Abstract
Objective: To analyze the expressions of Bcl-2, B7-H1, EGFR and VEGF in colorectal cancer for the further investigation of their correlations with the clinical pathological features of colorectal cancer. Method: Fresh colorectal cancer tissues and the expressions of Bcl-2, B7-H1, VEGF and EGFR in paraneoplastic normal mucosal tissues of 57 cases were tested by immunohistochemisty method, and the results were analyzed by SPSS10.0. Results: 1. Compared with paraneoplastic normal tissues, the expressions of Bcl-2 and B7-H1 in colorectal cancer tissues increased significantly with significant difference (P<0.05), while the expression of EGFR and VEGF in colorectal cancer tissues showed no significant difference with those in paraneoplastic normal tissue (P>0.05); 2. The correlation with clinical pathological features: there was significant difference of expression rates of EGFR between different genders (P<0.05); the expressions of BCL-2 and B7-H1 in colorectal cancer of the high- and medium- differentiated groups were significantly higher than those of the low-differentiated group, and the difference was significant (P<0.01); compared with the colorectal cancer patients without lymph node metastasis (Dukes stage A+B), the expression of B7-H1 in patients with lymph node metastasis (Dukes stage C+D) was significantly higher (P<0.05); 3. Within the high- and medium- differentiated colorectal cancer tissues, Bcl-2 expression rate in B7-H1 negative group was higher than the positive group with significant difference (P<0.01). Conclusions: In colorectal carcinoma, Bcl-2, B7-H1, EGFR and VEGF were all expressed, independent from age and depth of invasion. However, the expression level of Bcl-2 and B7-H1 correlated with tissue differentiation, and the latter also had correlation with tumor staging. Meanwhile, the short-term follow-up showed that high expression of Bcl-2/B7-H1 existed in death cases. Therefore, the expression detection of Bcl-2, B7-H1 might provide a clear understanding of the biological behavior of colorectal cancer, and was important for the diagnosis, treatment and prognosis judgment of colorectal cancer.
Keywords: Bcl-2, B7-H1, colorectal cancer, pathologic staging, lymph node metastasis
Introduction
The occurrence and invasion of colorectal cancer is a complex interaction process of multiple factors and stages in vitro and in vivo, many abnormal changes in molecular level, such as cell growth, apoptosis regulation, angiogenesis and immune evasion, might appear and involved multiple genes.
Bcl-2 is an important gene regulating apoptosis, and recent studies have shown that itself has no activities of proliferating and promoting cell deterioration, while in the conditions of no growth factors or neurotrophic factors, it could increase the opportunities of chromosomal aberration and viral infection through inhibiting apoptosis to prolong cell survival time, which would lead to cell canceration and contribute to tumorigenesis and development [1-3].
B7-H1, also known as PD-L1, a new member of B7 family, could significantly regulate T and B cell response [4]. The studies found that B7-H1 molecules could be expressed in a variety of tumor tissues in humans, promoting the apoptosis of cytotoxic T lymphocytes (CTL), and involved in the tumor immune escape process [5]. In vitro, B7-H1 could not only induce the apoptosis of T cells, playing an important role in tumor immune evasion through inhibiting Th1-based immune response with its inhibitory receptor PD-1, but also might promote T cell proliferation and cytokine secretion, playing positive and negative dual regulatory roles through non-PD-1 receptor-mediated costimulatory signals.
Angiogenesis refers to the formation of new blood vessels from the already existing blood vessels. Today, we know that through tumor growth, angiogenesis involves from the first stage to the final stage of the development of cancer, namely distant metastasis [6]. Vascular endothelial growth factor (VEGF) is an important factor for angiogenesis, playing roles at every stage of angiogenesis [6-8]. VEGF is a highly specific vascular endothelial cell mitogen, also known to be the strongest VEGF promoting factor, showing strong role in regulating blood vessel. The epidermal growth factor receptor (EGFR) is the expression product of proto-oncogene C-erbB-1, and its overexpression is related to cell proliferation and canceration. EGFR could also promote tumor angiogenesis through upregulating VEGF levels. Casanova [9] found that in the evolution of tumor, EGFR would promote or synergize tumor angiogenesis through increasing the expression of VEGF, and removed the role of EGFR would hinder the further blood vessels development.
Bcl-2, B7-H1, EGFR and VEGF are important regulators promoting tumor growth, development and transfer, there were many reports about the relationships between Bcl-2, EGFR and VEGF with colorectal cancer clinical stage and differentiation degree, while there were rare reports about the relationships between B7-H1 and colorectal cancer domesticly, and no simultaneous detection report was found about the relationships of the above 4 factors with colorectal cancer. Therefore, we applied immunohistochemistry method to simultaneously detect the expression of Bcl-2, B7-H1, VEGF and EGFR in colorectal cancer tissues and (or) paraneoplastic normal tissues, for the purpose of analysis of their relationships with clinical pathological features of colorectal cancer, and exploring the interaction and significance among these 4 factors.
Materials and methods
Clinical data
57 cases, male 35 cases and females 22 cases, were collected from Jan. 2010 to Jan. 2011, Baotou Tumor Hospital, with complete clinical data of radical surgery of colorectal cancer for the removal of fresh tissues and paraneoplastic normal tissue (5 cm more to the cancer tissue), with average age 60±14 years old, among them 31 cases of rectal cancer (including anal cancer) and 26 cases of colon cancer. 33 cases were identified as high- and medium- differentiated adenocarcinoma in accordance with the WHO pathology classification standards, and 24 cases were low-differentiated adenocarcinoma. According to Dukes Staging, 32 cases were identified as A+B stage (without lymph node metastasis and distant metastasis), and 25 cases as C+D (with lymph node metastasis but no distant metastasis). The patients had not received preoperative radiotherapy and chemotherapy treatment before.
Method
Making paraffin sections, after dewaxing and hydration, antigen retrieval was carried out. All patients were stained using 2-step EnviSion Method in accordance with EnviSion kit (DAKO Corporation, USA) instructions, adding antibody I and biotinylated antibody II ordinally, then using DAB for colouration and hematoxylin for afterstaining, then using alcohol for gradual dehydration, hyalinized, dry naturally after mounted with neutral resin, and observed under the microscope, using PBS buffer instead of antibody I as the negative sample. The BCL-2 antibody I and VEGF antibody I used in the study were rabbit monoclonal antibodies (Beijing Zhongshan Golden Bridge Biotechnology Company); B7-H1 antibody I was rabbit monoclonal antibody (Long Island biotechnology company); EGFR antibody I was mouse monoclonal antibody (Beijing Zhongshan Golden Bridge Biotechnology company). All monoclonal antibodies were prepared according to strict instructions (specify each antibody dilution level), and the pre-experiments were done to verify the effects.
Results determination
Double blind method was used for film-reading: EGFR was brownish yellow fine particle, expressed in the cell membrane and cytoplasm; VEGF was expressed in the cytoplasm as brown granular; B7-H1 was expressed on the cell membrane and cytoplasm as pale yellow to brown fine particle; Bcl-2’s positive expression was also a brown particle, mainly locating in the cytoplasm. Positive cells semi-quantitative grading method was used to judge the results: scored the results according to the percentage of positive cells accounted for the whole tumor cells, <5% as 0, 5%-25% as 1 point, 26%-50% as 2 points, 51%-75% as 3 points, >75% as 4 points; then scored the results according to positive cells dyeing shade, uncolored as 0, pale yellow as 2 points, brown as 3 points, and finally the above 2 scores were multiplied, 0 was identified as negative, 1-4 points as (+), 5-8 points as (++), 9-12 (+++), (+) and over were seen as positive cases. Results were shown in Figures 1 and 2.
Figure 1.
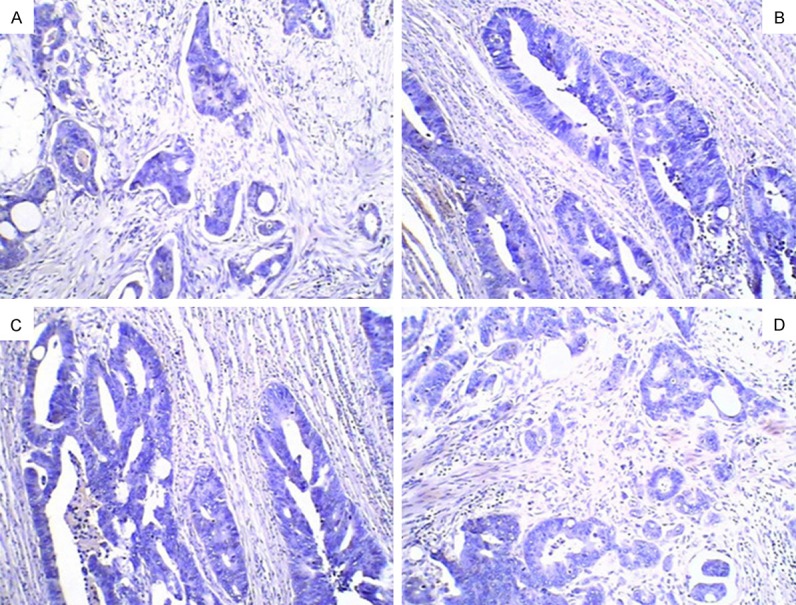
Negative expression in the colorectal cancer tissues. A. Bcl-2 negative expression in the colorectal cancer tissues ×200; B. B7-H1 negative expression in the colorectal cancer tissues ×200; C. EGFR negative expression in the colorectal cancer tissues ×200; D. VEGF negative expression in the colorectal cancer tissues ×200.
Figure 2.
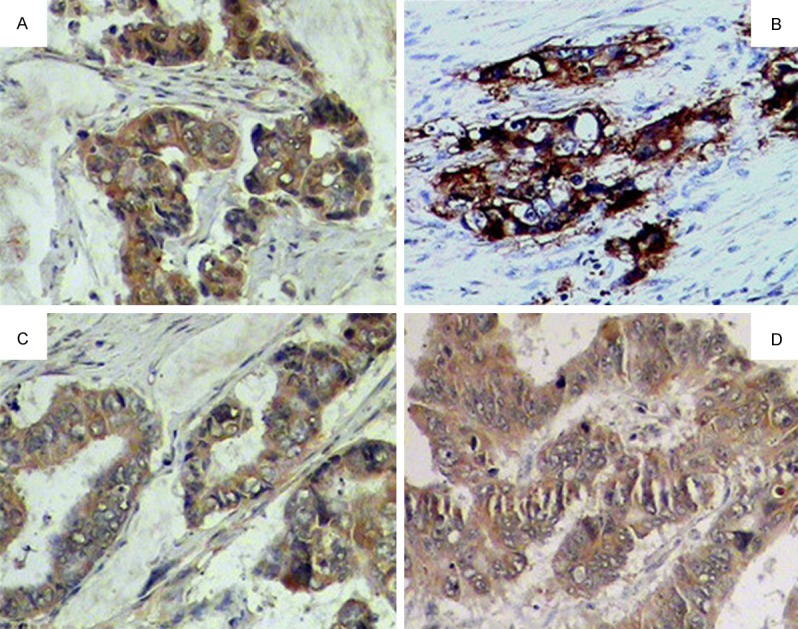
Positive expression in the colorectal cancer tissues. A. Bcl-2 positive expression in the colorectal cancer tissues ++ ×200; B. B7-H1 positive expression in the colorectal cancer tissues +++ ×200; C. EGFR positive expression in the colorectal cancer tissues ++ ×200; D. VEGF positive expression in the colorectal cancer tissues + ×200.
Follow-up situation
Follow-up was carried out since the surgery finished, and ended on July 1st, 2011. The whole were 6-18 months, and 48 cases still lived at the end of follow-up with good condition, efficacy evaluation as PR or NC; 5 cases were lost; the 4 dead patients survived 4 to 8 months.
Statistically
Using Excel to create the database, and all data were analyzed using the statistical software SPSS13.0 ×2 test and related statistical analysis, P<0.05 was set up for statistically significant differences.
Results
Expression of BCL-2 and B7-H1 in colorectal cancer tissues and paraneoplastic normal tissues
BCL-2 positive expression rate in 57 cases of colorectal cancer tissues and paraneoplastic normal tissues was 75.43% (43/57) and 52.63% (30/57), respectively. B7-H1 positive expression rate in 57 cases of colorectal cancer tissues and paraneoplastic normal tissues was 45.61% (26/57) and 15.79% (9/57), respectively. The positive expression rates of BCL-2 and B7-H1 in colorectal cancer tissues were higher than those in paraneoplastic normal tissues, and the difference was statistically significant (P<0.05). Note: expression of EGFR and VEGF in 57 cases of colorectal cancer tissues and paraneoplastic normal tissues showed no statistically significant difference (P>0.05).
Clinicopathological relationships of Bcl-2, B7-H1, EGFR and VEGF with colorectal cancer
Through X2 test, positive expression rates of BCL-2 and B7-H1 had statistically significant difference in different differentiated degrees of colorectal cancer (*P<0.01); the positive expression rate of B7-H1, in the different Dukes stage with lymph node metastasis of colorectal cancer, showed statistically significant difference (**P<0.05); compared with female, the positive expression rate of EGFR in colorectal cancer tissues of male patients had statistically significant difference (P<0.05); the expression of VEGF had no significant differences in different age, gender, depth of invasion, stage and lymph node metastasis of colorectal cancer patients. See Table 1. (In this study, the main emphasis of Dukes stage D was distant lymph node metastasis, but not distant metastases.)
Table 1.
The relationship with clinicopathological features of Bcl-2, B7-H1, EGFR and the VEGF expres-sion in colorectal cancer tissues
| Clinical pathology parameter | n | Bcl-2 (+) | B7-H1 (+) | EGFR (+) | VEGF (+) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| n (%) | x2 | p | n (%) | x2 | p | n (%) | x2 | p | n (%) | x2 | p | ||
| Sex | |||||||||||||
| Male | 35 | 24 (68.6) | 2.308 | 0.129 | 15 (42.9) | 0.753 | 0.386 | 7 (20.0)*** | 5.016 | 0.025 | 26 (74.3) | 2.397 | 0.122 |
| Female | 22 | 19 (86.4) | 11 (50.0) | 0 (0.00) | 20 (90.9) | ||||||||
| Age | |||||||||||||
| ≥60 | 36 | 27 (75.0) | 0.01 | 0.92 | 14 (38.9) | 1.781 | 0.182 | 3 (8.3) | 1.413 | 0.234 | 30 (83.3) | 0.435 | 0.51 |
| <60 | 21 | 16 (76.2) | 12 (57.1) | 4 (19.6) | 16 (76.2) | ||||||||
| Infiltration depth | |||||||||||||
| Under serosa | 8 | 4 (50.0) | 3.25 | 0.071 | 4 (50.0) | 0.072 | 0.92 | 0 (0.00) | 1.303 | 0.254 | 7 (87.5) | 0.276 | 0.599 |
| Outside serosa and serosa | 49 | 39 (79.6) | 22 (44.9) | 7 (14.3) | 39 (79.6) | ||||||||
| Differentiated degree | |||||||||||||
| the highly- and medium-differentiated | 33 | 21 (63.6)* | 6.605 | 0.01 | 8 (24.2)* | 14.43 | 0.001 | 4 (12.1) | 0.002 | 0.966 | 25 (75.8) | 0.464 | 0.496 |
| the low-differentiated | 24 | 22 (91.7) | 18 (75.0) | 3 (12.5) | 21 (87.5) | ||||||||
| Dukes stages | |||||||||||||
| A+B | 27 | 22 (81.5) | 1.011 | 0.315 | 8 (29.6) | 5.284 | 0.022 | 2 (7.4) | 0.182 | 0.67 | 23 (85.2) | 0.662 | 0.416 |
| C+D | 30 | 21 (70.0) | 18 (60.0)** | 5 (16.7) | 23 (76.7) | ||||||||
| Lymph node metastasis | |||||||||||||
| Yes | 30 | 21 (70.0) | 1.011 | 0.315 | 18 (60.0)** | 5.284 | 0.022 | 5 (16.7) | 0.182 | 0.67 | 23 (76.7) | 0.662 | 0.416 |
| No | 27 | 22 (81.5) | 8 (29.6) | 2 (7.4) | 23 (85.2) | ||||||||
P<0.05;
P<0.05.
P values: a statistical method of Statistics.
Correlation between the expression of Bcl-2 and B7-H1 in the high- and medium- differentiated colorectal cancer tissues
In the high- and medium differentiated colorectal cancer tissues, Bcl-2 positive expression of B7-H1 negative group was higher than that of B7-H1 positive group, with statistically significant differences (*P<0.01), indicating that in high- and medium-differentiated colorectal cancer tissues, the expressions of Bcl-2 and B7-H1 was negatively correlated, in Table 2. Note: because the expressions of Bcl-2 and B7-H1 in low-differentiated colorectal cancer tissues were not obviously correlated, it would not be discussed in detail.
Table 2.
The relationship of Bcl-2 and B7-H1 expression Within the highly- and medium- differentiated colorectal cancer tissues
| Bcl-2 Positive number | Bcl-2 Negative number | X2 | P | |
|---|---|---|---|---|
| B7-H1 Positive group | 4 | 3 | 8.450 | 0.003 |
| B7-H1 Negative group | 17* | 8 |
P<0.01.
P values: a statistical method of Statistics.
In the study, we did have a multivariate analysis, but in addition to the correlation found in the expressions of BCL-2 and B7-H1 in the high- and medium-differentiated groups, there were no other valuable findings. In order to prevent the article was unfocused, the other findings was omitted.
Survival time
Till Jul. 1st 2011, the follow up towards 57 cases of colorectal cancer patients was 6-18 months, and till the follow-up cutoff date, 48 cases still lived with good condition and no recurrence; 5 cases lost; the 4 dead survived for 4 to 8 months. The positive rates of Bcl-2 and (or) B7-H1 in the 4 dead were 100%, and showed a strong positive. Because the number of dead cases was low and follow-up time was short, its practical significance was not yet clear, and the next step would be continued for the 3- and 5-year survival of the patients.
Discussion
The Bcl-2 proto-oncogene was found in follicular lymphoma, and a high level of bcl-2 protein could inhibit cell death, extending cell life and anti-apoptosis [10]. The abnormal Bcl-2 expression allows cells to escape apoptosis, leading to malignant transformation of cells [11]. In the study of various tumors, there were different reports about the expression of Bcl-2 and tumor grading, staging and prognosis [12].
Dong et al [13] found that B7-H was not only expressed in normal tissues, but also in ovarian cancer tissues. Though B7-H1 mRNA could be detected in many tissues, its protein expression in normal tissue was limited to the cells sourced from macrophage system [5], while it could be detected in human lung cancer, ovarian cancer, colorectal cancer, melanoma, head and neck squamous cell carcinoma, breast cancer, stomach cancer, bladder cancer, renal cell carcinoma and other tumors. Hua et al [14] found that B7-H1 protein had significantly higher positive expression rate in colon carcinoma than in the adjacent tissues, and another report [15] also confirmed this and found that B7-Hl expression had no significant association with patient gender, age, histological type, grade and stage, while had association with lymph node metastasis, suggesting that B7-H1 was indeed involved in the development of colon cancer invasion and metastasis. B7-Hl expression could be seen as one of the indicators reflecting the colon cancer prognosis and predicting risk of recurrence.
In this study, Bcl-2 and B7-Hl synchronous detection was done in cancer tissue samples and paraneoplastic normal tissues of 57 patients with colorectal cancer surgery, the results showed that: compared with paraneoplastic normal tissues, the positive expression rates of BCL-2 and B7-H1 significantly increased in colorectal cancer tissues. The difference was statistically significant (P<0.05). At the same time, the relationship between the two factors and the clinical pathological features revealed that BCL-2 and B7-H1 expression in colorectal cancer tissues was negatively correlated with the differentiation degree, the higher the degree, the lower the expression. This conclusion was consistent with previous domestic and international researches. In addition, this study also found that B7-H1 protein expression in colorectal cancer with lymph node metastasis (Dukes stage C+D) was higher than without lymph node metastasis (Dukes stage A+B). The above conclusions were not entirely consistent with the past researches, the difference might be due to experimental methods or methods of selecting cases of clinical and pathological grouping. But these were able to reveal that BCL-2 could induce the inhibition of apoptosis of tumor cells, as well as B7-H1-mediated tumor cell immune escape was indeed one of the important molecular biological mechanisms of colorectal cancer development, invasion and metastasis. The combined detection of BCL-2 and B7-H1 expression might provide a reference for the prognosis and metastasis of patients with colorectal cancer.
In the present study, we discovered for the first time that in high- and medium-differentiated colorectal cancer tissues, Bcl-2 protein expression of B7-H1 negative group was higher than B7-H1 positive group with statistically significant difference (P<0.01), indicating that in high- and medium-differentiated colorectal cancer tissues, the expression of Bcl-2 and B7-H1 expression might be negative correlated in colorectal cancer tissues, which was not found in the previous literatures. The reason was still unclear, and still being explored whether it could be used to predict histological differentiation in colorectal cancer.
EGFR has expression and overexpression in neck squamous cell carcinoma, breast cancer, gastric cancer, lung cancer, prostate cancer, kidney cancer, ovarian cancer, bladder cancer and other epithelial tumors, and associated with clinical progression, invasion and metastasis [16,17]. VEGF has low expression in normal adult and animal tissues, but higher expression in fetus and the organizations with ongoing physiological blood vessel growth. VEGF has higher expression in the tumor, promoting tumor angiogenesis, playing an important role to maintain the continuous growth of tumor and forming the basis of metastasis [18], therefore, VEGF expression level was positively correlated with malignancy, metastasis and microvascular density, etc, and a negative correlation with the prognosis of patients. In this study, the correlation between the expression of VEGF and EGFR and clinical pathological features, such as tumor size, lymph node metastasis and Dukes stage, was not found, nor was the correlation between their expression. Compared with the previous reports, it might be related to the study sample size and sample related. We had come to the conclusion that the positive expression rate of EGFR in men colorectal cancer tissues was found higher than those in women, while yet there were not reports related to gender differences in VEGF or EGFR in colorectal cancer.
In summary, the occurrence and development of colorectal cancer is a multi-factor, multi-stage, multi-gene and mutational pathological process. Its mechanism has not yet been fully elucidated, and closely related to a variety of gene abnormal expression. Bcl-2 and B7-H1 play important roles in colorectal cancer occurrence and development, contributing to early diagnosis and prognosis of colorectal cancer. But there is no strong evidence to support whether the expression of them has a certain relevance or not. The effects of VEGF and EGFR on determining tumor staging, treatment and prognosis, as well as synergy in tumorigenesis and tumor progression, though confirmed by most studies, has not reached a similar results except for the gender difference, the reason might because of less number of samples and specimen heterogeneity. In the next step, the sample size should be increased. Furthermore, the follow-up time of this study was relatively short, leading to no clear correlation between high expression of the above factors and the prognosis, which might be more prominent with the depth of the follow-up.
Acknowledgements
Supported by Baotou Health Scientific Fund (2010s2001-5).
Disclosure of conflict of interest
None.
References
- 1.Krecicki T, Fraczek M, Kozlak J, Zatonski T, Jelen M, Dus D. Bcl-xI protein expression in laryngeal squamous cell Carcinoma. Clin Otolaryngol Allied Sci. 2004;29:55–58. doi: 10.1111/j.1365-2273.2004.00780.x. [DOI] [PubMed] [Google Scholar]
- 2.Zhao WL, Daneshpouy ME, Mounier N, Brière J, Leboeuf C, Plassa LF, Turpin E, Cayuela JM, Ameisen JC, Gisselbrecht C, Janin A. Prognostic significance of Bcl-xL gene expression and apoptotic cell counts in follicular lymphoma. Blood. 2007;103:695–697. doi: 10.1182/blood-2003-06-1901. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe J, Kushihata F, Honda K, Sugita A, Tateishi N, Mominoki K, Matsuda S, Kobayashi N. Prognostic significance of bcl-xL in human hepatocellular carcinoma. Surgery. 2008;135:604–612. doi: 10.1016/j.surg.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Wang S, Bajorath J, Flies DB, Dong H, Honjo T, Chen L. Molecular modeling and functional mapping o f B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J Exp Med. 2003;197:1083–1091. doi: 10.1084/jem.20021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 6.Bremnes RM, Camps C, Sirera R. Angiogenesis in non-small cell lung cancer: the prognostic impact of neoangiogenesis and the cytokines VEGFand bFGF in tumours and blood. Lung Cancer. 2006;51:143–158. doi: 10.1016/j.lungcan.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N. Vascular endothelial growth factor. Eur J Cancer. 1996;32:2413–2422. doi: 10.1016/s0959-8049(96)00387-5. [DOI] [PubMed] [Google Scholar]
- 8.Yuan A, Yu CJ, Kuo SH, Chen WJ, Lin FY, Luh KT, Yang PC, Lee YC. Vascular endothelial growth factor 189 mRNA isoform expression specifically correlates with tumor angiogenesis, patient survival, and postoperative relapse in non-small-cell lung cancer. J. Clin. Oncol. 2001;19:432–441. doi: 10.1200/JCO.2001.19.2.432. [DOI] [PubMed] [Google Scholar]
- 9.Casanova ML, Larcher F, Casanova B, Murillas R, Fernández-Aceñero MJ, Villanueva C, Martínez-Palacio J, Ullrich A, Conti CJ, Jorcano JL. Acriticalrol for ras mediate depidermal growth factor receptor dependent angio genesis in mouse skin carcinogenesis. Cancer Res. 2002;62:3402–3407. [PubMed] [Google Scholar]
- 10.Colombel M, Symmans F, Gil S, O’Toole KM, Chopin D, Benson M, Olsson CA, Korsmeyer S, Buttyan R. Det ect ion of the apoptosissuppressing oncoprotein blc-2 in hormone-refractory human prostate cancer. Am J Pathol. 1993;143:390–400. [PMC free article] [PubMed] [Google Scholar]
- 11.O zak, Tani E, Ikemoto H, Kitagawa H, Fujikawa H. Activative of stress-activation of stress-activated protein kinase/c-Jun NH 2-terminalkinase and P38 kinase in calphostin C-induced apoptosis requirescaspase-3-like proleases, but is dispensable for cell death. J Bio Chem. 1999;274:5310–5317. doi: 10.1074/jbc.274.9.5310. [DOI] [PubMed] [Google Scholar]
- 12.De Angelis PM, Stokke T, Thorstensen L, Lothe RA, Clausen OP. Apoptosis and expression of BAX, and BCL- 2 apoptotic regulatory proteins in colorectal carcinomas, and association with p53 genotype /phenotype. Curr Cancer Drug T argets. 2004;4:65. doi: 10.1136/mp.51.5.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, costimulation T cell proliferation and secretion of IL-10. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 14.Hua D, Sun J, Mao Y, Chen LJ, Wu YY, Zhang XG. B7-H1 expression is associated with expansion of regulatory T cells in colorectal carcinoma. World J Gastroenterol. 2012;18:971–978. doi: 10.3748/wjg.v18.i9.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carneiro A, Isinger A, Karlsson A, Johansson J, Jönsson G, Bendahl PO, Falkenback D, Halvarsson B, Nilbert M. Pprognostic impact of array-based genomic profiles in esophageal squamous cell cancer. BMC Cancer. 2008;11:98–102. doi: 10.1186/1471-2407-8-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matkovic B, Juretic A, Separovic V, Novosel I, Separovic R, Gamulin M, Kruslin B. Immunohistochemical analysis of ER, PR, HER-2, CK5/6, P63 and EGFR antigen expression in Medullary breast cancer. Tumori. 2008;94:838–841. doi: 10.1177/030089160809400611. [DOI] [PubMed] [Google Scholar]
- 18.Rmali KA, Puntis MC, Jiang WC. Tumouassociated angiogenesis in human colorectalr. Colorectal Dis. 2007;9:3–7. doi: 10.1111/j.1463-1318.2006.01089.x. [DOI] [PubMed] [Google Scholar]


