Abstract
Some studies have shown the usability of neoadjuvant chemotherapy (NAC) in gastric cancer (GC). Nevertheless there are a few predictive markers of the effectiveness of NAC in GC. The aim of this study is to assess the predictive impact of organic cation transporter 2 (OCT2) expression on response to neoadjuvant chemotherapy (NAC) in gastric cancer. We retrospectively assessed 66 patients with advanced gastric cancer received NAC with S-1/cisplatin or paclitaxel/cisplatin. Expression levels of OCT2 were assessed by immunohistochemistry in pre-chemotherapy biopsies and correlated with clinicopathologic parameters including pathologic response. High expression level of OCT2 (OCT2high) was significantly associated with intestinal type according to Laurén classification (P = 0.03) and low histologic grade (P = 0.03). In univariate analysis of the entire cohort, no variables showed any significant association with a response, although intestinal type (P = 0.09), low histologic grade (P = 0.09), and OCT2high (P = 0.07) tended to be more frequent in responders compared with non-responders. When the two treatment groups were separately assessed in the univariate analysis, a significantly higher rate of OCT2high was observed in responders compared with non-responders in the S-1/cisplatin group (P = 0.001). In addition, multivariate analysis identified OCT2high as the sole independent predictor of response (P = 0.04). However, in the paclitaxel/cisplatin group, no variables were associated with response. Taken together, our results suggest that OCT2high may represent a potential predictor of response to NAC with S-1/cisplatin in gastric cancer.
Keywords: Organic cation transporter 2, neoadjuvant chemotherapy, S-1 plus cisplatin, gastric cancer, immunohistochemistry
Introduction
Although the incidence of gastric cancer (GC) has declined over the last few years, GC still remains a significant cause of cancer death worldwide [1]. Clinical studies have shown that neoadjuvant chemotherapy (NAC) is feasible and improves clinical outcomes of patients with locally advanced GC [2]. Advantages of NAC include a high rate of complete surgical resection, downstaging, and avoidance of unnecessary surgery [2]. In Japan, NAC is an investigational treatment only permitted in clinical trials [2]. Among several NAC regimens, S-1, a fluoropyrimidine containing 5-fluorouracil (5-FU) prodrug, plus cisplatin (S-1/CDDP) and paclitaxel plus CDDP (PTX/CDDP) are promising regimens [3-5]. Nevertheless, it has been pointed out that there is a risk of delaying surgery in patients who do not respond to NAC, and thus the identification of predictors of NAC response is essential for selecting the appropriate treatment strategy.
To the best of our knowledge, only a few studies on predictive markers of the effectiveness of NAC with S-1/CDDP in primary GC and no studies on PTX/CDDP have been undertaken to date.
Thymidylate synthase (TS) is an essential DNA synthetic enzyme that is suppressed by 5-fluoro-deoxyuridine-monophosphate, an active metabolite of 5-FU, and has received attention as a possible predictor of resistance to fluoropyrimidines [6,7]. However, characterization of the role of high TS expression in NAC resistance with S-1/CDDP has been confusing [8,9].
The solute carrier (SLC) transporters are imperative for the cellular uptake of endogenous compounds, xenobiotics, and clinically important drugs [10-12]. Since the facilitated uptake system via SLC transporters is an important mechanism for the responsiveness to anticancer drugs, expression levels of SLC transporters may help predict an individual’s susceptibility to certain treatments. Organic cation transporters (OCTs; encoded by SLC22 genes) play a critical role in the cellular uptake of endogenous cationic substrates, hydrophilic exogenous xenobiotics, and platinum anticancer drugs [11]. For instance, organic cation transporter 2 (OCT2), also called SLC22A2, is a critical determinant in uptake and consequent cytotoxicity of CDDP and oxaliplatin [13-15]. OCT2 is strongly expressed in renal proximal tubule cells, and uptake of CDDP, mediated by OCT2, is essential to explain selective organ toxicity of CDDP [15]. On the other hands, our research group recently found that high OCT2 expression was significantly correlated with longer progression-free survival in patients with metastatic colorectal cancer treated with first-line fluorouracil/leucovorin/oxaliplatin (FOLFOX)-based chemotherapy [16]. However, the clinicopathologic role of OCT2 in GC remains to be elucidated.
Therefore, in this study, we immunohistochemically assessed the impact of the OCT2 expression level in GC for predicting response to NAC with S-1/CDDP or PTX/CDDP regimens.
Materials and methods
Patients and chemotherapy
Fifty-six patients with advanced GC who received NAC between 2001 and 2006 at the Kanagawa Cancer Center Hospital were recruited for this study. The patients were treated with CDDP-based NAC with S-1/CDDP or PTX/CDDP regimens. Two or four courses of these regimens were administered, depending on the response to NAC and resectability of the tumor. In the S-1/CDDP regimen, S-1 80 mg/m2 was orally administered twice daily for the first 3 weeks of a 4-week cycle and CDDP 60 mg/m2 was intravenously administered on day 8 of each cycle [3,5]. In the PTX/CDDP regimen, paclitaxel 80 mg/m2 and cisplatin 25 mg/m2 were given on days 1, 8, and 15 over a 4-weekly cycle [3,4].
Prior to NAC, patients underwent an endoscopic biopsy of their tumor. Written informed consent was obtained from all patients and the Ethics Committees of Kobe University Graduate School of Health Sciences and Kanagawa Cancer Center Hospital approved the study.
Tumor specimens and histologic assessment
Pre-chemotherapy biopsies with multiple pieces and post-chemotherapy resection materials were fixed in 10% formalin and embedded in paraffin, and 3-μm-thick sections were prepared. Sections were stained with hematoxylin and eosin, to assess histopathologic features and response to NAC. Histologic grade was defined as well (G1), moderately (G2), poorly differentiated (G3), or undifferentiated (G4), and reflected the poorest grade within the tissue. G1 and G2 were defined as low histologic grade, and G3 and G4 as high histologic grade.
Pathologic response to NAC was evaluated using post-chemotherapy resection materials, according to the Japanese Classification of Gastric Carcinoma [17] as follows: grade 0, no effect; grade 1, slight effect (grade 1a, viable tumor cells occupy more than 2/3 of the entire cancer area; grade 1b, viable tumor cells remain in more than 1/3 but less than 2/3 of the entire cancer area); grade 2, considerable effect (viable tumor cells remain in less than 1/3 of the entire cancer area); and grade 3, complete response (no viable tumor cells remain). Patients with tumors showing grades 2 and 3 were defined as responders, and patients with tumors showing grades 0, 1a, and 1b as non-responders.
Protein expression and gene amplification of human epidermal growth factor receptor type 2 (HER2) were analyzed using immunohistochemistry and fluorescence in situ hybridization (FISH), respectively. Tumors with strong positive staining in at least one cancer cell cluster (five or more cells) or FISH ratio of > 2.2 were regarded as positive for HER2.
Immunohistochemistry of OCT2
Sections of biopsy specimens were deparaffinized in xylene and then immersed in a graded alcohol series, and rehydrated in tap water. Heat-induced antigen retrieval (HIAR) method was applied using a pressure cooker for 10 min at 120°C in 0.001 mol/L EDTA (pH 8.0). After HIAR, the sections were cooled to room temperature (RT) for 30 min. Sections were then rinsed in tap water followed by phosphate-buffered saline (PBS; pH 7.2).
The sections were subsequently incubated with an anti-human OCT2 rabbit polyclonal antibody (1:800 dilution; Atlas Antibodies, Stockholm, Sweden) overnight at RT. After rinsing with PBS, sections were incubated with the Histofine Simple Stain MAX-PO (Nichirei Bioscience, Tokyo, Japan) as a secondary reagent for 1 h at RT. Thereafter, reaction products were developed using diaminobenzidine solution (Dako, Glostrup, Denmark) and the nuclei were lightly counterstained with Mayer’s hematoxylin. A negative control was included in each run without applying the primary antibodies. Sections of normal kidney were used as a positive control.
Assessment of OCT2 level
All of the immunostaining results were assessed by three investigators (A.N., R.T., and S.K.) who had no knowledge of the clinicopathologic details of the patients. The staining intensity on cell membranes was graded on a scale of 0-3 (0, none; 1, weak; 2, moderate; 3, strong). The percentage of positive tumor cells was scored as follows: 0, 0%; 1, 1-10%; 2, 11-50%; 3, 51-100%. A final semi-quantitative score (0-6) was derived by adding the intensity score and the percentage score. According to the cutoff score determined by receiver operating characteristic curve analysis, the cutoff threshold was set at 4 using the best sensitivity and specificity. Scores of 0-3 were considered low expression level (OCT2low) and scores of 4-6 were considered high expression level (OCT2high). In the event of disagreement, the three investigators reevaluated the immunostained sections and discussed the interpretation until agreement was reached.
Statistical analysis
The Fisher’s exact test was used to evaluate the association of OCT2 level with patient age and sex, tumor localization, Laurén classification, histologic grade, HER2 status, and NAC regimen. The Fisher’s exact test was also used to determine the association of pathologic response with patient age and sex, tumor localization, Laurén classification, histologic grade, HER2 status, NAC regimen, and OCT2 level. A logistic regression model for multivariate analysis was performed to identify independent predictors of response. All variables assessed on the univariate analysis were included in the multivariate analysis. Differences with a P-value < 0.05 were considered statistically significant. All analyses were performed using SPSS 21.0 software (SPSS Inc., Chicago, IL, USA).
Results
Patient and tumor characteristics
Pre-chemotherapy characteristics of patients and tumors are shown in Table 1. The median age of the patients was 64 (range, 40-77) years and the study population included 33 men and 23 women. Twenty-six patients had tumors located in the proximal third, 13 had tumors in the middle third, and 11 had tumors in the distal third. In six patients, the tumors diffusely involved the entire stomach. Regarding Laurén classification, 11 tumors were intestinal, 36 were diffuse, and 9 were mixed type. Three patients had G1 tumors, 8 had G2 tumors, 32 had G3 tumors, and 13 had G4 tumors. HER2 was evaluable in 47 of the 56 tumors and was positive in only two tumors. Twenty-eight patients received the S-1/CDDP regimen and the remaining 28 received the PTX/CDDP regimen. No significant differences in characteristics were detected between the S-1/CDDP and PTX/CDDP groups.
Table 1.
Patient and tumor characteristics
| Characteristic | Total N = 56 | S-1/CDDP N = 28 | PTX/CDDP N = 28 |
|---|---|---|---|
| Age (years) | |||
| Median | 64 | 61 | 64 |
| Range | 40-77 | 41-79 | 40-77 |
| Sex | |||
| Man | 33 | 16 | 17 |
| Woman | 23 | 12 | 11 |
| Tumor localization | |||
| Proximal | 26 | 10 | 16 |
| Non proximal | 30 | 18 | 12 |
| Laurén classification | |||
| Intestinal | 11 | 5 | 6 |
| Non-intestinal | 45 | 23 | 22 |
| Histologic grade | |||
| G1/G2 | 11 | 5 | 6 |
| G3/G4 | 45 | 23 | 22 |
| HER2 status1 | |||
| Positive | 2 | 0 | 2 |
| Negative | 45 | 23 | 22 |
Human epidermal growth factor receptor type 2 (HER2) was evaluable in 47 of the 56 patients.
NAC: neoadjuvant chemotherapy; CDDP: cisplatin; PTX: paclitaxel.
Association of OCT2 level with clinicopathologic parameters
OCT2high was observed in 41 (73%) of the 56 pre-chemotherapy biopsy specimens. As shown in Table 2, OCT2high was significantly associated with intestinal type according to Laurén classification (P = 0.03) and low histologic grade (P = 0.03). However, no significant association with OCT2 level was detected for age, sex, tumor localization, HER2 status, or NAC regimen.
Table 2.
Association between OCT2 level and clinicopathologic parameters
| OCT2 | ||||
|---|---|---|---|---|
|
|
||||
| Variables | No. | High (%) | Low (%) | P-value |
| Age (years) | ||||
| ≥ 60 | 38 | 30 (79) | 8 (21) | 0.20 |
| < 60 | 18 | 11 (61) | 7 (39) | |
| Sex | ||||
| Man | 33 | 26 (79) | 7 (21) | 0.36 |
| Woman | 23 | 15 (65) | 8 (35) | |
| Tumor localization | ||||
| Proximal | 26 | 19 (73) | 7 (27) | 1.00 |
| Non proximal | 30 | 22 (73) | 8(27) | |
| Laurén classification1 | ||||
| Intestinal | 11 | 11 (100) | 0 (0) | 0.032 |
| Non-intestinal | 45 | 30 (67) | 15 (33) | |
| Histologic grade1 | ||||
| G1/G2 | 11 | 11 (100) | 0 (0) | 0.032 |
| G3/G4 | 45 | 30 (67) | 15 (33) | |
| HER2 status3 | ||||
| Positive | 2 | 1 (50) | 1 (50) | 0.54 |
| Negative | 45 | 31 (69) | 14 (31) | |
| NAC regimen | ||||
| S-1/CDDP | 28 | 20 (71) | 8 (29) | 1.00 |
| PTX/CDDP | 28 | 21 (75) | 7 (25) | |
Laurén classification and histologic grade data showed the same pattern;
Statistically significant;
Human epidermal growth factor receptor type 2 (HER2) was evaluable in 47 of the 56 patients.
OCT2: organic cation transporter 2; NAC: neoadjuvant chemotherapy; CDDP: cisplatin; PTX: paclitaxel.
Univariate analysis of association of clinicopathologic parameters or OCT2 level with pathologic response to NAC in the entire cohort
Thirty-one patients (55%) were classified as responders (grade 2 in 28 patients and grade 3 in three patients) and the remaining 25 (45%) as non-responders (grade 0 in 9 patients, grade 1a in 12 patients, and grade 1b in 4 patients). Table 3 shows univariate analysis of the association of clinicopathologic parameters or OCT2 level with pathologic response to CDDP-based NAC in the entire cohort. Intestinal type (P = 0.09), low histologic grade (P = 0.09), and OCT2high (P = 0.07) tended to be more frequent in responders compared with non-responders, but this was not statistically significant. However, age, sex, tumor localization, HER2 status, and NAC regimen showed no association with response.
Table 3.
Univariate analysis of the association between clinicopathologic parameters or OCT2 level and chemotherapeutic response in the entire cohort
| Variables | No. | Res. (%) | Non-res. (%) | P-value |
|---|---|---|---|---|
| Age (years) | ||||
| ≥ 60 | 38 | 22 (58) | 16 (42) | 0.77 |
| < 60 | 18 | 9 (50) | 9 (50) | |
| Sex | ||||
| Man | 33 | 20 (61) | 13 (39) | 0.42 |
| Woman | 23 | 11 (48) | 12 (52) | |
| Tumor localization1 | ||||
| Proximal | 26 | 14 (54) | 12 (46) | 1.00 |
| Non-proximal | 30 | 17 (57) | 13 (43) | |
| Laurén classification2 | ||||
| Intestinal | 11 | 9 (82) | 2 (18) | 0.09 |
| Non-intestinal | 45 | 22 (49) | 23 (51) | |
| Histologic grade2 | ||||
| G1/G2 | 11 | 9 (82) | 2 (18) | 0.09 |
| G3/G4 | 45 | 22 (49) | 23 (51) | |
| HER2 status3 | ||||
| Positive | 2 | 1 (50) | 1 (50) | 1.00 |
| Negative | 45 | 24 (53) | 21 (47) | |
| NAC regimen | ||||
| S-1/CDDP | 28 | 17 (61) | 11 (39) | 0.59 |
| PTX/CDDP | 28 | 14 (50) | 14 (50) | |
| OCT2 level | ||||
| High | 41 | 26 (63) | 15 (37) | 0.07 |
| Low | 15 | 5 (33) | 10 (67) |
Even when diffuse/entire vs. non-diffuse/entire was analyzed instead of proximal vs. non-proximal, a significant association was not detected;
Laurén classification and histologic grade data showed the same pattern;
Human epidermal growth factor receptor type 2 (HER2) was evaluable in 47 of the 56 patients.
OCT2: organic cation transporter 2; Res.: responder; Non-res.: non-responder; NAC: neoadjuvant chemotherapy; CDDP: cisplatin; PTX: paclitaxel.
Univariate analysis of the association of clinicopathologic parameters or OCT2 level with pathologic response to NAC according to chemotherapy regimen
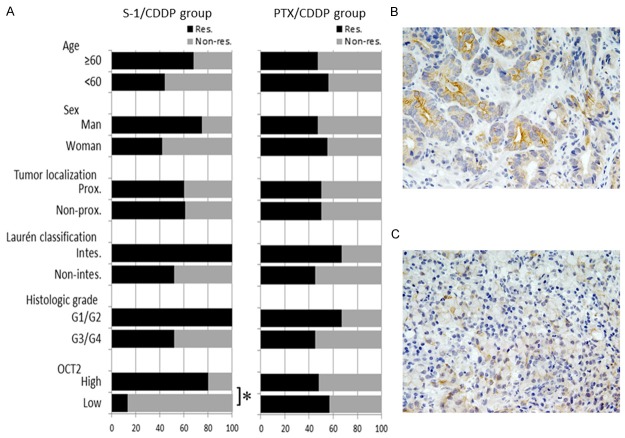
The S-1/CDDP and PTX/CDDP groups were subjected separately to further analysis (Table 4; Figure 1A). HER2 was not included in this analysis, because there were no HER2-positive tumors in the S-1/CDDP group. In the S-1/CDDP group, a significantly higher rate of OCT2high was observed in responders compared with non-responders (80% vs. 20%; P = 0.001). Accuracy of the OCT2 level for predicting response to S-1/CDDP chemotherapy was 82%: 23 (16 responders with OCT2high and 7 non-responders with OCT2low) of the 28 patients. Figure 1B and 1C show representative patterns of OCT2 expression in pre-chemotherapy biopsy specimens from a responder and a non-responder, respectively. However, no significant association with response was detected for age, sex, tumor localization, Laurén classification, or histologic grade. Conversely, in the PTX/CDDP group, no variables were associated with response.
Table 4.
Univariate analysis of the association between clinicopathologic parameters or OCT2 level and chemotherapeutic response according to NAC regimen
| S-1/CDDP group | PTX/CDDP group | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Variables | No. | Res. (%) | Non-res. (%) | P-value | No. | Res. (%) | Non-res. (%) | P-value |
| Age (years) | ||||||||
| ≥ 60 | 19 | 13 (68) | 6 (32) | 0.41 | 19 | 9 (47) | 10 (53) | 1.00 |
| < 60 | 9 | 4 (44) | 5 (56) | 9 | 5 (56) | 4 (44) | ||
| Sex | ||||||||
| Man | 16 | 12 (75) | 4 (25) | 0.12 | 17 | 8 (47) | 9 (53) | 1.00 |
| Woman | 12 | 5 (42) | 7 (58) | 11 | 6 (55) | 5 (45) | ||
| Tumor localization1 | ||||||||
| Proximal | 10 | 6 (60) | 4 (40) | 1.00 | 16 | 8 (50) | 8 (50) | 1.00 |
| Non-proximal | 18 | 11 (61) | 7 (39) | 12 | 6 (50) | 6 (50) | ||
| Laurén classification2 | ||||||||
| Intestinal | 5 | 5 (100) | 0 (0) | 0.13 | 6 | 4 (67) | 2 (33) | 0.65 |
| Non-intestinal | 23 | 12 (52) | 11 (48) | 22 | 10 (45) | 12 (55) | ||
| Histologic grade2 | ||||||||
| G1/G2 | 5 | 5 (100) | 0 (0) | 0.13 | 6 | 4 (67) | 2 (33) | 0.65 |
| G3/G4 | 23 | 12 (52) | 11 (48) | 22 | 10 (45) | 12 (55) | ||
| OCT2 level | ||||||||
| High | 20 | 16 (80) | 4 (20) | 0.0013 | 21 | 10 (48) | 11 (53) | 1.00 |
| Low | 8 | 1 (13) | 7 (88) | 7 | 4 (57) | 3 (43) | ||
Human epidermal growth factor receptor type 2 (HER2) was not included because of no HER2-positive tumors in the S-1/CDDP group.
Even when Diffuse/entire vs. Non-diffuse/entire was analyzed instead of Proximal vs. Non-proximal, a significant association was not detected;
Laurén classification and histologic grade data showed the same pattern;
Statistically significant.
OCT2: organic cation transporter 2; NAC: neoadjuvant chemotherapy; CDDP: cisplatin; PTX: paclitaxel; Res.: responder; Non-res.: non-responder.
Figure 1.
Association between OCT2 expression level and pathologic response to NAC with S-1/CDDP. A: A significantly higher rate of OCT2high was detected in responders (black bar) compared with non-responders (gray bar) in the S-1/CDDP group (P = 0.001). However, no variables were associated with response in the PTX/CDDP group. B: OCT2 immunostaining of a biopsy specimen from a responder showing OCT2high status. Strong staining was observed in the cell membrane of a number of tumor cells. C: OCT2 immunostaining of a biopsy specimen from a non-responder showing OCT2low status. Only a few of the tumor cells showed positive staining. *P = 0.001; CDDP: csiplatin; PTX: paclitaxel; Res: responder; Non-res: non-responder; Prox: proximal localization; Intes: intestinal type.
Multivariate analysis of predictors of response to NAC in the S-1/CDDP group
We included age, sex, tumor localization, Laurén classification, and OCT2 level in multivariate analysis; histologic grade was not included to avoid multicollinearity due to the same data distribution as Laurén classification (Table 5). This analysis demonstrated that OCT2high was the sole independent predictor of response (odds ratio [OR], 47.59; 95% confidence interval, 1.15-2.00 × 103; P = 0.04). However, age, sex, and tumor localization were not independent predictors. Unfortunately, the OR for chemotherapeutic response in the Laurén classification could not be calculated because the data contained zero: no patients with intestinal-type tumors were classified as non-responders.
Table 5.
Multivariate logistic regression analysis of predictive factors for chemotherapeutic response in the S-1/CDDP group
| Variables | OR (95% CI) | P-value |
|---|---|---|
| Age | ||
| ≥ 60 vs. < 60 | 1.11 (0.08-16.55) | 0.94 |
| Sex | ||
| Man vs. Woman | 9.44 (0.60-148.31) | 0.11 |
| Tumor localization | ||
| Proximal vs. Non-proximal | 0.20 (0.01-3.32) | 0.26 |
| Laurén classification | ||
| Intestinal vs. Non-intestinal | –1 | –1 |
| OCT2 level | ||
| High vs. Low | 47.59 (1.15-2.00 × 103) | 0.042 |
Histologic grade was not included because it represents the same data as Laurén classification.
Odds ratio (OR) in the Laurén classification could not be calculated because the data contained zero;
Statistically significant.
CDDP: cisplatin; CI: confidence interval; OCT2: organic cation transporter 2.
Discussion
Although S-1/CDDP and PTX/CDDP are promising NAC regimens for primary GC, to the best of our knowledge, only a few studies on biomarkers for predicting the effectiveness of NAC with S-1/CDDP and no studies on PTX/CDDP exist to date. Kamoshida and colleagues [8], using an immunohistochemical technique, found no significant association between pre-chemotherapy TS expression and pathologic response to NAC with S-1/CDDP. In their study, they further analyzed the combination of TS and p53 expression and indicated that the TS- and/or p53-high phenotypes are a strong predictor of NAC resistance with S-1/CDDP. Conversely, Miyazaki et al. indicated that pre-chemotherapy expression level of TS, analyzed by enzyme-linked immunosorbent assay, was significantly higher in non-responders than in responders [9]. Nevertheless, clinicians might find more value in identifying predictors of response to NAC in GC rather than those of resistance.
Because the influx of drug molecules via SLC transporters is an important determinant of intracellular drug concentrations, it may influence the sensitivity of tumor cells to cytotoxic anticancer agents. For example, OCT2 is a critical determinant in uptake and consequent cytotoxicity of CDDP [13-15]. Therefore, in this study, we immunohistochemically assessed the association of OCT2 expression levels with pathologic response to NAC with S-1/CDDP or PTX/CDDP in GC.
In the univariate analysis of the entire cohort, no variables showed any significant association with a response, although intestinal type (P = 0.09), low histologic grade (P = 0.09), and OCT2high (P = 0.07) tended to be more frequent in responders compared with non-responders. Two previous studies with large samples showed intestinal type and low histologic grade were associated with pathologic response to NAC with CDDP-based regimens [19,20]. Thus, an insufficient sample size may be one of the main causes of the lack of statistical significance in our results.
Nevertheless, we also considered that there may be effects of the two different groups of NAC regimens in our cohort, and assessed a predictive value of clinicopathologic parameters and OCT2 level according to NAC regimen. In the S-1/CDDP group, OCT2high was the only significant predictor of response in univariate (P = 0.001) and multivariate analyses (P = 0.04). However, in the PTX/CDDP group, OCT2 had no effect on response to NAC. This finding suggests that independent predictors of response to CDDP-based NAC may differ according to the individual chemotherapeutic regimen. However, we can only speculate as to the reasons for the lack of an association between the OCT2 expression and response in the PTX/CDDP group. We hypothesize that the predictability of OCT2 for response might be influenced by the combination of PTX. A clinical study has suggested that paclitaxel may help alleviate platinum resistance in ovarian cancer [18]. Thus, PTX might play a greater role than CDDP in patients treated with the PTX/CDDP regimen.
To the best of our knowledge, this is the first study underlining the potential role of SLC transporter expression for predicting response to NAC in GC. However, this study is limited by its retrospective design, small number of patients, and lack of survival analysis, which precludes drawing any firm conclusions. In the future, our results require validation with another large population and also in prospective studies. However, we believe that our result may assist in decision making in GCs for screening GC patients who are more likely to respond to NAC with S-1/CDDP.
Another finding of our study was that OCT2high was significantly associated with low histologic grade; i.e., well and moderate differentiation. Interestingly, an in vitro study using human embryonic stem cells demonstrated that expression levels of OCT2 mRNA in differentiated embryonic stem cells with embryoid body formation was markedly increased compared with undifferentiated cells [21]. However, the underlying mechanisms involved in OCT2 expression during the differentiation of GC cells have not been elucidated and thus further studies are required.
In summary, our study suggests that OCT2high in pre-chemotherapy biopsy specimens may be a potential predictor of pathologic response to NAC with S-1/CDDP in GC. Thus, NAC with S-1/CDDP may be recommended for patients with GC showing OCT2high.
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research (Grant No. 25460455) from the Japan Society for the Promotion of Science.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Yoshikawa T, Rino Y, Yukawa N, Oshima T, Tsuburaya A, Masuda M. Neoadjuvant chemotherapy for gastric cancer in Japan: a standing position by comparing with adjuvant chemotherapy. Surg Today. 2014;44:11–21. doi: 10.1007/s00595-013-0529-1. [DOI] [PubMed] [Google Scholar]
- 3.Yoshikawa T, Tsuburaya A, Morita S, Kodera Y, Ito S, Cho H, Miyashita Y, Sakamoto J. A comparison of multimodality treatment: two or four courses of paclitaxel plus cisplatin or S-1 plus cisplatin followed by surgery for locally advanced gastric cancer, a randomized Phase II trial (COMPASS) Jpn J Clin Oncol. 2010;40:369–372. doi: 10.1093/jjco/hyp178. [DOI] [PubMed] [Google Scholar]
- 4.Tsuburaya A, Nagata N, Cho H, Hirabayashi N, Kobayashi M, Kojima H, Munakata Y, Fukushima R, Kameda Y, Shimoda T, Oba K, Sakamoto J. Phase II trial of paclitaxel and cisplatin as neoadjuvant chemotherapy for locally advanced gastric cancer. Cancer Chemother Pharmacol. 2013;71:1309–1314. doi: 10.1007/s00280-013-2130-0. [DOI] [PubMed] [Google Scholar]
- 5.Yoshikawa T, Tanabe K, Nishikawa K, Ito Y, Matsui T, Kimura Y, Hirabayashi N, Mikata S, Iwahashi M, Fukushima R, Takiguchi N, Miyashiro I, Morita S, Miyashita Y, Tsuburaya A, Sakamoto J. Induction of a pathological complete response by four courses of neoadjuvant chemotherapy for gastric cancer: early results of the randomized phase II COMPASS Trial. Ann Surg Oncol. 2014;21:213–219. doi: 10.1245/s10434-013-3055-x. [DOI] [PubMed] [Google Scholar]
- 6.Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 7.Fareed KR, Kaye P, Soomro IN, Ilyas M, Martin S, Parsons SL, Madhusudan S. Biomarkers of response to therapy in oesophago-gastric cancer. Gut. 2009;58:127–143. doi: 10.1136/gut.2008.155861. [DOI] [PubMed] [Google Scholar]
- 8.Kamoshida S, Suzuki M, Shimomura R, Sakurai Y, Komori Y, Uyama I, Tsutsumi Y. Immunostaining of thymidylate synthase and p53 for predicting chemoresistance to S-1/cisplatin in gastric cancer. Br J Cancer. 2007;96:277–283. doi: 10.1038/sj.bjc.6603546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyazaki I, Kawai T, Harada Y, Moriyasu F. A predictive factor for the response to S-1 plus cisplatin in gastric cancer. World J Gastroenterol. 2010;16:4575–4582. doi: 10.3748/wjg.v16.i36.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burger H, Loos WJ, Eechoute K, Verweij J, Mathijssen RH, Wiemer EA. Drug transporters of platinum-based anticancer agents and their clinical significance. Drug Resist Updat. 2011;14:22–34. doi: 10.1016/j.drup.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Cutler MJ, Choo EF. Overview of SLC22A and SLCO families of drug uptake transporters in the context of cancer treatments. Curr Drug Metab. 2011;12:793–807. doi: 10.2174/138920011798357060. [DOI] [PubMed] [Google Scholar]
- 12.Sprowl JA, Mikkelsen TS, Giovinazzo H, Sparreboom A. Contribution of tumoral and host solute carriers to clinical drug response. Drug Resist Updat. 2012;15:5–20. doi: 10.1016/j.drup.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yonezawa A, Masuda S, Yokoo S, Katsura T, Inui K. Cisplatin and oxaliplatin but not carboplatin and nedaplatin, are substrates for human organic cation transporters (SLC22A1-3 and multidrug and toxin extrusion family) J Pharmacol Exp Ther. 2006;319:879–886. doi: 10.1124/jpet.106.110346. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S, Lovejoy KS, Shima JE, Lagpacan LL, Shu Y, Lapuk A, Chen Y, Komori T, Gray JW, Chen X, Lippard SJ, Giacomini KM. Organic cation transporters are determinants of oxaliplatin cytotoxicity. Cancer Res. 2006;66:8847–8857. doi: 10.1158/0008-5472.CAN-06-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burger H, Zoumaro-Djayoon A, Boersma AW, Helleman J, Berns EM, Mathijssen RH, Loos WJ, Wiemer EA. Differential transport of platinum compounds by the human organic cation transporter hOCT2 (hSLC22A2) Br J Pharmacol. 2010;159:898–908. doi: 10.1111/j.1476-5381.2009.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tatsumi S, Matsuoka H, Hashimoto Y, Hatta K, Maeda K, Kamoshida S. Organic cation transporter 2 and tumor budding as independent prognostic factors in metastatic colorectal cancer patients treated with oxaliplatin-based chemotherapy. Int J Clin Exp Pathol. 2014;7:204–212. [PMC free article] [PubMed] [Google Scholar]
- 17.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 18.Smith S, Su D, de la Longrais IA R, Schwartz P, Puopolo M, Rutherford TJ, Mor G, Yu H, Katsaros D. ERCC1 genotype and phenotype in epithelial ovarian cancer identify patients likely to benefit from paclitaxel treatment in addition to platinum-based therapy. J. Clin. Oncol. 2007;25:5172–5179. doi: 10.1200/JCO.2007.11.8547. [DOI] [PubMed] [Google Scholar]
- 19.Becker K, Langer R, Reim D, Novotny A, zum Büschenfelde CM, Engel J, Friess H, Höfler H. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas. Ann Surg. 2011;253:934–939. doi: 10.1097/SLA.0b013e318216f449. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt T, Sicic L, Blank S, Becker K, Weichert W, Bruckner T, Parakonthun T, Langer R, Büchler MW, Siewert JR, Lordick F, Ott K. Prognostic value of histopathological regression in 850 neoadjuvantly treated oesophagogastric adenocarcinomas. Br J Cancer. 2014;110:1712–1720. doi: 10.1038/bjc.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mai X, Mai Q, Li T, Zhou C. Dynamic expression patterns of imprinted genes in human embryonic stem cells following prolonged passaging and differentiation. J Assist Reprod Genet. 2011;28:315–323. doi: 10.1007/s10815-010-9524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]