis coli (APC) is a frequent event that initiates the formation of aberrant crypt foci (reviewed in [1]) and results in stabilization and nuclear translocation of β-catenin. Subsequently, β-catenin activates the transcription of its target genes [1,2]. One of the β-catenin target genes is ITF2, encoding the basic helix-loop-helix (bHLH) protein and transcription factor ITF-2B [3].
Apart from APC, KRAS is frequently mutated in colorectal tumors. Activating mutations are present in approximately 40% of all colorectal tumors and result in promoting the growth of adenomatous lesions [1,4]. KRAS is able to phosphorylate β-catenin and induce the dissociation of β-catenin and E-cadherin, resulting in induction of transcriptionally active β-catenin [1,2,5]. In clinical therapies targeting EGFR signalling with monoclonal antibodies, detection of KRAS mutations is a robust predictive marker of therapy resistance.
Another useful prognostic marker is the microsatellite status: When compared to colorectal carcinomas patients with chromosomal instable carcinomas (CIN), CRC patients with microsatellite instable (MSI) carcinomas have been shown to have a better prognosis [3,6]. Furthermore, patients with MSI appear not to benefit from adjuvant therapy with 5-FU, therefore analyzing the MSI status can be used to discriminate between low- (MSI) and and high-risk (microsatellite stable (MSS)) patients and treat the patients accordingly [7]. Apart from patients with MSI, patients with intact chromosome 18q are classified as low-risk patients [1,8]. Interestingly, this chromosomal arm also contains the gene ITF2 [2,9].
In normal colonic epithelium, ITF-2B protein is hardly detectable. In contrast, in our previous study we demonstrated that increased levels of ITF-2B occur in adenomas where it was found in the cytoplasm and nucleus. However, with increasing carcinoma stages, protein levels of ITF-2B are frequently reduced [3,9], suggesting that ITF2 acts as a tumor suppressor gene [1,4,9]. Here, we investigated the correlation between ITF-2B protein levels in colorectal carcinomas and overall or progression free survival with respect to KRAS mutational and MSI status. Our data suggest that detection of cytoplasmic ITF-2B levels can be used as a prognostic marker for patients with colorectal carcinomas and identify individuals with a greater risk of poor overall survival and disease recurrence.
Materials and methods
Clinical samples
This study included carcinoma material from patients with colorectal adenocarcinomas exhibiting moderate differentiation (G2 according to WHO), comprising T-categories T2 and T3 and having neither nodal (N0) nor distant metastasis (M0) at the time of diagnosis. Patient material was taken from the archives of the University of Munich. Only patients were considered who underwent intentionally curative surgical resection between 1994 and 2004. Follow up data were available from the Tumorregister München. To reduce effects directly related to surgery, specimens of patients who died within 6 months after surgical resection were excluded. The final case collection contained tissue from 213 patients, of whom 91 (43%) died from colorectal carcinomas within 5 years of diagnosis. The survival data of 166 cases (78%) was censored as case follow up was discontinued or patients died of reasons other than colorectal carcinomas. Case characteristics are summarized in Table 1. The study complied with the requirements of the local ethics committee.
Table 1.
Clinicopathological characteristics of the investigated colorectal carcinomas cases (n=213)
| Variable | Number of cases | % |
|---|---|---|
| Gender | ||
| Male | 118 | 55 |
| Female | 95 | 45 |
| Age, y | ||
| < 65 | 74 | 35 |
| ≥ 65 | 139 | 65 |
| T-category | ||
| T2 | 34 | 16 |
| T3 | 179 | 84 |
| Cancer specific survival, y | ||
| < 5 | 91 | 43 |
| ≥ 5 | 122 | 57 |
| Censored | 166 | 78 |
Tissue microarray technique
Tissue microarrays (TMA) from colorectal carcinomas were generated as described previously [10].
Immunohistochemistry
Paraffin sections (5 μm) were stained with anti-β-catenin monoclonal mouse antibody (1:150, BD Biosciences, NJ, USA) and ITF-2B antibody [1,9] using standard immunohistochemical procedures.
Analyses of KRAS mutations
Analyses of KRAS exon 2 codon 12/13 were done as previously described [1,4,11].
Microsatellite stability analysis
Status of microsatellite stability [microsatellite stability (MSS) or high-grade microsatellite instability (MSIH)] was investigated as previously described analyzing the two mononucleotide repeat markers BAT-25 and BAT-26 [6,12-14].
Evaluation of ITF-2B and β-catenin immunohistochemistry
ITF-2B staining was positive in the nucleus of carcinoma cells consistent with its function as transcription factor. Additionally we found a cytoplasmic staining which was evaluated regarding positivity of staining: score 0: no staining, score 1: positive staining (Figure 1Aa, 1Ab). Nuclear staining was evaluated regarding intensity of staining: score 0: no or weak staining, score 1: strong staining (Figure 1Ac, 1Ad). Nuclear β-catenin staining was evaluated as previously described [7,15]. Membranous β-catenin staining was not considered in the evaluation. To exclude intraobserver variability, an observer who had no prior knowledge of prognosis or other clinicopathological variables evaluated the specimens thrice.
Figure 1.
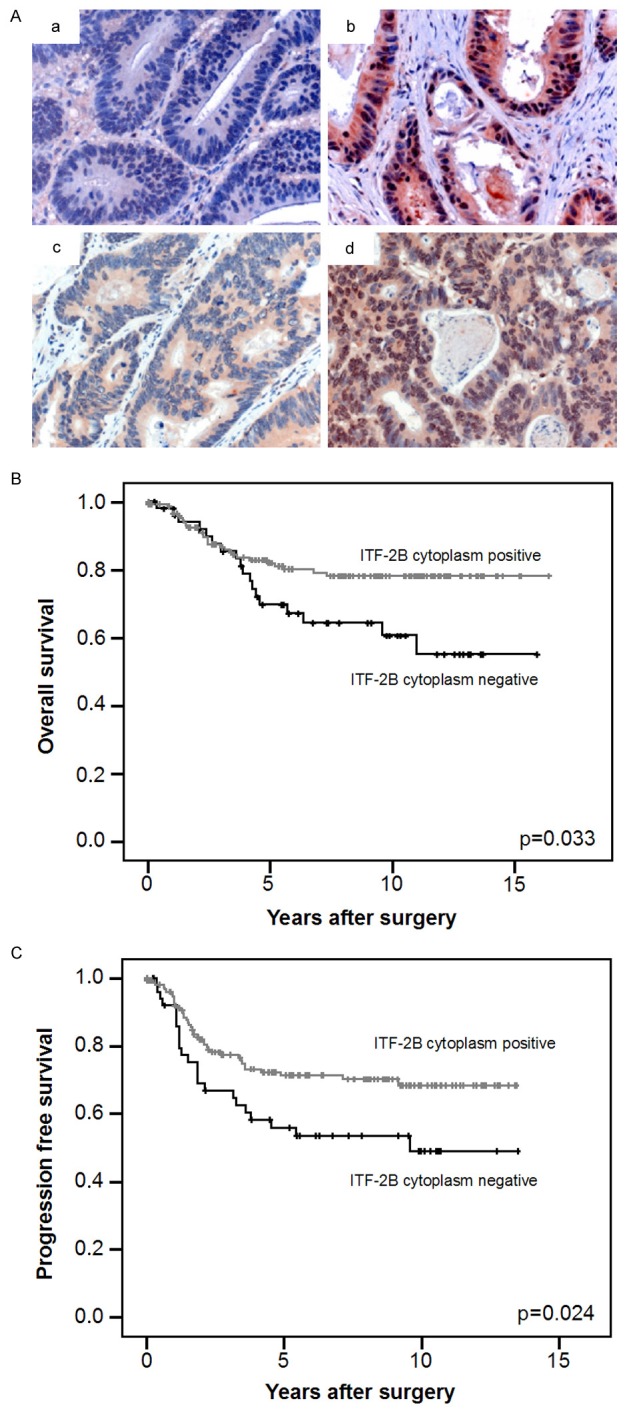
Correlation of ITF-2B protein levels with survival. (A) (a, b) Cytoplasmic ITF-2B levels in human colorectal carcinomas: 52 (24%) cases displayed no cytoplasmic ITF-2B (a), whereas 161 (76%) showed cytoplasmic ITF-2B (b). (c, d) Intensity of nuclear ITF-2B protein staining in human colorectal carcinomas: 105 cases (49%) showed no or low intensity of nuclear ITF-2B staining (c), whereas 108 cases (51%) displayed high ITF-2B protein levels (d). (Original magnification: 400 ×). (B) Cytoplasmic ITF-2B levels correlated with overall survival. Kaplan-Meier plot of colorectal carcinomas specimens (n=213) demonstrated significant (log-rank test) better survival with cytoplasmic ITF-2B levels (P=0.033). (C) Cytoplasmic ITF-2B levels correlated with progression free survival. Kaplan-Meier plot of colorectal carcinomas specimens demonstrated significant (log-rank test) better survival with cytoplasmic ITF-2B levels (P=0.024).
Statistical analyses
Cross-tabulations were calculated using Fisher’s exact test. Kaplan-Meier analysis was employed to estimate cancer specific survival. Significance of the Kaplan-Meier statistic was tested applying the log-rank test. Multivariate analysis was done by using the multivariate Cox regression model. P-values < 0.05 were considered statistically significant. Statistics were performed using SPSS statistical software (version 15.0; SPSS Inc., Chicago, IL).
Results
ITF-2B protein levels in colorectal carcinomas
To investigate the localization of ITF-2B in human colorectal carcinomas, we evaluated the protein levels by immunostaining of tissue microarrays. ITF-2B was found to be positive in the cytoplasm of 161 cases (76%), whereas 52 (24%) cases displayed no cytoplasmic ITF-2B levels. No or low intensity of nuclear ITF-2B staining was found in 105 cases (49%), whereas 108 cases (51%) displayed strong nuclear ITF-2B staining. Cytoplasmic ITF-2B levels were significantly correlated with intensity of nuclear ITF-2B staining (P=0.001).
Because ITF2 has been identified as a β-catenin target gene, we next evaluated ITF-2B protein levels in relation to nuclear β-catenin levels. 162 (81%) cases were positive for nuclear β-catenin staining, while 38 (19%) were negative, which is in accordance to the literature [9,15]. Two types of nuclear β-catenin staining patterns have been defined: 1) Colorectal carcinomas with conserved intratumoral regulation and heterogeneous distribution of β-catenin. 2) Colorectal carcinomas with a homogeneous distribution of β-catenin, which showed no intratumoral regulation of β-catenin. Cytoplasmic detection of ITF-2B was found more often in cases with conserved β-catenin regulation (P=0.033). Intensity of nuclear ITF-2B staining did not correlate with nuclear β-catenin levels (P=0.355).
Detection of cytoplasmic ITF-2B in colorectal carcinomas correlates with patient survival
In Kaplan-Meier analyses, cytoplasmic ITF-2B positivity was associated with a significantly better overall and progression free survival of patients with colorectal carcinomas as compared to cytoplasmic ITF-2B negativity (P=0.033 and P=0.024, respectively; Figure 1B and 1C). Intensity of nuclear ITF-2B staining, however, was not significantly correlated with overall and progression free survival (P=0.308 and P=0.261, respectively). Age (P=0.511), gender (P=0.688) and T-category (P=0.762) were not significantly associated with outcome. In a multivariate Cox regression analysis including gender, age and T-category, cytoplasmic ITF-2B negativity indicated an independent relative risk of 1.91 of poor overall survival and of 1.75 of disease recurrence compared to ITF-2B positivity (P=0.033 in each case).
Cytoplasmic ITF-2B staining in microsatellite stable (MSS) colorectal carcinomas correlated with patient survival
Since colorectal carcinomas with high levels of microsatellite instability (MSI-H) have a good prognosis, we analyzed if ITF-2B levels were correlated with survival or disease recurrence of the affected patients. For MSI analyses material was available in 177 cases. 63 (36%) cases showed microsatellite instability. Within this group of microsatellite instable cases no correlation was found between cytoplasmic ITF-2B levels and intensity of nuclear ITF-2B staining with β-catenin regulation pattern, clinicopathological variables or patients’ survival (data not shown).
When comparing cytoplasmic ITF-2B levels and intensity of nuclear ITF-2B staining in microsatellite stable (MSS) cases with the clinicopathological variables age, gender, T-category and KRAS mutation status of the carcinoma, no correlation was observed applying Fisher’s exact test (data not shown). In Kaplan-Meier analyses cytoplasmic ITF-2B positivity and intensity of nuclear ITF-2B staining were associated with a significant better overall survival of patients with MSS colorectal carcinomas (P=0.013 and P=0.033 respectively; Supplementary Figure 1). In contrast, cytoplasmic ITF-2B positivity and intensity of nuclear ITF-2B staining were not associated with progression free survival (P=0.092 and P=0.105, respectively). In multivariate Cox regression analysis, cytoplasmic ITF-2B negativity in MSS carcinomas indicated an independent relative risk of 2.58 compared to ITF-2B positivity of poor overall survival (P=0.023). Intensity of nuclear staining proved to be an independent factor for overall survival in a multivariate Cox regression analyses (HR=1.55; P=0.045).
Cytoplasmic ITF-2B levels in KRAS mutated colorectal carcinomas correlates with patient survival
Because KRAS is capable of dissociating β-catenin and E-cadherin and thereby promotes the transcription of β-catenin target genes, for example the ITF2 gene, we were interested to find out if patients with KRAS mutations benefit from ITF-2B protein. Material for KRAS mutational analyses was available in 180 cases. 68 (38%) cases displayed KRAS mutation in exon 2 codon 12 or codon 13. Within this group of KRAS mutated cases, 51 (75%) cases showed positive cytoplasmic ITF-2B levels. Cytoplasmic ITF-2B was significantly found more often in cases with conserved β-catenin regulation (P=0.021). No association was found with age (P=0.552), gender (P=0.556), T-category (P=0.656) and MSI (P=0.272). In accordance with a previous publication [9,16], the comparison of the survival curves for patients with KRAS mutations and wild-type KRAS did not show a significant difference in overall survival (P=0.640; Figure 2A). In KRAS wild-type cases no correlation was found between cytoplasmic ITF-2B levels and patients’ overall and progression free survival (P=0.635 and P=0.917, respectively). Remarkably, in Kaplan-Meier analyses cytoplasmic ITF-2B levels correlated significantly with a better overall and progression free survival of patients with KRAS mutations (P=0.001 and P=0.023, respectively; Figure 2B and 2C). Cytoplasmic ITF-2B negativity indicated an independent relative risk of 4.71 compared to ITF-2B positivity for overall survival and 2.57 for progression free survival in a multivariate Cox regression analysis including gender, age and T-category (P=0.002 and P=0.024, respectively).
Figure 2.
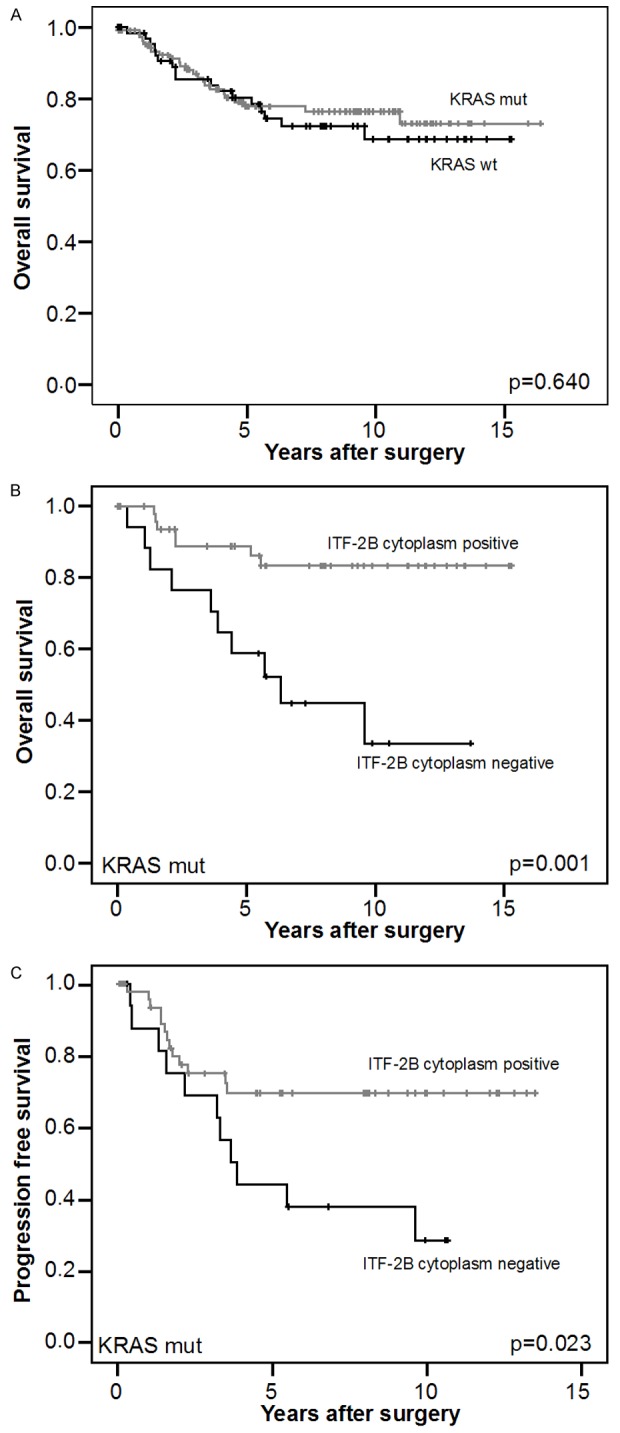
Correlation of ITF-2B protein levels with survival in KRAS mutated cases. A. Kaplan-Meier plot of patients with known KRAS status (n=180) demonstrated no significant (log-rank test) better survival of patients with KRAS wild-type (KRAS wt) versus patients with KRAS mutations (KRAS mut) (P=0.640). B. Cytoplasmic ITF-2B levels correlated with good overall survival in KRAS mut cases. Kaplan-Meier plot of colorectal carcinomas specimens demonstrated significant (log-rank test) better survival with cytoplasmic ITF-2B levels (P=0.001). C. Cytoplasmic ITF-2B levels correlated with good progression free survival in KRAS mut cases. Kaplan-Meier plot of colorectal carcinomas specimens demonstrated significant (log-rank test) better survival with cytoplasmic ITF-2B levels (P=0.023).
Discussion
Aberrant activation of β-catenin occurs early in colorectal carcinogenesis and contributes to the development of adenomatous lesions. Deregulation of β-catenin also results in enhanced transcription of the gene ITF2, encoding the protein ITF-2B, in adenomas [9]. However, with increasing carcinoma stages ITF-2B levels are strongly reduced, suggesting that reduction of ITF-2B levels might be necessary for the progression of adenomas to colorectal carcinomas [9,10]. This notion is supported by previous publications suggesting that patients with an intact chromosomal arm 18q have a better prognosis [8]. Furthermore, Shin et al. found that loss of ITF-2B in colorectal carcinoma tissues was associated with poor patient outcomes [17]. To extend these data, we analyzed a well-characterized, homogeneous collection of T2 and T3, N0, M0 human colorectal carcinomas, since the majority of late stage carcinomas frequently lost ITF-2B protein and have a poor prognosis in general. Our results demonstrated that patients with colorectal carcinomas have a better survival if their carcinomas show cytoplasmic ITF-2B protein levels. In addition, cytoplasmic ITF-2B negativity proved to be an independent prognostic marker for poor survival. The observation that detection of cytoplasmic ITF-2B is correlated with better survival is surprising since ITF-2B has been primarily described as a transcription factor. A similar distribution pattern has been described for the bHLH protein BHLHB2: In normal pancreatic cells, it is detected in the nucleus. However, in cancer cells BHLHB2 was also localized in the cytoplasm [18]. Furthermore, patients with endometrial cancer showed predominantly cytoplasmic localization of the bHLH protein TWIST [19]. This data suggests that the bHLH protein ITF-2B protein also has cytoplasmic functions that interfere with the development of carcinomas and recurring diseases.
Interestingly, high cytoplasmic ITF-2B levels in colorectal carcinomas with KRAS mutations were associated with a better survival compared to corresponding carcinomas lacking cytoplasmic ITF-2B. Additionally patients with MSS carcinomas showed increased overall survival rates if these carcinomas were positive for ITF-2B either in the nucleus or in the cytoplasm. These observations support the notion that ITF-2B interferes with the progression of colorectal carcinomas. In contrast patients with microsatellite instability did not benefit from ITF-2B positivity confirming that MSI colorectal carcinomas are low-risk carcinomas.
In summary, we conclude that detection of cytoplasmic ITF-2B levels in colorectal carcinomas is a useful prognostic marker to identify patients that have increased overall survival rates and a lower risk to suffer from disease recurrence. However, prospective studies have to confirm the prognostic value of ITF-2B detection and mouse models would help to elucidate the effect of KRAS on the amount of ITF-2B and the role of ITF-2B in this context [20].
Acknowledgements
We thank A. Heier and A. Sendelhofert for their excellent technical assistance. This project was supported by the grant KO1826/5 to F.T.K. by the German Research Council (DFG).
Disclosure of conflict of interest
The authors declare no conflict of interest.
Abbreviations
- APC
adenomatous polyposis coli
- bHLH
basic helix-loop-helix
- CIN
chromosomal instability
- CRC
colorectal carcinomas
- EGFR
epidermal growth factor receptor
- 5-FU
5-Fluorouracil
- G
grading
- HR
hazard ratio
- KRAS
Kirsten rat sarcoma
- MSI
microsatellite instable
- MSS
microsatellite stable
- TMA
tissue microarray
Supporting Information
References
- 1.Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 2.Herbst A, Jurinovic V, Krebs S, Thieme SE, Blum H, Göke B, Kolligs FT. Comprehensive analysis of β-catenin target genes in colorectal carcinoma cell lines with deregulated Wnt/β-catenin signaling. BMC Genomics. 2014;15:74. doi: 10.1186/1471-2164-15-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolligs FT, Nieman MT, Winer I, Hu G, Van Mater D, Feng Y, Smith IM, Wu R, Zhai Y, Cho KR, Fearon ER. ITF-2, a downstream target of the Wnt/TCF pathway, is activated in human cancers with beta-catenin defects and promotes neoplastic transformation. Cancer Cell. 2002;1:145–55. doi: 10.1016/s1535-6108(02)00035-1. [DOI] [PubMed] [Google Scholar]
- 4.Phelps RA, Chidester S, Dehghanizadeh S, Phelps J, Sandoval IT, Rai K, Broadbent T, Sarkar S, Burt RW, Jones DA. A two-step model for colon adenoma initiation and progression caused by APC loss. Cell. 2009;137:623–34. doi: 10.1016/j.cell.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinch MS, Clark GJ, Der CJ, Burridge K. Tyrosine phosphorylation regulates the adhesions of ras-transformed breast epithelia. J Cell Biol. 1995;130:461–71. doi: 10.1083/jcb.130.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walther A, Houlston R, Tomlinson I. Association between chromosomal instability and prognosis in colorectal cancer: a meta-analysis. Gut. 2008;57:941–50. doi: 10.1136/gut.2007.135004. [DOI] [PubMed] [Google Scholar]
- 7.Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, Hamilton SR, Laurent-Puig P, Gryfe R, Shepherd LE, Tu D, Redston M, Gallinger S. Tumor microsatelliteinstability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–57. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Popat S, Houlston RS. A systematic review and meta-analysis of the relationship between chromosome 18q genotype, DCC status and colorectal cancer prognosis. Eur J Cancer. 2005;41:2060–70. doi: 10.1016/j.ejca.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 9.Herbst A, Bommer GT, Kriegl L, Jung A, Behrens A, Csanadi E, Gerhard M, Bolz C, Riesenberg R, Zimmermann W, Dietmaier W, Wolf I, Brabletz T, Göke B, Kolligs FT. ITF-2 is disrupted via allelic loss of chromosome 18q21, and ITF-2B expression is lost at the adenoma-carcinoma transition. Gastroenterology. 2009;137:639–648. 648.e1–9. doi: 10.1053/j.gastro.2009.04.049. [DOI] [PubMed] [Google Scholar]
- 10.Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–7. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 11.Neumann J, Zeindl-Eberhart E, Kirchner T, Jung A. Frequency and type of KRAS mutations in routine diagnostic analysis of metastatic colorectal cancer. Pathol Res Pract. 2009;205:858–62. doi: 10.1016/j.prp.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–57. [PubMed] [Google Scholar]
- 13.Deschoolmeester V, Baay M, Wuyts W, Van Marck E, Van Damme N, Vermeulen P, Lukaszuk K, Lardon F, Vermorken JB. Detection of microsatellite instability in colorectal cancer using an alternative multiplex assay of quasimonomorphic mononucleotide markers. J Mol Diagn. 2008;10:154–9. doi: 10.2353/jmoldx.2008.070087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kriegl L, Jung A, Horst D, Rizzani A, Jackstadt R, Hermeking H, Gallmeier E, Gerbes AL, Kirchner T, Göke B, De Toni EN. Microsatellite instability, KRAS mutations and cellular distribution of TRAIL-receptors in early stage colorectal cancer. PLoS One. 2012;7:e51654. doi: 10.1371/journal.pone.0051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horst D, Budczies J, Brabletz T, Kirchner T, Hlubek F. Invasion associated up-regulation of nuclear factor kappaB target genes in colorectal cancer. Cancer. 2009;115:4946–58. doi: 10.1002/cncr.24564. [DOI] [PubMed] [Google Scholar]
- 16.Andreyev HJ, Norman AR, Cunningham D, Oates J, Dix BR, Iacopetta BJ, Young J, Walsh T, Ward R, Hawkins N, Beranek M, Jandik P, Benamouzig R, Jullian E, Laurent-Puig P, Olschwang S, Muller O, Hoffmann I, Rabes HM, Zietz C, Troungos C, Valavanis C, Yuen ST, Ho JW, Croke CT, O’Donoghue DP, Giaretti W, Rapallo A, Russo A, Bazan V, Tanaka M, Omura K, Azuma T, Ohkusa T, Fujimori T, Ono Y, Pauly M, Faber C, Glaesener R, de Goeij AF, Arends JW, Andersen SN, Lövig T, Breivik J, Gaudernack G, Clausen OP, De Angelis PD, Meling GI, Rognum TO, Smith R, Goh HS, Font A, Rosell R, Sun XF, Zhang H, Benhattar J, Losi L, Lee JQ, Wang ST, Clarke PA, Bell S, Quirke P, Bubb VJ, Piris J, Cruickshank NR, Morton D, Fox JC, Al-Mulla F, Lees N, Hall CN, Snary D, Wilkinson K, Dillon D, Costa J, Pricolo VE, Finkelstein SD, Thebo JS, Senagore AJ, Halter SA, Wadler S, Malik S, Krtolica K, Urosevic N. Kirsten ras mutations in patients with colorectal cancer: the “RASCAL II” study. Br J Cancer. 2001;85:692–6. doi: 10.1054/bjoc.2001.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin HW, Choi H, So D, Kim YI, Cho K, Chung HJ, Lee KH, Chun YS, Cho CH, Kang GH, Kim WH, Park JW. ITF2 Prevents Activation of the βcatenin-TCF4 Complex in Colon Cancer Cells and Levels Decrease with Tumor Progression. Gastroenterology. 2014;147:430–442. e8. doi: 10.1053/j.gastro.2014.04.047. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Reiser-Erkan C, Michalski CW, Raggi MC, Quan L, Yupei Z, Friess H, Erkan M, Kleeff J. Hypoxia inducible BHLHB2 is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Biochem Biophys Res Commun. 2010;401:422–8. doi: 10.1016/j.bbrc.2010.09.070. [DOI] [PubMed] [Google Scholar]
- 19.Kyo S, Sakaguchi J, Ohno S, Mizumoto Y, Maida Y, Hashimoto M, Nakamura M, Takakura M, Nakajima M, Masutomi K, Inoue M. High Twist expression is involved in infiltrative endometrial cancer and affects patient survival. Hum Pathol. 2006;37:431–8. doi: 10.1016/j.humpath.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Janssen KP, Alberici P, Fsihi H, Gaspar C, Breukel C, Franken P, Rosty C, Abal M, El Marjou F, Smits R, Louvard D, Fodde R, Robine S. APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology. 2006;131:1096–109. doi: 10.1053/j.gastro.2006.08.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


