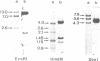Abstract
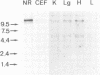
Non-dividing neuroretina cells from chicken embryos are induced to proliferate after a long latency, following infection with Rous associated virus type 1, an avian retrovirus which does not carry a transforming gene. We have isolated from these proliferating cells an acutely mitogenic retrovirus, designated IC10, which contains a novel oncogene. Nucleotide sequencing showed that the IC10 virus has transduced 1101 nucleotides of cellular origin inserted between the gag and env genes of RAV-1. This oncogene, designated v-Rmil, is 70.1% homologous to v-mil. v-Rmil encodes a protein of 40,976 daltons sharing 83.8% homology with the catalytic domain of the v-mil protein. Divergence with the v-mil gene product is observed at the NH2- and COOH-terminal portions of the v-Rmil protein. Restriction analysis of normal chicken DNA indicated that v-Rmil is derived from a cellular gene distinct from c-mil. The c-Rmil gene is transcribed through a major mRNA, greater than 10 kb in length, that is detected at much higher levels in neuroretinas, as compared to other embryonic tissues.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechade C., Calothy G., Pessac B., Martin P., Coll J., Denhez F., Saule S., Ghysdael J., Stéhelin D. Induction of proliferation or transformation of neuroretina cells by the mil and myc viral oncogenes. Nature. 1985 Aug 8;316(6028):559–562. doi: 10.1038/316559a0. [DOI] [PubMed] [Google Scholar]
- Beck T. W., Huleihel M., Gunnell M., Bonner T. I., Rapp U. R. The complete coding sequence of the human A-raf-1 oncogene and transforming activity of a human A-raf carrying retrovirus. Nucleic Acids Res. 1987 Jan 26;15(2):595–609. doi: 10.1093/nar/15.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. doi: 10.1016/s0092-8674(85)80098-2. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Oppermann H., Seeburg P., Kerby S. B., Gunnell M. A., Young A. C., Rapp U. R. The complete coding sequence of the human raf oncogene and the corresponding structure of the c-raf-1 gene. Nucleic Acids Res. 1986 Jan 24;14(2):1009–1015. doi: 10.1093/nar/14.2.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calothy G., Pessac B. Growth stimulation of chicl embryo neuroretinal cells infected with Rous sarcoma virus: relationship to viral replication and morphological transformation. Virology. 1976 May;71(1):336–345. doi: 10.1016/0042-6822(76)90117-3. [DOI] [PubMed] [Google Scholar]
- Calothy G., Poirier F., Dambrine G., Mignatti P., Combes P., Pessac B. Expression of viral oncogenes in differentiating chick embryo neuroretinal cells infected with avian tumor viruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):983–990. doi: 10.1101/sqb.1980.044.01.106. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coll J., Righi M., Taisne C., Dissous C., Gegonne A., Stehelin D. Molecular cloning of the avian acute transforming retrovirus MH2 reveals a novel cell-derived sequence (v-mil) in addition to the myc oncogene. EMBO J. 1983;2(12):2189–2194. doi: 10.1002/j.1460-2075.1983.tb01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes P. C., Privat A., Pessac B., Calothy G. Differentiation of chick embryo neuroretina cells in monolayer cultures. An ultrastructural study. I. Seven-day retina. Cell Tissue Res. 1977 Dec 13;185(2):159–173. doi: 10.1007/BF00220661. [DOI] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier C., Denhez F., Coll J., Amouyel P., Quatannens B., Begue A., Stehelin D., Saule S. Induction of proliferation of neuroretina cells by long terminal repeat activation of the carboxy-terminal part of c-mil. Mol Cell Biol. 1987 May;7(5):1995–1998. doi: 10.1128/mcb.7.5.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert F., Dupont de Dinechin S., Righi M., Stehelin D. The second oncogene mil of avian retrovirus MH2 is related to the src gene family. EMBO J. 1984 Jun;3(6):1333–1338. doi: 10.1002/j.1460-2075.1984.tb01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Ikawa S., Fukui M., Ueyama Y., Tamaoki N., Yamamoto T., Toyoshima K. B-raf, a new member of the raf family, is activated by DNA rearrangement. Mol Cell Biol. 1988 Jun;8(6):2651–2654. doi: 10.1128/mcb.8.6.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa F., Takaku F., Nagao M., Sugimura T. The complete primary structure of the rat A-raf cDNA coding region: conservation of the putative regulatory regions present in rat c-raf. Oncogene Res. 1987 Aug;1(3):243–253. [PubMed] [Google Scholar]
- Jansen H. W., Bister K. Nucleotide sequence analysis of the chicken gene c-mil, the progenitor of the retroviral oncogene v-mil. Virology. 1985 Jun;143(2):359–367. doi: 10.1016/0042-6822(85)90376-9. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Taylor S. S., Sefton B. M. Direct evidence that oncogenic tyrosine kinases and cyclic AMP-dependent protein kinase have homologous ATP-binding sites. Nature. 1984 Aug 16;310(5978):589–592. doi: 10.1038/310589a0. [DOI] [PubMed] [Google Scholar]
- Kan N. C., Flordellis C. S., Mark G. E., Duesberg P. H., Papas T. S. Nucleotide sequence of avian carcinoma virus MH2: two potential onc genes, one related to avian virus MC29 and the other related to murine sarcoma virus 3611. Proc Natl Acad Sci U S A. 1984 May;81(10):3000–3004. doi: 10.1073/pnas.81.10.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen M., Sippel A. E., Trachmann C., Bister K. Primary structure of the chicken c-mil protein:identification of domains shared with or absent from the retroviral v-mil protein. Oncogene. 1988 Feb;2(2):179–185. [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Mölders H., Defesche J., Müller D., Bonner T. I., Rapp U. R., Müller R. Integration of transfected LTR sequences into the c-raf proto-oncogene: activation by promoter insertion. EMBO J. 1985 Mar;4(3):693–698. doi: 10.1002/j.1460-2075.1985.tb03685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessac B., Calothy G. Transformation of chick embryo neuroretinal cells by Rous sarcoma virus in vitro: induction of cell proliferation. Science. 1974 Aug;185(4152):709–710. doi: 10.1126/science.185.4152.709. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F. Determination of nucleotide sequences in DNA. Science. 1981 Dec 11;214(4526):1205–1210. doi: 10.1126/science.7302589. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stavnezer E., Gerhard D. S., Binari R. C., Balazs I. Generation of transforming viruses in cultures of chicken fibroblasts infected with an avian leukosis virus. J Virol. 1981 Sep;39(3):920–934. doi: 10.1128/jvi.39.3.920-934.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]