Abstract
The IASLC/ATS/ERS classification system was proposed in 2011 to improve the histological subtypes of lung adenocarcinoma, while the prognostic value of the combination of histological predominant subtypes is not consistent. IMP3 is an oncofetal protein which has been proved associated with aggressive tumor behavior in malignancies, but few reports were investigated in lung adenocarcinoma. The aim of this study is to explore the prognostic value of the IASLC/ATS/ERS classification and IMP3 expression in lung adenocarcinoma of Chinese cases. A total of 196 cases were classified according to the IASLC/ATS/ERS classification system and immunohistochemically analyzed by using a monoclonal antibody against IMP3. Univariate survival analysis indicated patients with solid-predominant subtype had shorter disease-free survival (P = 0.003) and overall survival (P = 0.014) compared to those with non-solid predominant subtype. Multivariate survival analysis revealed that solid-predominant subtype could be an independent prognostic factor for disease-free survival (HR: 1.22, 95% CI: 1.05-1.41; P = 0.008). Analysis of IMP3 expression showed that IMP3 was more frequently overexpressed in tumors with advanced pTNM stage (P < 0.001), larger tumor size (P = 0.036), poorer histological differentiation (P < 0.001), lymph node metastasis (P < 0.001), and solid-predominant subtype (P < 0.001). Survival analysis also confirmed that patients in IMP3 high-expression group had both worse disease-free survival (P = 0.039) and overall survival (P = 0.029) than those in IMP3 low-expression group. Our results illustrated that solid-predominant subtype according to the IASLC/ATS/ERS classification is an independent prognostic factor, and IMP3 overexpression is associated with aggressive tumor behavior and poor clinical outcome in lung adenocarcinoma.
Keywords: Lung adenocarcinoma, subtype, IMP3, survival
Introduction
Lung cancer is the leading cause of cancer mortality with high incidence all over the world. Although the overall incidence of lung cancer is falling in western countries, it still remains the biggest cause of cancer mortality [1,2]. In China, the mortality rate caused by lung cancer has taken the first place of all the malignancies and shown increasing trend in both urban and rural areas [3]. In recent decades, the most common histological type of non-small cell lung carcinoma (NSCLC) is lung adenocarcinoma, accounting for 70% of NSCLC and nearly half of all lung carcinoma [4]. Due to the considerable heterogeneity, a histological classification criteria is in urgent need for lung adenocarcinoma to achieve more personalized treatment and better therapeutic effect.
In 2011, the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS) proposed a new classification system for lung adenocarcinoma [5]. Recently, quite a few of studies have demonstrated prognostic value of the new IASLC/ATS/ERS classification in both Caucasian and Asian populations [4,6-11]. However, the prognostic value of the combination of histological predominant subtypes is not consistent, and still remains for further study.
Insulin-like growth factor II mRNA-binding protein 3 (IGF2BP3/IMP3), also known as L523S or KOC (K homology domain containing protein overexpressed in cancer), is a member of the insulin-like growth factor II (IGF-II) mRNA-binding protein (IMP) family, which is composed of IMP1, IMP2, and IMP3 [12]. IMP family members play a pivotal role in RNA trafficking and stabilization, cell growth, and cell migration during embryogenesis [13]. As an oncofetal protein, IMP3 is normally expressed during embryonic development and then re-expressed in cancers. IMP3 promotes tumor cell proliferation through an insulin-like growth factor II-dependent pathway [14], and having a major influence on tumor cell invasion as well [15]. IMP3 re-expressed is widely detected in a variety of malignancies, which is also associated with aggressive biological behavior of tumors and poor survival of patients, including in renal cell carcinoma, cervical carcinoma, breast carcinoma, colorectal adenocarcinoma, hepatocellular carcinoma, intrahepatic cholangiocarcinoma, thyroid carcinoma, tongue carcinoma, urothelial carcinoma of the bladder, gastric adenocarcinoma, and prostate carcinoma [16-26].
Although there are numerous reports confirming the relationship between IMP3 expression and malignancies, few studies investigated IMP3 expression in lung carcinomas, let alone lung adenocarcinoma. The published studies have demonstrated that IMP3 expression is associated with advanced stage of disease, higher histologic grade, lymph node metastasis, distant metastasis and solid histological pattern in lung carcinomas [27-31].
The aim of our study was to explore the prognostic significance of the new IASLC/ATS/ERS classification system and IMP3 expression in patients with completely resected stage I to III invasive lung adenocarcinoma.
Materials and methods
Patients
From January 2007 to December 2012, all patients diagnosed and then underwent complete resection of lung adenocarcinoma in Fudan University Shanghai Cancer Center were reviewed. The use of human lung adenocarcinoma specimens was approved by the institutional review board of Fudan University Shanghai Cancer Center. Inclusion criteria covered that 1) Primary invasive lung adenocarcinoma which had been proved by pathological examination after complete resection; 2) Age < 80 years; 3) pTNM stage: from stage I to stage III; 4) Patients who were included in survival analysis had been followed up over 16 months (Death or tumor recurrence was occurred in at least 1/3 cases). A total of 243 patients were eligible for this study. Clinical features (including gender, age, clinical stage, and survival data) of all patients were available. Tumor stage was determined according to the 7th Revision TNM Classification [32].
Histological classification
Resected specimens were formalin fixed and stained with hematoxylin and eosin, and all slides were independently evaluated by two pathologists. The IASLC/ATS/ERS classification for lung adenocarcinoma was used for histological classification. Each case was reviewed by using comprehensive histological subtyping, and the percentage of each histological component was recorded semi-quantitatively in 5% increments. Repeated examination was used to resolve discrepancies in assessment of histological subtype between the two pathologists. A few patients with invasive mucinous adenocarcinoma were excluded from analysis because the IASLC/ATS/ERS criteria suggested that patients with specific variant subtypes should be separated from patients with invasive adenocarcinoma [5]. Cases with two (or even more) types of histological predominant patterns with similar percentages were also excluded from analysis. Ultimately, the remaining 196 patients were enrolled in our study.
Immunohistochemical analysis
In all cases, immunohistochemical tests were performed on 5-μm-thick formalin-fixed, paraffin-embedded tissue sections using a rabbit monoclonal antibody against IMP3 (clone EPR5111; Abcam; dilution 1:100). Each section was deparaffinized in a series of xylene baths and then rehydrated using a graded alcohol series. Sections were subjected to 5 min steam-heat-induced epitope retrieval in presence of 10 mM sodium citrate buffer (pH 6.0), and incubated overnight with primary anti-IMP3 antibody at 4°C. Tissues were then incubated with a biotinylated anti-rabbit secondary antibody. The avidin-biotin complex/HRP (ABC/HRP) was used along with DAB chromogen to visualise protein expression, and hematoxylin for counter-staining. Adjacent normal-appearing bronchial epithelium within each tissue section served as an internal reference. IMP3 is known to exhibit a predominantly cytoplasmic staining. All sections were independently evaluated by two pathologists using a semi-quantitative system based on the H-index [33,34]: 3 × percentage of strongly staining cells + 2 × percentage of moderately staining cells + percentage of weakly staining cells, giving “composite scores” that ranged from 0 to 300. All the cases were classified by the composite scores. Cases with the scores of 0 to 100 were interpreted as negative/mildly positive, 101 to 200 as moderately positive, and 201 to 300 as strongly positive.
Statistical analysis
Statistical calculations were performed using the Statistical Package for the Social Sciences (SPSS) software (version 20). Categorical variables were compared by the Pearson’s chi-square test, while continuous variables were compared by the independent-sample t test. The prognostic influence of variables was evaluated by Kaplan-Meier method and log-rank test in univariate survival analysis. Multivariate survival analysis was performed with the Cox proportional hazards model to evaluate the independent prognostic factors for lung adenocarcinoma. A two-sided p value of less than 0.05 was considered to be statistically significant.
Results
Association between IASLC/ATS/ERS classification and clinicopathologic variables
Mean age of the 196 patients when undergoing complete resection was 57.8 ± 8.9 years (mean ± SD), and 118 (60.2%) cases were male while 78 (39.8%) cases female. According to the IASLC/ATS/ERS classification, acinar-predominant subtype was the most common (105 cases, 53.6%), followed by the solid-predominant (41 cases, 20.9%), papillary-predominant (30 cases, 15.3%), lepidic-predominant (14 cases, 7.1%) and micropapillary-predominant (6 cases, 3.1%). All of the lepidic-predominant cases were distributed in well/moderate histological differentiation, T1-T2, N0-N1, and pTNM stage I-III. Pairwise comparison showed the lepidic-predominant subtype was significantly different from the other subtypes in histological differentiation, pTNM stage, T stage and N stage, which implied lepidic-predominant subtype was tent to associate with small tumor size, well histological differentiation, early pTNM stage and non-metastatic regional lymph nodes.
Correlation of the five histological patterns with clinicopathologic variables was showed in Table 1, which revealed the considerable differences in histological differentiation (P < 0.001), pTNM stage (P = 0.001), T stage (P = 0.004) and N stage (P = 0.015).
Table 1.
Association between the IASLC/ATS/ERS classification and clinicopathologic variables
| Variables | Numbers of patients | Lepidic predominant | Acinar predominant | Papillary predominant | Micropapillary predominant | Solid predominant | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Numbers (%) | 196 | 14 | (7.1%) | 105 | (53.6%) | 30 | (15.3%) | 6 | (3.1%) | 41 | (20.9%) | |
| Age (years, mean ± SD) | 57.8 ± 8.9 | 61.2 ± 10.7 | 58.3 ± 7.8 | 57.7 ± 10.3 | 56.0 ± 14.0 | 55.9 ± 9.0 | 0.48 | |||||
| Gender | ||||||||||||
| Male | 118 | 7 | (50.0%) | 56 | (53.3%) | 18 | (60.0%) | 5 | (83.3%) | 32 | (78.0%) | 0.05 |
| Female | 78 | 7 | (50.0%) | 49 | (46.7%) | 12 | (40.0%) | 1 | (16.7%) | 9 | (22.0%) | |
| Differentiation | ||||||||||||
| Well | 19 | 7 | (50.0%) | 8 | (7.6%) | 4 | (13.3%) | 0 | (0.0%) | 0 | (0.0%) | < 0.001 |
| Moderate | 102 | 7 | (50.0%) | 70 | (66.7%) | 20 | (66.7%) | 2 | (33.3%) | 3 | (7.3%) | |
| Poor | 75 | 0 | (0.0%) | 27 | (25.7%) | 6 | (20.0%) | 4 | (66.7%) | 38 | (92.7%) | |
| pTNM stage | ||||||||||||
| I | 87 | 13 | (92.9%) | 51 | (48.6%) | 10 | (33.3%) | 0 | (0.0%) | 13 | (31.7%) | 0.001 |
| II | 41 | 1 | (7.1%) | 17 | (16.2%) | 8 | (26.7%) | 4 | (66.7%) | 11 | (26.8%) | |
| III | 68 | 0 | (0.0%) | 37 | (35.2%) | 12 | (40.0%) | 2 | (33.3%) | 17 | (41.5%) | |
| T stage | ||||||||||||
| 1 | 72 | 11 | (78.6%) | 41 | (39.0%) | 8 | (26.7%) | 1 | (16.7%) | 11 | (26.8%) | 0.004 |
| 2 | 103 | 3 | (21.4%) | 55 | (52.4%) | 17 | (56.7%) | 4 | (66.7%) | 24 | (58.5%) | |
| 3 | 17 | 0 | (0.0%) | 6 | (5.7%) | 4 | (13.3%) | 1 | (16.7%) | 6 | (14.6%) | |
| 4 | 4 | 0 | (0.0%) | 3 | (2.9%) | 1 | (3.3%) | 0 | (0.0%) | 0 | (0.0%) | |
| N stage | ||||||||||||
| 0 | 102 | 13 | (92.9%) | 56 | (53.3%) | 15 | (50.0%) | 2 | (33.3%) | 16 | (39.0%) | 0.015 |
| 1 | 30 | 1 | (7.1%) | 15 | (14.3%) | 3 | (10.0%) | 2 | (33.3%) | 9 | (22.0%) | |
| 2 | 60 | 0 | (0.0%) | 32 | (30.5%) | 12 | (40.0%) | 2 | (33.3%) | 14 | (34.1%) | |
| 3 | 4 | 0 | (0.0%) | 2 | (1.9%) | 0 | (0.0%) | 0 | (0.0%) | 2 | (4.9%) | |
Association between IASLC/ATS/ERS classification and clinical outcome of lung adenocarcinoma
The range of follow-up time for all patients was 16.5 to 69.0 months. During the five-year follow-up after complete resection, 56 (58.3%) patients suffered from relapse or metastasis, while 31 (32.3%) patients died. The mean disease-free survival (DFS) was 32.0 months (95% CI: 26.9-37.1), and the mean overall survival (OS) was 45.8 months (95% CI: 40.7-50.9).
Univariate survival analysis (Table 2) indicated that histological differentiation, pTNM stage and N stage were significant prognostic factors for DFS (P = 0.019, P < 0.001, P = 0.001, respectively) and OS (P = 0.023, P < 0.001, P < 0.001, respectively). Kaplan-Meier survival curves overlapped according to the five histological subtypes of invasive lung adenocarcinoma (Figure 1A and 1B). Therefore, we divided them into two groups of solid-predominant subtype and non-solid predominant subtype as reported by Yanagawa et al. [35]. The result revealed that patients with solid-predominant subtype had shorter DFS (P = 0.003) and OS (P = 0.014) compared to those with non-solid predominant subtype (Figure 1C and 1D).
Table 2.
Univariate analysis for disease-free survival and overall survival
| Variables | Numbers (%) | 5-year DFS | p-value | 5-year OS | p-value | |
|---|---|---|---|---|---|---|
| Age | ||||||
| ≤ 55 | 40 | (41.7%) | 19.1% | 0.405 | 36.2% | 0.166 |
| > 55 | 56 | (58.3%) | 24.4% | 61.9% | ||
| Gender | ||||||
| Male | 52 | (54.2%) | 16.8% | 0.705 | 43.0% | 0.420 |
| Female | 44 | (45.8%) | 29.7% | 66.9% | ||
| Differentiation | ||||||
| Well/moderate | 58 | (60.4%) | 39.8% | 0.019 | 69.7% | 0.023 |
| Poor | 38 | (39.6%) | 0.0% | 37.5% | ||
| Histological subtype | ||||||
| Non-solid | 75 | (78.1%) | 29.2% | 0.003 | 60.5% | 0.014 |
| Solid | 21 | (21.9%) | 16.9% | 29.6% | ||
| pTNM stage | ||||||
| Ι | 28 | (29.2%) | 49.9% | < 0.001 | 87.8% | < 0.001 |
| II-III | 68 | (70.8%) | 11.7% | 38.4% | ||
| T stage | ||||||
| 1-2 | 84 | (87.5%) | 26.7% | 0.274 | 57.7% | 0.492 |
| 3-4 | 12 | (12.5%) | 20.8% | 33.3% | ||
| N stage | ||||||
| 0 | 36 | (37.5%) | 46.1% | 0.001 | 84.9% | < 0.001 |
| ≥ 1 | 60 | (62.5%) | 11.3% | 33.4% | ||
| IMP3 expression | ||||||
| Low | 53 | (55.2%) | 35.2% | 0.039 | 64.8% | 0.029 |
| High | 43 | (44.8%) | 0.0% | 40.9% | ||
Figure 1.
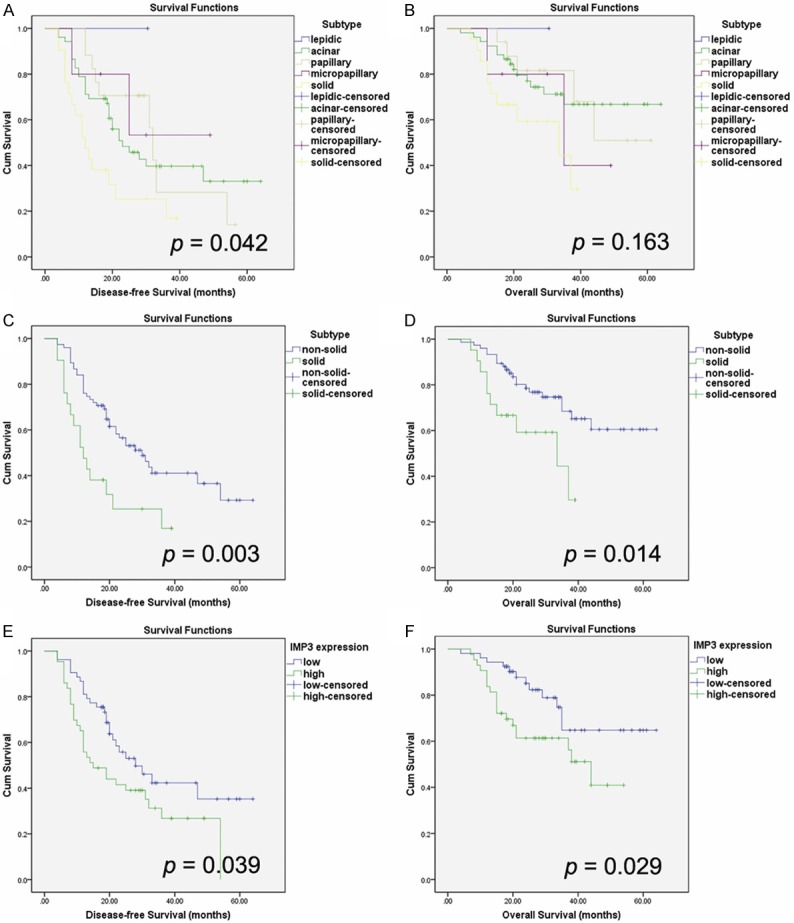
Kaplan-Meier survival curves for disease-free survival and overall survival. A, B: Kaplan-Meier survival curves overlapped according to the five histological subtypes of invasive lung adenocarcinoma. C, D: Solid-predominant subtype was associated with shorter disease-free survival (P = 0.003) and overall survival (P = 0.014) compared with non-solid predominant subtype. E, F: High-expression of IMP3 was associated with worse disease-free survival (P = 0.039) and overall survival (P = 0.029) in lung adenocarcinoma.
Multivariate survival analysis (Table 3) showed both the IASLC/ATS/ERS classification (solid-predominant vs. non-solid predominant) and pTNM stage were statistically significant predictors of DFS (HR: 1.22, 95% CI: 1.05-1.41, P = 0.008; HR: 3.26, 95% CI: 1.59-6.70, P = 0.001), while only the pTNM stage was the independent prognostic factor for OS (HR: 8.11, 95% CI: 1.92-34.23; P = 0.004).
Table 3.
Multivariate analysis for disease-free survival and overall survival
| Variables | Disease-free survival | Overall survival | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age(> 55 vs. ≤ 55) | 0.72 | 0.42-1.23 | 0.224 | 0.57 | 0.28-1.17 | 0.124 |
| Gender(female vs. male) | 1.27 | 0.72-2.25 | 0.416 | 1.23 | 0.57-2.68 | 0.602 |
| Differentiation (poor vs. well/moderate) | 1.34 | 0.68-2.68 | 0.399 | 1.51 | 0.63-3.64 | 0.361 |
| Histological subtype (solid vs. non-solid) | 1.22 | 1.05-1.41 | 0.008 | 1.21 | 1.00-1.47 | 0.050 |
| pTNM stage (II-III vs. I) | 3.26 | 1.59-6.70 | 0.001 | 8.11 | 1.92-34.23 | 0.004 |
| IMP3 expression (high vs. low) | 0.99 | 0.52-1.89 | 0.977 | 1.14 | 0.50-2.60 | 0.763 |
Association between IMP3 expression and clinicopathologic variables
IMP3 protein exhibited a predominantly cytoplasmic staining in lung adenocarcinoma tissue, which was not observed in normal tissue of lung, including pneumocytes and other types of stromal cells [31]. We divided 196 cases into IMP3 high-expression (moderately/strongly positive) group and IMP3 low-expression (negative/mildly positive) group (Figure 2).
Figure 2.
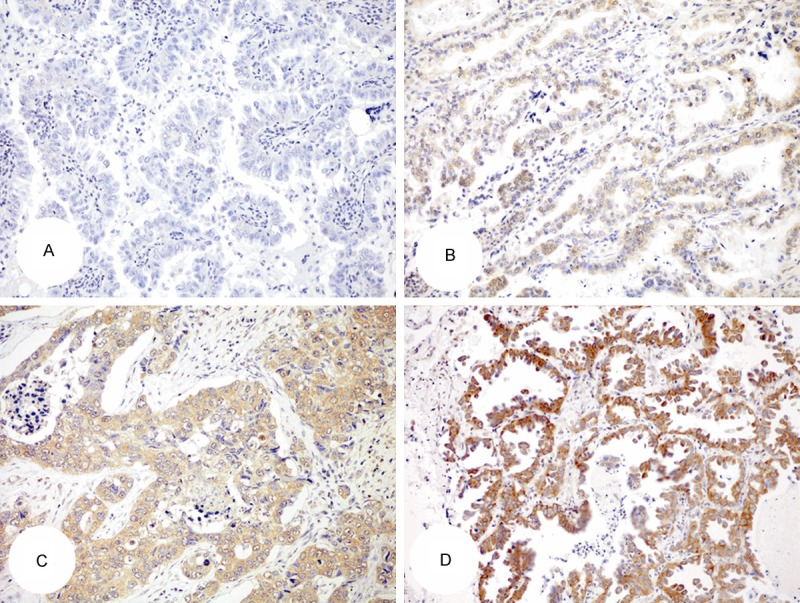
Expression of IMP3 in lung adenocarcinoma. A: Negative; B: Weakly staining; C: Moderately staining; D: Strongly staining. (Envision, × 200).
According to Table 4, the overall percentages of IMP3 high-expression and low-expression were 42.4% (83/196) versus 57.6% (113/196). Mean age of patients with IMP3 high-expression was 57.4 ± 9.5 years, while low-expression was 58.1 ± 8.4 years. IMP3 high-expression was most common seen in cases with pTNM stage III (70.6%), T4 (75.0%), N3 (100%), poor histological differentiation (72.0%) and solid-predominant subtype (78.0%). There were significant differences between IMP3 high-expression group and low-expression group in histological differentiation (P < 0.001), the IASLC/ATS/ERS classification subtypes (P < 0.001), pTNM stage (P < 0.001), T stage (P = 0.036) and N stage (P < 0.001), while no statistical significance in age or gender.
Table 4.
Association between IMP3 expression and clinicopathologic variables
| Variable | Numbers of patients | IMP3 low-expression | IMP3 high-expression | p-value | ||
|---|---|---|---|---|---|---|
| Numbers (%) | 196 | 113 | (57.6%) | 83 | (42.4%) | |
| Age (years, mean ± SD) | 57.8 ± 8.9 | 58.1 ± 8.4 | 57.4 ± 9.5 | 0.563 | ||
| Gender | ||||||
| Male | 118 | 62 | (52.5%) | 56 | (47.5%) | 0.075 |
| Female | 78 | 51 | (65.4%) | 27 | (34.6%) | |
| Differentiation | ||||||
| Well | 19 | 17 | (89.5%) | 2 | (10.5%) | < 0.001 |
| Moderate | 102 | 75 | (73.5%) | 27 | (26.5%) | |
| Poor | 75 | 21 | (28.0%) | 54 | (72.0%) | |
| Histological subtype | ||||||
| Lepidic | 14 | 14 | (100.0%) | 0 | (0.0%) | < 0.001 |
| Acinar | 105 | 73 | (69.5%) | 32 | (30.5%) | |
| Papillary | 30 | 15 | (50.0%) | 15 | (50.0%) | |
| Micropapillary | 6 | 2 | (33.3%) | 4 | (66.7%) | |
| Solid | 41 | 9 | (22.0%) | 32 | (78.0%) | |
| pTNM stage | ||||||
| I | 87 | 69 | (79.3%) | 18 | (20.7%) | < 0.001 |
| II | 41 | 24 | (58.5%) | 17 | (41.5%) | |
| III | 68 | 20 | (29.4%) | 48 | (70.6%) | |
| T stage | ||||||
| 1 | 72 | 45 | (62.5%) | 27 | (37.5%) | 0.036 |
| 2 | 103 | 62 | (60.2%) | 41 | (39.8%) | |
| 3 | 17 | 5 | (29.4%) | 12 | (70.6%) | |
| 4 | 4 | 1 | (25.0%) | 3 | (75.0%) | |
| N stage | ||||||
| 0 | 102 | 75 | (73.5%) | 27 | (26.5%) | < 0.001 |
| 1 | 30 | 19 | (63.3%) | 11 | (36.7%) | |
| 2 | 60 | 19 | (31.7%) | 41 | (68.3%) | |
| 3 | 4 | 0 | (0.0%) | 4 | (100.0%) | |
Association between IMP3 expression and clinical outcome of lung adenocarcinoma
Kaplan-Meier survival analysis showed that patients with IMP3 high-expression had shorter DFS and OS compared to those with IMP3 low-expression. Univariate survival analysis indicated IMP3 high-expression as a significant prognostic factor for both DFS (P = 0.039) and OS (P = 0.029) (Table 2; Figure 1E and 1F), but multivariate survival analysis showed IMP3 expression could not predict prognosis independently for DFS (HR: 0.99, 95% CI: 0.52-1.89; P = 0.977) or OS (HR: 1.14, 95% CI: 0.50-2.60; P = 0.763) (Table 3).
Discussion
Lung adenocarcinoma has become the major subtype of NSCLC during the past decades, which makes trouble in clinical decision because of the considerable heterogeneity. There are new biologically targeted chemotherapies targeting EGFR mutations and ALK fusion genes since activated gene mutations are more common found in lung adenocarcinoma. In spite of the new therapeutic agents and improved surgical technologies, survival for patients with lung adenocarcinoma remains unsatisfactory [36].
As invasive adenocarcinomas represent more than 70-90% of surgically resected lung cases, it is quite vital to present a practical way to classify these tumors [5]. According to the 2004 WHO classification, over 90% of lung adenocarcinoma should be identified as adenocarcinoma with mixed subtypes [37]. The clinical outcomes of patients diagnosed with “adenocarcinoma with mixed subtype” are diverse for the different components of histological patterns. The purpose of the IASLC/ATS/ERS classification is to provide an integrated approach to classification of the various types of lung adenocarcinoma. This new classification discontinued the term “mixed subtype”, and recommended the addition of micropapillary-predominant subtype in invasive lung adenocarcinoma.
In our study, the frequencies of lepidic-, acinar-, papillary-, micropapillary-, and solid-predominant patterns were 7.1%, 53.6%, 15.3%, 3.1% and 20.9%, respectively. The frequencies of these five predominant subtypes of invasive adenocarcinoma varied in the literature because of the interobserver variation shown by different pathologists around the world [6,7,38]. There was compact association between the predominant histological patterns and the clinicopathologic variables. The differences of the five histological subtypes were statistically significant in histological differentiation (P < 0.001), pTNM stage (P = 0.001), T stage (P = 0.004) and N stage (P = 0.015). Moreover, the lepidic-predominant subtype was significantly different from the other four subtypes, which implied lepidic-predominant subtype was tent to associate with small tumor size, well histological differentiation, early pTNM stage and non-metastatic regional lymph nodes.
The prognostic value of the new IASLC/ATS/ERS classification has been investigated in several studies [6-11,39]. Hung et al. [39] and Gu et al. [7] have demonstrated that solid-predominant and micropapillary-predominant subtypes were correlated with worse DFS and OS. Woo et al. [10] verified that solid-predominant, micropapillary-predominant and invasive mucinous subtypes had an independent prognostic value to predict post-operative recurrence. Yoshizawa et al. [6] have reported that solid-predominant, micropapillary-predominant and colloid subtypes indicated an increased risk of recurrence and worse OS. Yanagawa et al. [9] have also found solid-predominant subtype was an independent predictor of increased risk of recurrence.
Our results showed that patients with solid-predominant subtype had significantly worse DFS (P = 0.003) and OS (P = 0.014) compared to those with non-solid predominant subtypes (including lepidic-predominant, acinar- predominant, papillary-predominant and micropapillary-predominant). Solid-predominant subtype could also be an independent prognostic factor for DFS (HR: 1.22, 95% CI: 1.05-1.41; P = 0.008). However, the sample number of our study was still small. It remains to be further studied about the prognostic and predictive value of the IASLC/ATS/ERS classification system.
IMP3 is a 580-amino acid oncofetal RNA-binding protein, encoded by the IGF2BP3 gene located on chromosome 7p11.2 [40]. IMP3 contains 2 RNA recognition motifs and 4 K homology (KH) domains, and its function is implicated in cell growth and cell migration [13,41]. IMP3 is a cytoplasmic protein which binds to the 5’ untranslated region of the insulin-like growth factor II (IGF-II) leader-3 messenger RNA (mRNA), as a translational activator of IGF-II leader-3 mRNA, which normally controls cell proliferation [14]. IMP3 is believed to participate in the protection and intracellular distribution of IGF-II mRNA and thus has been implicated in regulating the production of IGF-II [16].
IMP3 is ubiquitously expressed during the early stage of embryogenesis, with only limited normal expression in postembryonic stages [42,43]. IMP3 expression is low or undetectable in postnatal tissues and virtually absent in adult tissues, the common exception of which is in placental intermediate trophoblasts [12,16].
IMP3 re-expression in human malignancies was first identified in pancreatic carcinoma in 1996 [44]. Since then, IMP3 has been detected in a variety of other tumors. Research has demonstrated that IMP3 can induce cell adhesion and invasion by stabilizing CD44 mRNA [15]. IMP3 is also a biomarker for tumor aggressive behavior and metastases [45,46]. Moreover, IMP3 overexpression in malignancies has been proved to correlate with poor survival of patients [23,24,33].
The expression of IMP3 in lung carcinomas has been studied in few reports. Bellezza et al. [30] have first reported IMP3 overexpression was correlated with advanced stages of disease, lymph nodes metastases, and higher histologic grades. Findeis-Hosey et al. [29] have also found that IMP3 was strongly expressed in a large proportion of poorly differentiated lung adenocarcinoma, and furthermore in the solid component of mixed subtype adenocarcinomas. Beljan Perak et al. [27] have demonstrated expression of IMP3 was correlated with solid subtype and with distant metastases regardless of histological subtype of lung adenocarcinoma. There are barely researches involving the correlation of IMP3 expression with clinical prognosis in lung carcinomas. Del Gobbo et al. [41] lately verified IMP3 as a marker of poor outcome in 74 patients with a diagnosis of lung neuroendocrine tumor.
In our work, analysis of IMP3 expression revealed that IMP3 was more frequently overexpressed in tumors with the advanced pTNM stage, larger tumor size, poorer histological differentiation, lymph node metastasis, and solid-predominant subtype. The ratio of IMP3 high-expression was increasing following the advance of pTNM stage (P < 0.001), T stage (P = 0.036) and N stage (P < 0.001). IMP3 high-expression was also associated with poor histological differentiation (P < 0.001). There were statistical differences of IMP3 expression among the five histological subtypes according to the IASLC/ATS/ERS classification (P < 0.001), and also between the solid-predominant subtype and non-solid predominant subtype (78.1% vs. 32.9%, P < 0.001). Our findings supported the concept that IMP3 overexpression was a marker of increased tumor aggressive behavior.
Beljan Perak et al. [27] have ever reported that patients with IMP3 positive lung adenocarcinoma had shorter time of OS, but the result was not statistically significant (P = 0.713). In our study, univariate survival analysis revealed that patients in IMP3 high-expression group had both shorter DFS (P = 0.039) and OS (P = 0.029) than those in IMP3 low-expression group, but there were still no sufficient evidences to support IMP3 expression as an independent prognostic factor.
In summary, solid-predominant subtype according to the IASLC/ATS/ERS classification is an independent prognostic factor, and IMP3 overexpression is associated with aggressive tumor behavior and poor clinical outcome in lung adenocarcinoma, which may probably affect the clinical personalized treatments or reveal a potential therapeutic target in the near future.
Acknowledgements
This study was supported by the grant from the Science and Technology Commission of Shanghai Municipality (No. 10DJ1400500, No. 10DJ1400501), National Clinical Key Discipline (2011-2015), Priority of Shanghai key discipline of medicine (2013-2015), Shanghai R&D pub-lic service platform construction projects (12DZ2295100), and the National Natural Science Foundation of China (81071791, 81201836).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Yang L, Parkin DM, Li L, Chen Y. Time trends in cancer mortality in China: 1987-1999. Int J Cancer. 2003;106:771–783. doi: 10.1002/ijc.11300. [DOI] [PubMed] [Google Scholar]
- 4.Okada M. Subtyping lung adenocarcinoma according to the novel 2011 IASLC/ATS/ERS classification: correlation with patient prognosis. Thorac Surg Clin. 2013;23:179–186. doi: 10.1016/j.thorsurg.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, Garg K, Austin JH, Asamura H, Rusch VW, Hirsch FR, Scagliotti G, Mitsudomi T, Huber RM, Ishikawa Y, Jett J, Sanchez-Cespedes M, Sculier JP, Takahashi T, Tsuboi M, Vansteenkiste J, Wistuba I, Yang PC, Aberle D, Brambilla C, Flieder D, Franklin W, Gazdar A, Gould M, Hasleton P, Henderson D, Johnson B, Johnson D, Kerr K, Kuriyama K, Lee JS, Miller VA, Petersen I, Roggli V, Rosell R, Saijo N, Thunnissen E, Tsao M, Yankelewitz D. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshizawa A, Sumiyoshi S, Sonobe M, Kobayashi M, Fujimoto M, Kawakami F, Tsuruyama T, Travis WD, Date H, Haga H. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol. 2013;8:52–61. doi: 10.1097/JTO.0b013e3182769aa8. [DOI] [PubMed] [Google Scholar]
- 7.Gu J, Lu C, Guo J, Chen L, Chu Y, Ji Y, Ge D. Prognostic significance of the IASLC/ATS/ERS classification in Chinese patients-A single institution retrospective study of 292 lung adenocarcinoma. J Surg Oncol. 2013;107:474–480. doi: 10.1002/jso.23259. [DOI] [PubMed] [Google Scholar]
- 8.Russell PA, Barnett SA, Walkiewicz M, Wainer Z, Conron M, Wright GM, Gooi J, Knight S, Wynne R, Liew D, John T. Correlation of mutation status and survival with predominant histologic subtype according to the new IASLC/ATS/ERS lung adenocarcinoma classification in stage III (N2) patients. J Thorac Oncol. 2013;8:461–468. doi: 10.1097/JTO.0b013e3182828fb8. [DOI] [PubMed] [Google Scholar]
- 9.Yanagawa N, Shiono S, Abiko M, Ogata SY, Sato T, Tamura G. New IASLC/ATS/ERS classification and invasive tumor size are predictive of disease recurrence in stage I lung adenocarcinoma. J Thorac Oncol. 2013;8:612–618. doi: 10.1097/JTO.0b013e318287c3eb. [DOI] [PubMed] [Google Scholar]
- 10.Woo T, Okudela K, Mitsui H, Tajiri M, Yamamoto T, Rino Y, Ohashi K, Masuda M. Prognostic value of the IASLC/ATS/ERS classification of lung adenocarcinoma in stage I disease of Japanese cases. Pathol Int. 2012;62:785–791. doi: 10.1111/pin.12016. [DOI] [PubMed] [Google Scholar]
- 11.Yoshizawa A, Motoi N, Riely GJ, Sima CS, Gerald WL, Kris MG, Park BJ, Rusch VW, Travis WD. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24:653–664. doi: 10.1038/modpathol.2010.232. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen J, Christiansen J, Lykke-Andersen J, Johnsen AH, Wewer UM, Nielsen FC. A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Mol Cell Biol. 1999;19:1262–1270. doi: 10.1128/mcb.19.2.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mueller-Pillasch F, Lacher U, Wallrapp C, Micha A, Zimmerhackl F, Hameister H, Varga G, Friess H, Buchler M, Beger HG, Vila MR, Adler G, Gress TM. Cloning of a gene highly overexpressed in cancer coding for a novel KHdomain containing protein. Oncogene. 1997;14:2729–2733. doi: 10.1038/sj.onc.1201110. [DOI] [PubMed] [Google Scholar]
- 14.Liao B, Hu Y, Herrick DJ, Brewer G. The RNAbinding protein IMP-3 is a translational activator of insulin-like growth factor II leader-3 mRNA during proliferation of human K562 leukemia cells. J Biol Chem. 2005;280:18517–18524. doi: 10.1074/jbc.M500270200. [DOI] [PubMed] [Google Scholar]
- 15.Vikesaa J, Hansen TV, Jonson L, Borup R, Wewer UM, Christiansen J, Nielsen FC. RNAbinding IMPs promote cell adhesion and invadopodia formation. EMBO J. 2006;25:1456–1468. doi: 10.1038/sj.emboj.7601039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann NE, Sheinin Y, Lohse CM, Parker AS, Leibovich BC, Jiang Z, Kwon ED. External validation of IMP3 expression as an independent prognostic marker for metastatic progression and death for patients with clear cell renal cell carcinoma. Cancer. 2008;112:1471–1479. doi: 10.1002/cncr.23296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei Q, Yan J, Fu B, Liu J, Zhong L, Yang Q, Zhao T. IMP3 expression is associated with poor survival in cervical squamous cell carcinoma. Hum Pathol. 2014;45:2218–2224. doi: 10.1016/j.humpath.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Sidoni A, Cartaginese F. IMP3 expression in triple-negative breast carcinoma. Hum Pathol. 2010;41:1355–1356. doi: 10.1016/j.humpath.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Lochhead P, Imamura Y, Morikawa T, Kuchiba A, Yamauchi M, Liao X, Qian ZR, Nishihara R, Wu K, Meyerhardt JA, Fuchs CS, Ogino S. Insulin-like growth factor 2 messenger RNA binding protein 3 (IGF2BP3) is a marker of unfavourable prognosis in colorectal cancer. Eur J Cancer. 2012;48:3405–3413. doi: 10.1016/j.ejca.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu S, Wu X, Zhou B, Xu Z, Qin J, Lu H, Lv L, Gao Y, Deng L, Yin J, Li G. IMP3 combined with CD44s, a novel predictor for prognosis of patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2014;140:883–893. doi: 10.1007/s00432-014-1639-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Y, Yang M, Jiang Z, Woda BA, Mercurio AM, Qin J, Huang X, Zhang F. IMP3 expression is associated with poor outcome and epigenetic deregulation in intrahepatic cholangiocarcinoma. Hum Pathol. 2014;45:1184–1191. doi: 10.1016/j.humpath.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Asioli S, Erickson LA, Righi A, Jin L, Volante M, Jenkins S, Papotti M, Bussolati G, Lloyd RV. Poorly differentiated carcinoma of the thyroid: validation of the Turin proposal and analysis of IMP3 expression. Mod Pathol. 2010;23:1269–1278. doi: 10.1038/modpathol.2010.117. [DOI] [PubMed] [Google Scholar]
- 23.Li HG, Han JJ, Huang ZQ, Wang L, Chen WL, Shen XM. IMP3 is a novel biomarker to predict metastasis and prognosis of tongue squamous cell carcinoma. J Craniofac Surg. 2011;22:2022–2025. doi: 10.1097/SCS.0b013e3182319750. [DOI] [PubMed] [Google Scholar]
- 24.Szarvas T, Vom DF, Niedworok C, Melchior-Becker A, Fischer JW, Singer BB, Reis H, Bankfalvi A, Schmid KW, Romics I, Ergun S, Rubben H. High insulin-like growth factor mRNA-binding protein 3 (IMP3) protein expression is associated with poor survival in muscleinvasive bladder cancer. Bju Int. 2012;110:E308–E317. doi: 10.1111/j.1464-410X.2012.11149.x. [DOI] [PubMed] [Google Scholar]
- 25.Damasceno EA, Carneiro FP, Magalhaes AV, Carneiro MV, Takano GH, Vianna LM, Seidler HB, Castro TM, Muniz-Junqueira MI, Amorim RF, Ferreira VM, Motoyama AB. IMP3 expression in gastric cancer: association with clinicopathological features and HER2 status. J Cancer Res Clin Oncol. 2014;140:2163–2168. doi: 10.1007/s00432-014-1850-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szarvas T, Tschirdewahn S, Niedworok C, Kramer G, Sevcenco S, Reis H, Shariat SF, Rubben H, Vom DF. Prognostic value of tissue and circulating levels of IMP3 in prostate cancer. Int J Cancer. 2014;135:1596–1604. doi: 10.1002/ijc.28808. [DOI] [PubMed] [Google Scholar]
- 27.Beljan Perak R, Durdov MG, Capkun V, Ivcevic V, Pavlovic A, Soljic V, Peric M. IMP3 can predict aggressive behaviour of lung adenocarcinoma. Diagn Pathol. 2012;7:165. doi: 10.1186/1746-1596-7-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Findeis-Hosey JJ, Xu H. Insulin-like growth factor II-messenger RNA-binding protein-3 and lung cancer. Biotech Histochem. 2012;87:24–29. doi: 10.3109/10520295.2011.591831. [DOI] [PubMed] [Google Scholar]
- 29.Findeis-Hosey JJ, Yang Q, Spaulding BO, Wang HL, Xu H. IMP3 expression is correlated with histologic grade of lung adenocarcinoma. Hum Pathol. 2010;41:477–484. doi: 10.1016/j.humpath.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Bellezza G, Cavaliere A, Sidoni A. IMP3 expression in non-small cell lung cancer. Hum Pathol. 2009;40:1205–1206. doi: 10.1016/j.humpath.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 31.Xu H, Bourne PA, Spaulding BO, Wang HL. High-grade neuroendocrine carcinomas of the lung express K homology domain containing protein overexpressed in cancer but carcinoid tumors do not. Hum Pathol. 2007;38:555–563. doi: 10.1016/j.humpath.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 33.Fadare O, Liang SX, Crispens MA, Jones HR, Khabele D, Gwin K, Zheng W, Mohammed K, Parkash V, Hecht JL, Desouki MM. Expression of the oncofetal protein IGF2BP3 in endometrial clear cell carcinoma: assessment of frequency and significance. Hum Pathol. 2013;44:1508–1515. doi: 10.1016/j.humpath.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Budwit-Novotny DA, McCarty KS, Cox EB, Soper JT, Mutch DG, Creasman WT, Flowers JL, McCarty KJ. Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986;46:5419–5425. [PubMed] [Google Scholar]
- 35.Yanagawa N, Shiono S, Abiko M, Ogata SY, Sato T, Tamura G. New IASLC/ATS/ERS classification and invasive tumor size are predictive of disease recurrence in stage I lung adenocarcinoma. J Thorac Oncol. 2013;8:612–618. doi: 10.1097/JTO.0b013e318287c3eb. [DOI] [PubMed] [Google Scholar]
- 36.Morgensztern D, Waqar S, Subramanian J, Gao F, Govindan R. Improving survival for stage IV non-small cell lung cancer: a surveillance, epidemiology, and end results survey from 1990 to 2005. J Thorac Oncol. 2009;4:1524–1529. doi: 10.1097/JTO.0b013e3181ba3634. [DOI] [PubMed] [Google Scholar]
- 37.Motoi N, Szoke J, Riely GJ, Seshan VE, Kris MG, Rusch VW, Gerald WL, Travis WD. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol. 2008;32:810–827. doi: 10.1097/PAS.0b013e31815cb162. [DOI] [PubMed] [Google Scholar]
- 38.Warth A, Muley T, Meister M, Stenzinger A, Thomas M, Schirmacher P, Schnabel PA, Budczies J, Hoffmann H, Weichert W. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J. Clin. Oncol. 2012;30:1438–1446. doi: 10.1200/JCO.2011.37.2185. [DOI] [PubMed] [Google Scholar]
- 39.Hung JJ, Yeh YC, Jeng WJ, Wu KJ, Huang BS, Wu YC, Chou TY, Hsu WH. Predictive value of the international association for the study of lung cancer/American Thoracic Society/ European Respiratory Society classification of lung adenocarcinoma in tumor recurrence and patient survival. J. Clin. Oncol. 2014;32:2357–2364. doi: 10.1200/JCO.2013.50.1049. [DOI] [PubMed] [Google Scholar]
- 40.Monk D, Bentley L, Beechey C, Hitchins M, Peters J, Preece M, Stanier P, Moore G. Characterization of the growth regulating gene IMP3, a candidate for Silver-Russell syndrome. J Med Genet. 2002;39:575–581. doi: 10.1136/jmg.39.8.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Del GA, Vaira V, Guerini RE, Palleschi A, Bulfamante G, Ricca D, Fiori S, Bosari S, Ferrero S. The oncofetal protein IMP3: a useful marker to predict poor clinical outcome in neuroendocrine tumors of the lung. J Thorac Oncol. 2014;9:1656–1661. doi: 10.1097/JTO.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 42.Wang T, Fan L, Watanabe Y, McNeill PD, Moulton GG, Bangur C, Fanger GR, Okada M, Inoue Y, Persing DH, Reed SG. L523S, an RNAbinding protein as a potential therapeutic target for lung cancer. Br J Cancer. 2003;88:887–894. doi: 10.1038/sj.bjc.6600806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mueller-Pillasch F, Pohl B, Wilda M, Lacher U, Beil M, Wallrapp C, Hameister H, Knochel W, Adler G, Gress TM. Expression of the highly conserved RNA binding protein KOC in embryogenesis. Mech Dev. 1999;88:95–99. doi: 10.1016/s0925-4773(99)00160-4. [DOI] [PubMed] [Google Scholar]
- 44.Gress TM, Muller-Pillasch F, Geng M, Zimmerhackl F, Zehetner G, Friess H, Buchler M, Adler G, Lehrach H. A pancreatic cancerspecific expression profile. Oncogene. 1996;13:1819–1830. [PubMed] [Google Scholar]
- 45.Jiang Z, Chu PG, Woda BA, Rock KL, Liu Q, Hsieh CC, Li C, Chen W, Duan HO, McDougal S, Wu CL. Analysis of RNA-binding protein IMP3 to predict metastasis and prognosis of renal-cell carcinoma: a retrospective study. Lancet Oncol. 2006;7:556–564. doi: 10.1016/S1470-2045(06)70732-X. [DOI] [PubMed] [Google Scholar]
- 46.Yantiss RK, Woda BA, Fanger GR, Kalos M, Whalen GF, Tada H, Andersen DK, Rock KL, Dresser K. KOC (K homology domain containing protein overexpressed in cancer): a novel molecular marker that distinguishes between benign and malignant lesions of the pancreas. Am J Surg Pathol. 2005;29:188–195. doi: 10.1097/01.pas.0000149688.98333.54. [DOI] [PubMed] [Google Scholar]


