Abstract
Although only a single serotype of hepatitis E virus (HEV), the causative agent of hepatitis E, has been identified, there is great genetic variation among the different HEV isolates reported. There are at least four major recognized genotypes of HEV: genotypes 1 and 2 are mainly restricted to humans and linked to epidemic outbreaks in nonindustrialized countries, whereas genotypes 3 and 4 are zoonotic in both developing and industrialized countries. Besides human strains, genotype 3 and 4 strains of HEV have been genetically characterized from swine, sika deer, mongooses, sheep, and rabbits. Currently, there are approximately 11,000 human and animal sequences of HEV available at the International Nucleotide Sequence Database Collaboration. HEV is the major cause of waterborne outbreaks of hepatitis in areas of poor sanitation. Additionally, it is responsible for sporadic cases of viral hepatitis in not only endemic but industrialized countries as well. Transmission of HEV occurs predominantly by the fecal-oral route, although parenteral and perinatal routes have been reported. HEV infection develops in most individuals as a self-limiting, acute, icteric hepatitis; with mortality rates around 1%. However, some affected individuals will develop fulminant hepatic failure, a serious condition that is frequently fatal without a liver transplant. This complication is particularly common when the infection occurs in pregnant women, where mortality rates rise dramatically to up to 25%. Among the preventive measures available to avoid HEV infection, two separate subunit vaccines containing recombinant truncated capsid proteins of HEV have been shown to be highly effective in the prevention of disease. One of them, HEV 239, was approved in China, and its commercialization by Innovax began in November 2012 under the name Hecolin®.
Keywords: Hepatitis E virus, Hepatitis E, HEV, Zoonosis
Introduction
Hepatitis E, caused by the hepatitis E virus (HEV), is an important public-health concern and is a major cause of enterically transmitted hepatitis worldwide. It is responsible for over 50% of acute viral hepatitis cases in endemic countries, and approximately 2 billion people, a third of the world’s population, live in areas endemic for HEV and are at risk of infection.1,2
Hepatitis E was first described in 1983 by Balayan et al.,3 who reproduced HEV infection in a healthy volunteer who ingested pooled stool extracts from patients presumed to have non-A, non-B hepatitis. Stool samples from said patient were analyzed using electron microscopy, and markers of acute hepatitis A and B infection were not detected. However, virus-like particles were visualized. The virus was cloned in 1990,4 and the first serologic test was produced in 1991.5
HEV infection in developing countries is primarily a waterborne illness, associated with large epidemics due to contaminated water and water supplies and poor sanitation conditions.6,7 In contrast, industrialized countries, including many European countries, USA, and Japan, acute hepatitis E occurs sporadically and the contamination pathways are not fully understood.7 Travel to endemic countries and the presence of different HEV strains in industrialized versus developing countries support an autochthonous origin of these sporadic cases.8,9
HEV is the only one of the major hepatitis viruses (A, B, C, and D) with an animal reservoir. The discovery of HEV in pigs (swine HEV), chickens (avian HEV), and, more recently, in rabbits, rodents, wild boars, ferrets, bats, sheep, and cutthroat trout as well as the successful experimental transmission of swine HEV to macaques (model for human HEV transmission) strongly support a zoonotic origin of hepatitis E. This theory is further strengthened by studies from Japan that have phylogenetically linked viruses recovered from uncooked meat (pigs and deer) and humans who became infected after consuming the meat.10 This review summarizes the current knowledge of hepatitis E.
Viral particle
Hepatitis E virus (HEV) is a nonenveloped virus with an icosahedral symmetry and is between 32 and 34 nm in diameter. The buoyant density of HEV is between 1.39 and 1.40 g/cm3 in CsCl, and its sedimentation coefficient is 183 S.11 HEV isolates are between 6.6 and 7.3 kb long (Fig. 1), and they are comprised of a single-stranded positive polarity ribonucleic acid (RNA) molecule with three open reading frames (ORFs).12 ORF1 is the longest reading frame at the 5’-end of the genome. ORF1 encodes a nonstructural polyprotein that includes a methyltransferase, a Y domain, a papain-like protease, a polyproline region (hypervariable region), a macro-domain, a helicase, and an RNA-dependent RNA polymerase. ORF2 is located at the 3’ end of the genome and encodes the major capside protein. Three glycosylation regions have been identified in ORF2, but the biological relevance of these potential modifications is unclear. ORF3 overlaps partially with ORF1 and ORF2 and encodes a phosphoprotein that modulates cellular activities.13 All ORFs are expressed during viral infection, as antibodies against these regions have been detected in naturally infected humans and in experimentally infected monkeys.14 Additionally, there are two untranslated regions (UTRs) in 3’ and 5’ terminal portions.
Fig. 1. Genomic organization of hepatitis E virus.
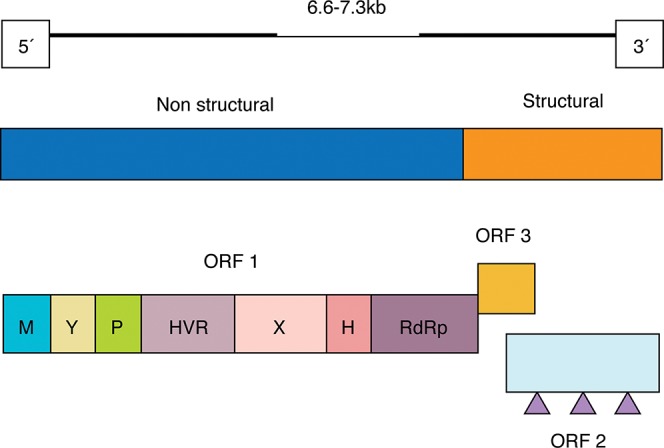
M, methyltransferase; Y, Y domain; P, papain-like cysteine protease; HVR, hypervariable region; X, macro-domain; H, helicase; RdRp, RNA-dependent RNA polymerase; , glicosilation site.
, glicosilation site.
Classification
Although only a single serotype has been identified to date, there is great genetic diversity between the different HEV isolates reported.15 Recent studies have proposed several classifications for HEV into different genotypes and subtypes.16 The last proposed classification by the International Committee of Taxonomic Virology17 classified HEV into the family Hepeviridae (Table 1). This family is divided into two genera, Orthohepevirus (all mammalian and avian HEV isolates) and Piscihepevirus (cutthroat trout HEV). Species within the genus Orthohepevirus are designated Orthohepevirus A (isolates from human, pig, wild boar, deer, mongoose, rabbit, and camel), Orthohepevirus B (isolates from chicken), Orthohepevirus C (isolates from rat, greater bandicoot, Asian musk shrew, ferret, and mink), and Orthohepevirus D (bat isolates). Within species Orthohepevirus A, there are four genotypes described that infect humans.
Table 1. Classification of the family Hepeviridae .
| Family | Genus | Species | Genotype | Source |
|---|---|---|---|---|
| Hepeviridae | Orthohepevirus | Orthohepevirus A | 1 |  |
| 2 |  |
|||
| 3 |  |
|||
| 4 |  |
|||
| 5 |  |
|||
| 6 |  |
|||
| 7 |  |
|||
| Orthohepevirus B | – |  |
||
| Orthohepevirus C | C1 |  |
||
| C2 |  |
|||
| Orthohepevirus D | – |  |
||
| Piscihepevirus | Piscihepevirus A | – |  |
|
Genotype 1 comprises the human Burma strain (prototype) and strains from Asia and Africa. Genotype 2 comprises a human Mexican strain (prototype) and several strains isolated in outbreaks from Nigeria and Chad. Genotype 3 comprises human and animal strains from the USA,18 Canada,19 Argentina,20 Spain,21,22 France,23 UK,24 Austria,25 the Netherlands,26 New Zealand,27 and others. Genotype 4 includes human and animal strains identified in China, Taiwan, Japan, India, Vietnam, France,28 and Italy.29 Genotypes 1 and 2 have been responsible for outbreaks in Asian and African countries and in Mexico. On the other hand, genotype 3 and 4 are responsible for the acute autochthonous cases30 reported in the USA, Argentina, European countries, Japan, and China.
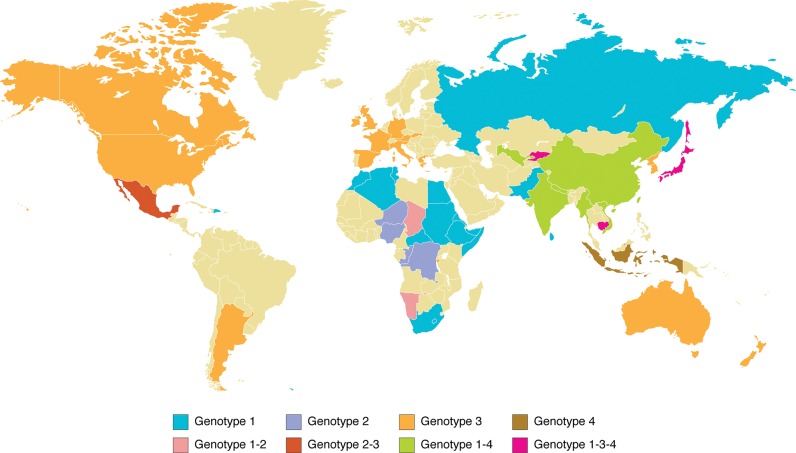
These four genotypes have been divided into different subtypes based on the phylogenetic analysis of many HEV isolates.16 Genotype 1 has been divided into five subtypes, genotype 2 into two subtypes, whereas genotypes 3 and 4 show a greater diversity and have been divided into 10 and seven subtypes, respectively. Genotypes 1 and 2 have been isolated in all human epidemic outbreaks in developing countries, whereas genotypes 3 and 4 have been isolated not only in humans but also in animals in both developing and industrialized countries (Fig. 2). These data support the hypothesis that genotypes 3 and 4 have a zoonotic nature.31
Fig. 2. Geographical distribution of hepatitis E virus genotypes.
The observed divergence among genotypes might be due to different routes of transmission: genotypes 1 and 2 produce epidemic outbreaks via efficient feco-oral transmission by means of contaminated water or food. These similar patterns of transmission may have produced similar degrees of genetic divergence. However, genotypes 3 and 4 are mainly circulating in different animal species and only infect humans occasionally, probably due to inefficient interspecies transmission of these subtypes. A possible explanation is that HEV has been circulating for a long time in different regions with varying degrees of evolution that depends on the species infected.31
Epidemiology
Like Hepatits A, hepatitis E has been traditionally thought to be enterically transmitted. This epidemiological pattern has been recorded in developing countries where epidemic outbreaks linked to contaminated drinking water have been reported. However, the epidemiology of hepatitis E in industrialized countries is different and has changed over time. When it was first reported in developed countries, it was linked to travel to endemic areas, but now, it is becoming an infection linked to an animal reservoir. Besides, the seroprevalence in industrialized countries is greater than it was previously thought when compared to the number of autochthonous cases reported. Indeed, several studies already define a clear separation between the two different types of HEV infection. Genotypes 1 and 2, prevalent in developing countries, are responsible for water-borne disease; whereas genotypes 3 and 4, prevalent in industrialized countries, produce food-borne zoonotic infection.32
HEV infection is endemic in the Center and Southeast of Asia. Several outbreaks have been reported in the Middle East, North and West Africa, and Central America (Mexico). In the rest of the world, HEV infection is considered infrequent and is restricted to people who have travelled to areas where HEV is endemic. HEV outbreaks are long lasting and can affect from hundreds to thousands of people. Outbreaks may vary from acute outbreaks to long lasting epidemics (>1 year). During these outbreaks, the percentage of the affected population may vary between 1% and 15%. The illness is more frequent in adults (3–30%) than in children (0.25–10%). These numbers suggest that anicteric and/or subclinical infections may be more frequent in children. Men are more commonly infected than women. Person-to-person transmission is rare and is more frequent in members of the same family who are infected by a common watersource.33
Sporadic hepatitis E is observed in nonepidemic countries where it constitutes 1–11% of acute cases of hepatitis and is mainly related to travelling to endemic zones,34 although an increasing number of autochthonous hepatitis has been recently reported.35,36
In industrialized countries, HEV seroprevalence rates between 1% and 5% have been reported in the healthy population. The high prevalence found in these countries does not correlate to the low incidence of acute hepatitis E cases in these areas. Therefore, whether seroreactivity in endemic areas is a reflection of subclinical infections, cross-reactions to other agents, false positives, a combination of all of them, or none remains uncertain. Another possibility is that the high seroprevalence found among healthy individuals is related to a subclinical infection with swine HEV, but further studies are required to evaluate the role of swine HEV in the epidemiology in developed areas.
An increasing number of studies suggest that hepatitis E is a zoonosis.37 Antibodies for HEV have been found in human population from nonendemic countries.38 These data raised the suspicion of animals as HEV reservoirs. Consistent with this, it has been reported by means of experimental infections that HEV is capable of crossing the species-barrier. Thus, primates have been infected with swine HEV;37 while pigs, lambs,39 and rats40 have infected with human HEV. Experimental inoculated pigs with a human isolate from the USA were rapidly viremic and seroconverted. These data suggest that this strain was well adapted in pigs and perhaps of swine origin. HEV is considered enzootic in a wide range of animals, including wild and domestic species.
Regarding serological data in animals, there are a number of studies reporting detection of antibodies against HEV in pigs from developing countries such as Nepal41 and Thailand.42 Antibodies against HEV have been found in swine from industrialized countries, including the USA,18 Canada,19 Korea,43 Taiwan,44 Australia, and New Zealand.27 Detection of antibodies in other species, such as poultry,45 dogs,44,46 cattle,46,47 deer,48 cats,49 rodents,47,50,51 and mongooses,52 have been reported as well. All this serological information suggests that certain animal species are exposed to HEV (or HEV-like agent), although the epidemiology of infection remains unclear.
The HEV genome has been detected in poultry,53 horses,54 pigs,18 deer, wild boars,55 rats,56 rabbits,57 and sheep.58 One possible explanation for the great genomic variability of HEV is an animal origin. Genomic sequencing and phylogenetic analysis of many HEV strains have shown a close relationship between swine and human isolates of the same geographic area (Fig. 2) in industrialized countries like Japan,55 Korea,59 United Kingdom,60 Taiwan,61 and the USA.18 Hepatitis E infection related to poorly cooked or raw swine liver consumption has been reported.62 There have been cases of hepatitis E infection by deer and wild boar meat ingestion as well.55,63 Because of its ability to cross the species-barrier, HEV constitutes an important Public Health problem, especially for swine workers who are at a higher risk of infection.64–66
Transmission
Hepatitis E is generally transmitted by the fecal-oral route, usually due to the consumption of contaminated water or food. In developing countries, this is the route of infection that leads to outbreaks. In contrast to the hepatitis A virus and other enteric viruses, human-to-human transmission of HEV is rare.28
Alternative transmission routes, such as transfusions and vertical transmission, are becoming increasingly relevant as more cases are reported each year. Transmission via blood transfusion has become one of the main transmission routes in developed countries. A research group in southeast England retrospectively screened a total of 225,000 blood donors, of which 79 were found to be viremic with genotype 3 HEV.67 Follow-up of 43 recipients showed 18 (42%) had evidence of infection, where only inmunosuppressed patients presented acute morbidity. Another increasingly common transmission route is vertical transmission from pregnant women to their children, especially since these women susceptible to HEV infection.68 A recent study suggests in developing countries that HEV may be responsible for more than 3,000 stillbirths each year, including fetal deaths linked to antenatal maternal mortality.69
Pathogenesis
From a clinical point of view, hepatitis E is similar to hepatitis A, with acute self-limiting and symptomatic presentation that varies in severity from subclinical to fulminant cases.31 Incubation period ranges from 2 weeks to 2 months with an average of 40 days, and viremia is transient, occurring mainly during the prodromic phase, and disappears at the onset of clinical symptoms (Fig. 3). Fecal excretion of the virus begins a few days (5 days on average) prior to jaundice and slows down at the onset of jaundice within 2 to 3 weeks.70
Fig. 3. Schematic summary of main pathogenetic events (virology, serology, disease) during acute HEV infection.
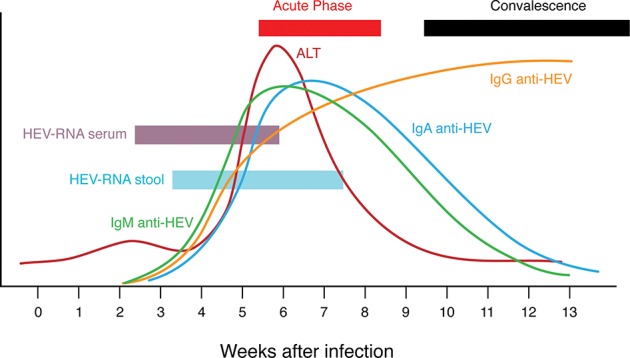
Most autochtonous cases of hepatitis E were reported in middle-aged and elderly men.7 Fulminant hepatitis was observed in 1–4% of the cases, which is a higher rate than that of hepatitis A.71 The mortality rate reached up to 25% in pregnant woman in some endemic areas (northern and central India, Pakistan).72,73
Host and viral factors that determine the severity of illness caused by HEV infection are not fully understood. Viral factors, such as the HEV strain (genotype or subtype), viral load, and other coinfections, might play a role in pathogenesis.74 It appears that genotype 3 and 4 strains are less pathogenic in humans relative to genotypes 1 and 2. A report from Japan found that patients infected with genotype 4 HEV (n=538) had a more severe illness than those infected with genotype 3.75 The patients with genotype 4 HEV infection had significantly higher peaks of alanine aminotransferase (ALT) levels and notably lower prothrombin activity. Fulminant hepatitis has not been described in genotype 2 HEV infection; however, this might merely be due to the limited data available for this genotype. Therefore, the differences in HEV genotypes between different geographical regions might explain the severity of infection during pregnancy. The viral inoculum dose may be relevant. In a study in primates, a larger viral inoculum dose was associated with more marked liver injury.70
Other factors involved in the severity of the disease are related to the host. In particular, pregnancy, use of contraceptives,9 age, and pre-existing liver disease clearly appear to be important. In addition, host immune response may also play a significant role.
More recently, other clinical forms of HEV diseases have been observed in patients under immunosuppressive conditions, such as transplant recipients76 or patients suffering from leukemia.77 Some of these patients developed chronic HEV infection progressing to cirrhosis.78 This clearly suggested that the atypical clinical and virological outcome of HEV infection in these cases could be related to immunosuppressive treatments, which might have resulted in suboptimal anti-HEV-specific immune responses and subsequent viral persistence. Such hypothesis has been evocated by Kamar et al.76 who found significantly reduced CD2, CD3, and CD4-lymphocyte counts in patients with chronic disease and in populations at risk of developing fulminant hepatic failure. Another population that might be at risk of chronic HEV infection are human immunodeficiency virus (HIV) patients with advanced immunodeficiency.79,80 In other studies, no increase in the prevalence of HEV infection was observed in this population,81,82 and only a few cases were reported,83 including a pregnant woman who recovered completely.84 In nonendemic regions, the number of hepatitis E cases in humans seems to be rapidly rising due to the inclusion of HEV among the differential diagnosis of hepatitis.85 Several studies have evaluated the consistency of HEV diagnostic tools86,87 in order to achieve a more reliable diagnostic. One retrospective study reported that the incidence of hepatitis E in France was stable over a period of 5 years, suggesting that hepatitis E is generally under-diagnosed.23
Most patients with autochthonous hepatitis E do not require a liver biopsy since they have a self-limiting disease. Some patients may present a more severe hepatitis with worsening liver blood tests and, therefore, a liver biopsy is sometimes helpful. There are few data on the hepatic histopathology of acute autochthonous hepatitis E, and such reports are limited to patients with severe disease.
Liver pathology of acute autochthonous hepatitis E in the noncirrhotic liver was similar to that seen in acute viral hepatitis, presenting lobular disarray with reticulin framework distortion. Portal tracts were expanded by a severe mixed polymorph and lymphocytic inflammatory infiltrate. Moderate to severe interface hepatitis and cholangitis were also present.88,89 In one study, three patients with autochthonous hepatitis E showed polymorphs concentrated at the periphery and interface of the liver, with lymphocytes, including aggregates, concentrated centrally.88 These findings might be helpful in distinguishing autochthonous hepatitis E from other causes of hepatitis such as autoimmune hepatitis. However, these findings were based on a small number of cases and require confirmation.
In patients with hepatitis E who have underlying cirrhosis, liver pathology findings were nonspecific90 and could easily be mistaken for alcoholic hepatitis in the context of established ethanolic cirrhosis.
In the small number of immunosuppressed transplant patients who have developed chronic infection with HEV, liver pathology showed progressive fibrosis and portal hepatitis with lymphocytic infiltration and piecemeal necrosis78,91 with progression to cirrhosis.
Pregnancy
Pregnant women are at increased risk of complications in HEV infection, with the risk increasing as the pregnancy progresses, often leading to fulminant hepatic failure and death in a high number of cases.92 Acute HEV infection is especially severe during second and third trimesters of pregnancy, and it may lead to fulminant hepatic failure and death in 30–100% of patients.93 There is also increased number of obstetric complications, e.g., premature rupture of membranes, postpartum hemorrhage, spontaneous abortions, and intrauterine fetal death. The fetal complications include prematurity and low birth weight. In a study from Bangladesh, it was observed that 19-25% of all maternal deaths and 7–13% of all neonatal deaths were associated with jaundice in pregnant women. Further, 58% of deaths in pregnant women with acute liver disease in hospitals was associated with HEV.94
The high mortality rate of acute hepatitis E in pregnancy is postulated to result from associated hormonal (estrogen and progesterone) and immunological changes, including downregulation of nuclear factor-kappa-B and shift in T helper-1 cells/T helper-2 cells (Th2) balance toward Th2, and host susceptibility factors that occur in pregnant state.95–97 Devhare et al. 98 studied the immune response to HEV infection and observed early cellular response in HEV infection and associated molecular mechanisms, suggesting a potential role of HEV infection triggered inflammatory response in host immune response and pathogenesis. They demonstrated upregulation of cytokine and chemokine genes and secretion of interleukin-6 (sIL-6), IL-8 and tumor necrosis factor-α in human epithelial cells infected with HEV. Tripathy et al. 99 found that elevated IL-1 α and sIL-2 receptor α (sIL-2R α) levels in the blood are pivotal in the pathogenesis of HEV. They also demonstrated involvement of the innate immune response at the site of infection. Furthermore, Srivastava et al. 100 suggested that interferon-γ secreting CD4 lymphocytes are involved in immune response and are related to intrahepatic sequestration of immune response. All these studies suggested that immune mediated destruction of hepatocytes is important in the pathogenesis of hepatitis E.
Moreover, a recent study101 demonstrated that HEV replicates in placenta, which could explain the high fetal and maternal mortality rate. Xia et al.102 confirmed these data in pregnant rabbits with a rabbit HEV isolate “in vitro”. They concluded that HEV infection could lead to adverse outcomes in pregnancy and vertical transmission, suggesting the necessity for pregnant women at risk of HEV infection to be vaccinated.
Laboratory diagnosis
Laboratory diagnosis tests to detect HEV infection include molecular techniques and electronic immunomicroscopy in feces and serum as well as serological assays to detect anti-HEV immunoglobulin IgM and IgG. HEV RNA is detectable in feces using reverse transcriptase polymerase chain reaction (RT-PCR) from 1 week before the onset of clinical signs to 2 weeks, although sometimes it has been detected up to 52 days after the onset of clinical signs. There are some real-time PCR assays to quantify HEV RNA in fecal and serum samples.103,104 These techniques have the advantage to reduce the diagnostic time (3 h) and show a high sensitivity (10 molecules complementary deoxyribonucleic acid (cDNA)/PCR). Electronic immunomicroscopy in feces is too arduous to perform and its sensitivity is very low, therefore, it is not used for routine diagnosis. HEV antigen has been detected in hepatic tissue from experimentally infected primates. Serologic diagnosis of HEV infection is usually carried out by means of enzyme linked immunoassays (ELISA). Synthetic peptides or recombinant proteins are used as antigens in these assays and correspond to immunodominant epitopes from ORF2 and/or ORF3 belonging to Mexico and/or Burma strains.
Anti-HEV IgM antibodies can be detected during the acute phase of the illness and can last approximately 4 or 5 months. IgG antibodies appear just after IgM levels rise, and they increase from the acute phase until the convalescent phase. IgG have been detected up to 4.5 years after the acute phase. Therefore, increased IgM levels are indicative of acute infection, whereas IgG levels are related to previous contact with HEV.41
Prevention and control
Efforts to prevent HEV concentrate on improving the sanitary status in developing countries, in order to limit contamination of drinking water by infected materials. Particular information and surveillance must be taken from populations at higher risk for fulminant hepatitis such as pregnant women and people with underlying liver conditions. In industrialized countries, where zoonotic transmissions may be the main route of hepatitis E infection, raw or undercooked meat consumers should be aware of the potential risk.
Other populations at risk, such as pregnant women, transplant recipients and individuals with underlying liver conditions, should be informed about the possible risk of consuming raw pork, deer, or wild boar meat or contact with infected pigs. Pork in these areas should be cooked thoroughly (71 °C/20 min) and appropriate safety measures should be taken during the storage, handling, and preparation of uncooked pork.105
Pig handlers and veterinarians who are exposed to HEV must take hygienic measures after handling animals. In swine, since HEV seems to be very contagious between animals,106 further investigation must be performed to determine which preventive biosecurity procedures would limit HEV dissemination. There are several other prevention strategies that could be considered, including the removal or reduction of viral burden eliminated by pigs via early weaning.107 Vaccination of pigs seems a less appropriate control option for the moment since there is no firm data available regarding the incidence of infection in human beings.
Another method of prevention is to enhance the surveillance strategy in commercial importation of swine. Recently, a genotype 4 virus was isolated in swine in Europe.108 Investigations on the origin of this genotype in Europe are needed. Since swine represent a large reservoir of HEV in nonendemic regions, pigs are probably a source of infection for sporadic cases of acute hepatitis E. Surveillance of swine, along with wild boar and deer reservoirs, should be performed until all the routes of human exposure to HEV are identified.
In order to prevent and control HEV waterborne outbreaks, the World Health Organization (WHO) has developed guidelines to make sure countries manage these situations as rapidly and effectively as possible. These control strategies are grouped in four major categories (Table 2) depending on the stage of the outbreak: prevention of exposure, prevention of infection, prevention of disease, and prevention of death.109
Table 2. WHO strategies for prevention and control of waterborne HEV outbreaks.
| Exposure | Infection | Disease | Death |
|---|---|---|---|
| • Improving quality and quantity of drinking water | • Prenatal tests in pregnant women | • Development of vaccines such as the Chinese 239 vaccine Hecolin® | • Prompt diagnosis and management of cases |
| • Treating and disposing of human wastes correctly | - | - | • Timely referral to a health-care facility |
| • Improving personal hygiene | - | - | • Avoid administration of unnecessary hepatotoxic drugs |
| • Preparing safe and clean food | - | - | - |
Vaccine
HEV infection can also be prevented with an effective human immunization program.110 Since HEV has only one serotype and natural infection leads to protective antibodies,110 HEV is a good candidate for the development of an effective vaccine.111 A large vaccination campaign in developing countries would reduce large waterborne epidemics for thousands of people.
In animal studies, several truncated recombinant HEV capsid proteins have been found to induce specific antibodies and to protect against liver injury following subsequent challenge with homologous and heterologous strains of the virus. An HEV DNA vaccine has also been shown to induce serum anti-HEV antibodies in cynomolgus macaques and protect against a heterologous challenge.112 These findings have led to the development of two separate subunit vaccines, which have been tested in clinical trials.
The first human vaccine contained virus like particles (VLPs) made up of a 56 kD truncated HEV ORF2 protein (amino acids 112–607) produced in Spodoptera frugiperda cells infected with a recombinant baculovirus. In a phase 1 trial, three doses of 1, 5, 20, or 40 μg of this recombinant protein, administered in an aluminium-adjuvanted formulation, induced production of anti-HEV antibodies among healthy volunteers.110 The antibody response was found to be dose-dependent. In a phase 2–3 efficacy trial, nearly 2,000 volunteer Nepalese soldiers who lacked detectable anti‐HEV antibodies randomly received either 20 μg of this vaccine or a matched placebo (given as three doses at 0, 1, and 6 months) and were followed-up for a median of 804 days.113 The study subjects were overwhelmingly (>99%) male and mostly young (mean age, 25 years). Clinically overt acute hepatitis E occurred less frequently among vaccine recipients who completed the three dose schedule than among placebo recipients, with a vaccine efficacy rate of 95%. Administration of two doses was associated with a somewhat lower efficacy rate of 86%. Adverse reactions were similar except for more frequent injection-site pain with the vaccine. This vaccine has not been commercialized.
The second vaccine, the HEV 239 vaccine, contains a more truncated HEV capsid protein (corresponding to aminoacids 368–606) expressed in Escherichia coli, which has been purified and adsorbed on aluminum hydroxide suspended in buffered saline solution.114 In a phase 2 human trial, all volunteers who lacked anti-HEV antibodies showed seroconversion 1 month after three doses of 20 μg each, administered at 0, 1, and 6 months, respectively.115 A large, community-based, randomized, double-blind, placebo-controlled, phase 3 trial of this vaccine has recently been completed in China.116 This study enrolled nearly 113,000 participants, aged 16–65 years and of either gender, irrespective of their anti-HEV antibody status. Among the approximately 97,000 participants who received three dose of the vaccine (30 μg each, at 0, 1, and 6 months), the protective efficacy rate was 100% during the next year. Even after two doses of the vaccine, 100% protection was noted, although these data were more limited.
The Chinese vaccine was shown to provide protection against genotype 4 HEV infections, even though it is based on genotype 1 of the virus. Whether these vaccines provide protection against genotype 3 virus strains prevalent in developed countries remains to be determined. The HEV 239 vaccine was approved in China by the State Food and Drug Administration (SFDA) in January 2012, and its commercialization by Innovax (Xiamen Innovax Biotech) started in November 2012 under the name of Hecolin®.117,118
A recent study published in the New England Journal of Medicine addressed the long-term efficacy of this vaccine.119 During 4.5 years, this group studied efficacy, immunogenicity, and safety of the vaccine compared to a control group receiving the hepatitis B virus (HBV) vaccine. As a result of this study, 60 cases of hepatitis E were identified, of which only seven belonged to the 239 vaccine group, proving the efficacy of the vaccine to be 87%.
This vaccine appears very useful for travelers from nonendemic countries to areas with high prevalence of hepatitis E. In industrialized countries, such vaccines may be useful for individuals who are at a high risk of severe disease following HEV infection, such as pregnant women, transplant recipients, people with pre-existing chronic liver disease, and immunosuppressed patients at risk of chronic HEV infection. Since patients with chronic liver disease are most at risk if infected, vaccination should be targeted at these individuals. Patients with chronic liver disease are currently being advised to receive vaccination against hepatitis A virus (HAV) and HBV. Including HEV vaccine in this program would seem logical, since superinfection with any of these viruses in the context of pre-existing chronic liver disease carries a higher mortality rate. The problem with this approach is that unidentified compensated chronic liver disease is common in the community; so all at-risk individuals would not be vaccinated. A more vigorous approach would be to vaccinate whole populations, possibly at the age of 40. This would be a huge undertaking and would need careful cost-benefit analysis before introduction. In addition, the vaccination might help to interrupt outbreaks of hepatitis E in highly endemic areas and among disadvantaged groups, such as flood affected and displaced populations.
Conclusions
Hepatitis E is an important public-health concern and is a major cause of enterically transmitted hepatitis worldwide. HEV mainly causes large outbreaks of acute hepatitis in endemic areas and sporadic cases in industrialized countries. Although most infections are subclinical, the clinical symptoms may range from acute hepatitis, chronic infection in immunosuppressed and transplant patients to severe acute failure in pregnant women. Transmission may occur via water, food and blood. Laboratory diagnosis relies on serological assays and testing for HEV RNA in blood. A vaccine has been developed in China and the results are promising.
Acknowledgements
Work cited in this review from the author’s laboratory was supported in part by grants from the Universidad CEU Cardenal Herrera (PRUCH 25/10, PRUCH 39/11 and Santander-PRUCH 19/12).
Abbreviations
- ALT
alanine aminotransferase
- cDNA
complementary deoxyribonucleic acid
- ELISA
enzyme linked immunoassay
- HAV
hepatitis A virus
- HBV
hepatitis B virus
- HEV
hepatitis E virus
- HIV
human immunodeficiency virus
- Ig
immunoglobulin
- ORFs
open reading frames
- RNA
ribonucleic acid
- RT-PCR
reverse transcriptase polymerase chain reaction
- SFDA
State Food and Drug Administration
- sIL-2R α
sIL-2 receptor α
- sIL-6
secretion of interleukin-6
- Th2
T helper-2
- UTRs
untranslated regions
- VLPs
virus like particles
- WHO
World Health Organization
References
- 1.Pérez-Gracia MT, Mateos-Lindemann ML. Hepatitis E. Current perspectives. Med Clin (Barc) 2012;139:404–411. doi: 10.1016/j.medcli.2012.02.013. 10.1016/j.medcli.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Pérez-Gracia MT, Mateos Lindemann ML, Montalvo Villalba MC. Hepatitis E: current status. Rev Med Virol. 2013;23:384–398. doi: 10.1002/rmv.1759. 10.1002/rmv.1759. [DOI] [PubMed] [Google Scholar]
- 3.Balayan MS, Andjaparidze AG, Savinskaya SS, Ketiladze ES, Braginsky DM, Savinov AP, et al. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology. 1983;20:23–31. doi: 10.1159/000149370. [DOI] [PubMed] [Google Scholar]
- 4.Reyes GR, Purdy MA, Kim JP, Luk KC, Young LM, Fry KE, et al. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science. 1990;247:1335–1339. doi: 10.1126/science.2107574. [DOI] [PubMed] [Google Scholar]
- 5.Yarbough PO, Tam AW, Fry KE, Krawczynski K, McCaustland KA, Bradley DW, et al. Hepatitis E Virus: identification of type-common epitopes. J Virol. 1991;65:5790–5797. doi: 10.1128/jvi.65.11.5790-5797.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arankalle VA, Tsarev SA, Chadha MS, Alling DW, Emerson SU, Banerjee K, et al. Age-specific prevalence of antibodies to hepatitis A and E viruses in Pune, India, 1982 and 1992. J Infect Dis. 1995;171:447–450. doi: 10.1093/infdis/171.2.447. [DOI] [PubMed] [Google Scholar]
- 7.Purcell RH, Emerson SU. Hepatitis E: an emerging awareness of an old disease. J Hepatol. 2008;48:494–503. doi: 10.1016/j.jhep.2007.12.008. 10.1016/j.jhep.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Pérez-Gracia MT, Mateos ML, Galiana C, Fernández-Barredo S, García A, Gómez MT, et al. Autochthonous hepatitis E infection in a slaughterhouse worker. Am J Trop Med Hyg. 2007;77:893–896. [PubMed] [Google Scholar]
- 9.Mateos Lindemann ML, Morales JG, Fernández-Barredo S, Domínguez MR, García de la Hoz F, Halfon P, et al. Fulminant hepatitis E in a woman taking oral contraceptive medication. Am J Trop Med Hyg. 2010;82:12–15. doi: 10.4269/ajtmh.2010.09-0436. 10.4269/ajtmh.2010.09-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyashita K, Kang JH, Saga A, Takahashi K, Shimamura T, Yasumoto A, et al. Three cases of acute or fulminant hepatitis E caused by ingestion of pork meat and entrails in Hokkaido, Japan: Zoonotic food-borne transmission of hepatitis E virus and public health concerns. Hepatol Res. 2012;42:870–878. doi: 10.1111/j.1872-034X.2012.01006.x. 10.1111/j.1872-034X.2012.01006.x. [DOI] [PubMed] [Google Scholar]
- 11.Li TC, Yamakawa Y, Suzuki K, Tatsumi M, Razak MA, Uchida T, et al. Expression and self-assembly of empty virus-like particles of hepatitis E virus. J Virol. 1997;71:7207–7213. doi: 10.1128/jvi.71.10.7207-7213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tam AW, Smith MM, Guerra ME, Huang CC, Bradley DW, Fry KE, et al. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology. 1991;185:120–131. doi: 10.1016/0042-6822(91)90760-9. 10.1016/0042-6822(91)90760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parvez MK, Al-Dosari MS. Evidence of MAPK-JNK1/2 activation by hepatitis E virus ORF3 protein in cultured hepatoma cells. Cytotechnology. 2015;67:545–550. doi: 10.1007/s10616-014-9785-1. 10.1007/s10616-014-9785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khudyakov YE, Favorov MO, Khudyakova NS, Cong ME, Holloway BP, Padhye N, et al. Artificial mosaic protein containing antigenic epitopes of hepatitis E virus. J Virol. 1994;68:7067–7074. doi: 10.1128/jvi.68.11.7067-7074.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlauder GG, Mushahwar IK. Genetic heterogeneity of hepatitis E virus. J Med Virol. 2001;2:282–292. doi: 10.1002/jmv.2031. 10.1002/jmv.2031. [DOI] [PubMed] [Google Scholar]
- 16.Lu L, Li C, Hagedorn CH. Phylogenetic analysis of global hepatitis E virus sequences: genetic diversity, subtypes and zoonosis. Rev Med Virol. 2006;16:5–36. doi: 10.1002/rmv.482. 10.1002/rmv.482. [DOI] [PubMed] [Google Scholar]
- 17.Smith DB, Simmonds P, International Committee on Taxonomy of Viruses Hepeviridae Study Group. Jameel S, Emerson SU, Harrison TJ, et al. Consensus proposals for classification of the family Hepeviridae. J Gen Virol. 2014;95:2223–2232. doi: 10.1099/vir.0.068429-0. 10.1099/vir.0.068429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng XJ, Purcell RH, Halbur PG, Lehman JR, Webb DM, Tsareva TS, et al. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci USA. 1997;94:9860–9865. doi: 10.1073/pnas.94.18.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoo D, Willson P, Pei Y, Hayes MA, Deckert A, Dewey CE, et al. Prevalence of hepatitis E virus antibodies in Canadian swine herds and identification of a novel variant of swine hepatitis E virus. Clin Diagn Lab Immunol. 2001;6:1213–1219. doi: 10.1128/CDLI.8.6.1213-1219.2001. 10.1128/CDLI.8.6.1213-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlauder GG, Frider B, Sookoian S, Castano GC, Mushahwar IK. Identification of 2 novel isolates of hepatitis E virus in Argentina. J Infect Dis. 2000;1:294–297. doi: 10.1086/315651. 10.1086/315651. [DOI] [PubMed] [Google Scholar]
- 21.Pérez-Gracia MT, Garcia-Valdivia MS, Galan F, Rodriguez-Iglesias MA. Detection of hepatitis E virus in patients sera in southern Spain. Acta Virol. 2004;48:197–200. [PubMed] [Google Scholar]
- 22.Fernandez-Barredo S, Galiana C, Garcia A, Vega S, Gomez MT, Perez-Gracia MT. Detection of hepatitis E virus shedding in feces of pigs at different stages of production using reverse transcription-polymerase chain reaction. J Vet Diagn Invest. 2006;18:462–465. doi: 10.1177/104063870601800506. [DOI] [PubMed] [Google Scholar]
- 23.Mansuy JM, Abravanel F, Miedouge M, Mengelle C, Merviel C, Dubois M, et al. Acute hepatitis E in south-west France over a 5-year period. J Clin Virol. 2009;44:74–77. doi: 10.1016/j.jcv.2008.09.010. 10.1016/j.jcv.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Banks M, Bendall R, Grierson S, Heath G, Mitchell J, Dalton H. Human and porcine hepatitis E virus strains, United Kingdom. Emerg Infect Dis. 2004;5:953–955. doi: 10.3201/eid1005.030908. 10.3201/eid1005.030908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Worm HC, Schlauder GG, Wurzer H, Mushahwar IK. Identification of a novel variant of hepatitis E virus in Austria: sequence, phylogenetic and serological analysis. J Gen Virol. 2000;12:2885–2890. doi: 10.1099/0022-1317-81-12-2885. [DOI] [PubMed] [Google Scholar]
- 26.Van der Poel WH, Verschoor F, van der Heide R, Herrera MI, Vivo A, Kooreman M, et al. Hepatitis E virus sequences in swine related to sequences in humans, The Netherlands. Emerg Infect Dis. 2001;6:970–976. doi: 10.3201/eid0706.010608. 10.3201/eid0706.010608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garkavenko O, Obriadina A, Meng J, Anderson DA, Benard HJ, Schroeder BA, et al. Detection and characterisation of swine hepatitis E virus in New Zealand. J Med Virol. 2001;65:525–529. 10.1002/jmv.2067. [PubMed] [Google Scholar]
- 28.Bouamra Y, Gérolami R, Arzouni JP, Grimaud JC, Lafforgue P, Nelli M, et al. Emergence of autochthonous infections with hepatitis E virus of genotype 4 in Europe. Intervirology. 2014;57:43–48. doi: 10.1159/000354801. 10.1159/000354801. [DOI] [PubMed] [Google Scholar]
- 29.Monne I, Ceglie L, DI Martino G, Natale A, Zamprogna S, Morreale A, et al. Hepatitis E virus genotype 4 in a pig farm, Italy, 2013. Epidemiol Infect. 2015;143:529–533. doi: 10.1017/S0950268814001150. 10.1017/S0950268814001150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pérez-Gracia MT, Suay B, Mateos-Lindemann ML. Hepatitis E: an emerging disease. Infect Genet Evol. 2014;22:40–59. doi: 10.1016/j.meegid.2014.01.002. 10.1016/j.meegid. [DOI] [PubMed] [Google Scholar]
- 31.Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145–154. doi: 10.1002/rmv.384. 10.1002/rmv.384. [DOI] [PubMed] [Google Scholar]
- 32.Teshale EH, Hu DJ, Holmberg SD. The Two Faces of Hepatitis E Virus. Clin Infect Dis. 2010;51:328–334. doi: 10.1086/653943. 10.1086/653943. [DOI] [PubMed] [Google Scholar]
- 33.Ducancelle A, Payan C, Nicand E, Le Guillou H, Calès P, Lunel-Fabiani F. Intrafamilial hepatitis E in France. J Clin Virol. 2007;39:51–53. doi: 10.1016/j.jcv.2007.02.007. 10.1016/j.jcv.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Piper-Jenks N, Horowitz HW, Schwartz E. Risk of hepatitis E infection to travellers. J Travel Med. 2000;4:194–199. doi: 10.2310/7060.2000.00059. 10.2310/7060.2000.00059. [DOI] [PubMed] [Google Scholar]
- 35.Ijaz S, Arnold E, Banks M, Bendal RP, Cramp ME, Cunningham R, et al. Non-travel-associated hepatitis E in England and wales: demographic, clinical, and molecular epidemiological characteristics. J Infect Dis. 2005;7:1166–1172. doi: 10.1086/444396. 10.1086/444396. [DOI] [PubMed] [Google Scholar]
- 36.Echevarría JM, Fogeda M, Avellón A. Diagnosis of acute hepatitis E by antibody and molecular testing: a study on 277 suspected cases. J Clin Virol. 2011;50:69–71. doi: 10.1016/j.jcv.2010.09.016. 10.1016/j.jcv.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 37.Meng XJ, Halbur PG, Shapiro MS, Govindarajan S, Bruna JD, Mushahwar IK, et al. Genetic and experimental evidence for cross-species infection by swine hepatitis E virus. J Virol. 1998;72:9714–9721. doi: 10.1128/jvi.72.12.9714-9721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas DL, Yarbough PO, Vlahov D, Tsarev SA, Nelson KE, Saah AJ, et al. Seroreactivity to hepatitis E virus in areas where the disease is not endemic. J Clin Microbiol. 1997;35:1244–1247. doi: 10.1128/jcm.35.5.1244-1247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Usmanov RK, Balaian MS, Dvoinikova OV, Alymbaeva DB, Zamiatina NA, Kazachkov, et al. [An experimental infection in lambs by the hepatitis E virus] Vopr Virusol. 1994;4:165–168. [PubMed] [Google Scholar]
- 40.Maneerat Y, Clayson ET, Myint KS, Young GD, Innis BL. Experimental infection of the laboratory rat with the hepatitis E virus. J Med Virol. 1996;2:121–128. doi: 10.1002/(SICI)1096-9071(199602)48:2<121::AID-JMV1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 41.Clayson ET, Innis BL, Myint KS, Narupiti S, Vaughn DW, Giri S, et al. Detection of hepatitis E virus infections among domestic swine in the Kathmandu Valley of Nepal. Am J Trop Med Hyg. 1995;53:228–232. doi: 10.4269/ajtmh.1995.53.228. [DOI] [PubMed] [Google Scholar]
- 42.Cooper K, Huang FF, Batista L, Rayo CD, Bezanilla JC, Toth TE, et al. Identification of genotype 3 hepatitis E virus (HEV) in serum and fecal samples from pigs in Thailand and Mexico, where genotype 1 and 2 HEV strains are prevalent in the respective human populations. J Clin Microbiol. 2005;43:1684–1688. doi: 10.1128/JCM.43.4.1684-1688.2005. 10.1128/JCM.43.4.1684-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi IS, Kwon HJ, Shin NR, Yoo HS. Identification of swine hepatitis E virus (HEV) and prevalence of anti-HEV antibodies in swine and human populations in Korea. J Clin Microbiol. 2003;8:3602–3608. doi: 10.1128/JCM.41.8.3602-3608.2003. 10.1128/JCM.41.8.3602-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu JC, Chen CM, Chiang TY, Tsai WH, Jeng WJ, Sheen IJ, et al. Spread of hepatitis E virus among different-aged pigs: two-year survey in Taiwan. J Med Virol. 2002;4:488–492. doi: 10.1002/jmv.2170. 10.1002/jmv.2170. [DOI] [PubMed] [Google Scholar]
- 45.Sun ZF, Larsen CT, Huang FF, Billam P, Pierson FW, Toth TE, et al. Generation and infectivity titration of an infectious stock of avian hepatitis E virus (HEV) in chickens and cross-species infection of turkeys with avian HEV. J Clin Microbiol. 2004;42:2658–2662. doi: 10.1128/JCM.42.6.2658-2662.2004. 10.1128/JCM.42.6.2658-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arankalle VA, Joshi MV, Kulkarni AM, Gandhe SS, Chobe LP, Rautmare SS, et al. Prevalence of anti-hepatitis E virus antibodies in different Indian animal species. J Viral Hepat. 2001;8:223–227. doi: 10.1046/j.1365-2893.2001.00290.x. 10.1046/j.1365-2893.2001.00290.x. [DOI] [PubMed] [Google Scholar]
- 47.Vitral CL, Pinto MA, Lewis-Ximenez LL, Khudyakov YE, dos Santos DR, Gaspar AM. Serological evidence of hepatitis E virus infection in different animal species from the Southeast of Brazil. Mem Inst Oswaldo Cruz. 2005;2:117–122. doi: 10.1590/s0074-02762005000200003. 10.1590/S0074-02762005000200003. [DOI] [PubMed] [Google Scholar]
- 48.Sonoda H, Abe M, Sugimoto T, Sato Y, Bando M, Fukui E, et al. Prevalence of hepatitis E virus (HEV) Infection in wild boars and deer and genetic identification of a genotype 3 HEV from a boar in Japan. J Clin Microbiol. 2004;42:5371–5374. doi: 10.1128/JCM.42.11.5371-5374.2004. 10.1128/JCM.42.11.5371-5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuno A, Ido K, Isoda N, Satoh Y, Ono K, Satoh S, et al. Sporadic acute hepatitis E of a 47-year-old man whose pet cat was positive for antibody to hepatitis E virus. Hepatology Research. 2003;3:237–242. doi: 10.1016/s1386-6346(03)00197-9. [DOI] [PubMed] [Google Scholar]
- 50.Favorov MO, Kosoy MY, Tsarev SA, Childs JE, Margolis HS. Prevalence of antibody to hepatitis E virus among rodents in the United States. J Infect Dis. 2000;181:449–455. doi: 10.1086/315273. 10.1086/315273. [DOI] [PubMed] [Google Scholar]
- 51.Kabrane-Lazizi Y, Fine JB, Elm J, Glass GE, Higa H, Diwan A, et al. Evidence for widespread infection of wild rats with hepatitis E virus in the United States. Am J Trop Med Hyg. 1999;61:331–335. doi: 10.4269/ajtmh.1999.61.331. [DOI] [PubMed] [Google Scholar]
- 52.Nakamura M, Takahashi K, Taira K, Taira M, Ohno A, Sakugawa H, et al. Hepatitis E virus infection in wild mongooses of Okinawa, Japan: demonstration of anti-HEV antibodies and a full-genome nucleotide sequenc. Hepatol Res. 2006;34:137–140. doi: 10.1016/j.hepres.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 53.Haqshenas G, Shivaprasad HL, Woolcock PR, Read DH, Meng XJ. Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. J Gen Virol. 2001;82:2449–2462. doi: 10.1099/0022-1317-82-10-2449. [DOI] [PubMed] [Google Scholar]
- 54.Saad MD, Hussein HA, Bashandy MM, Kamel HH, Earhart KC, Fryauff DJ, et al. Hepatitis E virus infection in work horses in Egypt. Infect, Genet Evol. 2007;7:368–373. doi: 10.1016/j.meegid.2006.07.007. 10.1016/j.meegid.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi M, Nishizawa T, Miyajima H, Gotanda Y, Iita T, Tsuda F, et al. Swine hepatitis E virus strains in Japan form four phylogenetic clusters comparable with those of Japanese isolates of human hepatitis E virus. J Gen Virol. 2003;4:851–862. doi: 10.1099/vir.0.18918-0. 10.1099/vir.0.18918-0. [DOI] [PubMed] [Google Scholar]
- 56.Li W, Guan D, Su J, Takeda N, Wakita T, Li TC, et al. High prevalence of rat hepatitis E virus in wild rats in China. Vet Microbiol. 2013;165:275–280. doi: 10.1016/j.vetmic.2013.03.017. 10.1016/j.vetmic.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 57.Zhao C, Ma Z, Harrison TJ, Feng R, Zhang C, Qiao Z, et al. A novel genotype of hepatitis E virus prevalent among farmed rabbits in China. J Med Virol. 2009;81:1371–1379. doi: 10.1002/jmv.21536. 10.1002/jmv.21536. [DOI] [PubMed] [Google Scholar]
- 58.Wu J, Si F, Jiang C, Li T, Jin M. Molecular detection of hepatitis E virus in sheep from southern Xinjiang, China. Virus Genes. 2015;50:410–417. doi: 10.1007/s11262-015-1194-9. 10.1007/s11262-015-1194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahn JM, Kang SG, Lee DY, Shin SJ, Yoo HS. Identification of novel human hepatitis E virus (HEV) isolates and determination of the seroprevalence of HEV in Korea. J Clin Microbiol. 2005;7:3042–3048. doi: 10.1128/JCM.43.7.3042-3048.2005. 10.1128/JCM.43.7.3042–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ijaz S, Arnold E, Banks M, Bendal RP, Cramp ME, Cunningham R, et al. Non-travel-associated hepatitis E in England and wales: demographic, clinical, and molecular epidemiological characteristics. J Infect Dis. 2005;7:1166–1172. doi: 10.1086/444396. 10.1086/444396. [DOI] [PubMed] [Google Scholar]
- 61.Hsieh SY, Meng XJ, Wu YH, Liu ST, Tam AW, Lin DY, et al. Identity of a novel swine hepatitis E virus in Taiwan forming a monophyletic group with Taiwan isolates of human hepatitis E virus. J Clin Microbiol. 1999;37:3828–3834. doi: 10.1128/jcm.37.12.3828-3834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yazaki Y, Mizuo H, Takahashi M, Nishizawa T, Sasaki N, Gotanda Y, et al. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J Gen Virol. 2003;84:2351–2357. doi: 10.1099/vir.0.19242-0. 10.1099/vir.0.19242-0. [DOI] [PubMed] [Google Scholar]
- 63.Tei S, Kitajima N, Takahashi K, Mishiro S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet. 2003;362:371–373. doi: 10.1016/S0140-6736(03)14025-1. 10.1016/S0140-6736(03)14025-1. [DOI] [PubMed] [Google Scholar]
- 64.Withers MR, Correa MT, Morrow M, Stebbins ME, Seriwatana J, Webster WD, et al. Antibody levels to hepatitis E virus in North Carolina swine workers, non-swine workers, swine, and murids. Am J Trop Med Hyg. 2002;66:384–388. doi: 10.4269/ajtmh.2002.66.384. [DOI] [PubMed] [Google Scholar]
- 65.Galiana C, Fernandez-Barredo S, Garcia A, Gomez MT, Perez-Gracia MT. Occupational exposure to hepatitis E virus (HEV) in swine workers. Am J Trop Med Hyg. 2008;78:1012–1015. [PubMed] [Google Scholar]
- 66.Galiana C, Fernandez-Barredo S, Perez-Gracia MT. Prevalence of hepatitis E virus (HEV) and risk factors in pig workers and blood donors. Enferm Infect Microbiol Clin. 2010;28:602–607. doi: 10.1016/j.eimc.2010.01.010. 10.1016/j.eimc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 67.Hewitt PE, Ijaz S, Brailsford SR, Brett R, Dicks S, Haywood B, et al. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet. 2014;384:1766–1773. doi: 10.1016/S0140-6736(14)61034-5. 10.1016/S0140-6736(14)61034-5. [DOI] [PubMed] [Google Scholar]
- 68.Kumar S, Subhadra S, Singh B, Panda BK. Hepatitis E virus: the current scenario. Int J Infect Dis. 2013;17:e228–e233. doi: 10.1016/j.ijid.2012.11.026. 10.1016/j.ijid.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 69.Krain LJ, Atwell JE, Nelson KE, Labrique AB. Fetal an neonatal health consequences of vertically transmitted hepatitis E virus infection. Am J Trop Med Hyg. 2014;90:365–370. doi: 10.4269/ajtmh.13-0265. 10.4269/ajtmh.13-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aggarwal R, Kini D, Sofat S, Naik SR, Krawczynski K. Duration of viraemia and faecal viral excretion in acute hepatitis E. Lancet. 2000;356:1081–1082. doi: 10.1016/S0140-6736(00)02737-9. 10.1016/S0140-6736(00)02737-9. [DOI] [PubMed] [Google Scholar]
- 71.Krawczynski K, Aggarwal R, Kamili S. Hepatitis E. Infect Dis Clin North Am. 2000;14:669–687. doi: 10.1016/s0891-5520(05)70126-4. [DOI] [PubMed] [Google Scholar]
- 72.Hussaini SH, Skidmore SJ, Richardson P, Sherratt LM, Cooper BT, O’Grady JG. Severe hepatitis E infection during pregnancy. J Viral Hepat. 1997;4:51–54. doi: 10.1046/j.1365-2893.1997.00123.x. [DOI] [PubMed] [Google Scholar]
- 73.Khuroo MS, Kamili S, Jameel S. Vertical transmission of hepatitis E virus. Lancet. 1995;345:1025–1026. doi: 10.1016/s0140-6736(95)90761-0. [DOI] [PubMed] [Google Scholar]
- 74.Renou C, Pariente A, Nicand E, Pavio N. Pathogenesis of hepatitis E in pregnancy. Liver Int. 2008;28:1465. doi: 10.1111/j.1478-3231.2008.01885.x. author reply 1466. 10.1111/j.1478-3231.2008.01885.x. [DOI] [PubMed] [Google Scholar]
- 75.Ohnishi S, Kang JH, Maekubo H, Arakawa T, Karino Y, Toyota J, et al. Comparison of clinical features of acute hepatitis caused by hepatitis E virus (HEV) genotypes 3 and 4 in Sapporo, Japan. Hepatol Res. 2006;36:301–307. doi: 10.1016/j.hepres.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 76.Kamar N, Selves J, Mansuy JM, Ouezzani L, Peron JM, Guitard J, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811–817. doi: 10.1056/NEJMoa0706992. 10.1056/NEJMoa0706992. [DOI] [PubMed] [Google Scholar]
- 77.Le Coutre P, Meisel H, Hofmann J, Rocken C, Vuong GL, Neuburger S, et al. Reactivation of hepatitis E infection in a patient with acute lymphoblastic leukaemia after allogeneic stem cell transplantation. Gut. 2009;58:699–702. doi: 10.1136/gut.2008.165571. 10.1136/gut.2008.165571. [DOI] [PubMed] [Google Scholar]
- 78.Kamar N, Mansuy JM, Cointault O, Selves J, Abravanel F, Danjoux M, et al. Hepatitis E virus-related cirrhosis in kidney- and kidney-pancreas-transplant recipients. Am J Transplant. 2008;8:1744–1748. doi: 10.1111/j.1600-6143.2008.02286.x. 10.1111/j.1600-6143.2008.02286.x. [DOI] [PubMed] [Google Scholar]
- 79.Mateos-Lindemann ML, Gonzalez-Galdámez A, Bordallo-Cardona M, Pérez-Gracia MT. Are HIV-infected patients a high-risk population for hepatitis E virus infection in Spain? Enferm Infecc Microbiol Clin. 2012;30:582–583. doi: 10.1016/j.eimc.2012.03.010. 10.1016/j.eimc.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 80.Mateos-Lindemann ML, Diez-Aguilar M, Galdamez AL, Galán JC, Moreno A, Pérez-Gracia MT. Patients infected with HIV are at high-risk for hepatitis E virus infection in Spain. J Med Virol. 2014;86:71–74. doi: 10.1002/jmv.23804. 10.1002/jmv.23804. [DOI] [PubMed] [Google Scholar]
- 81.Madejon A, Vispo E, Bottecchia M, Sanchez-Carrillo M, Garcia-Samaniego J, Soriano V. Lack of hepatitis E virus infection in HIV patients with advanced immunodeficiency or idiopathic liver enzyme elevations. J Viral Hepat. 2009;16:895–896. doi: 10.1111/j.1365-2893.2009.01138.x. 10.1111/j.1365-2893.2009.01138.x. [DOI] [PubMed] [Google Scholar]
- 82.Renou C, Lafeuillade A, Pavio N, Nicand E. Response to Madejon et al.: Are HIV-infected patients at risk of HEV infection? J Viral Hepat. 2010;17:380. doi: 10.1111/j.1365-2893.2009.01203.x. 10.1111/j.1365-2893.2009.01203.x. [DOI] [PubMed] [Google Scholar]
- 83.Colson P, Dhiver C, Gerolami R. Hepatitis E virus as a newly identified cause of acute viral hepatitis during human immunodeficiency virus infection. Clin Microbiol Infect. 2008;14:1176–1180. doi: 10.1111/j.1469-0691.2008.02102.x. 10.1111/j.1469-0691.2008.02102.x. [DOI] [PubMed] [Google Scholar]
- 84.Thoden J, Venhoff N, Miehle N, Klar M, Huzly D, Panther E, et al. Hepatitis E and jaundice in an HIV-positive pregnant woman. AIDS. 2008;22:909–910. doi: 10.1097/QAD.0b013e3282f7cb9a. 10.1097/QAD.0b013e3282f7cb9a. [DOI] [PubMed] [Google Scholar]
- 85.Mateos-Lindemann ML, Diez-Aguilar M, González-Galdamez A, Graus-Morales J, Moreno-Zamora A, Pérez-Gracia MT. Acute, chronic and fulminant hepatitis E: Seven years of experience (2004-2011) Enferm Infecc Microbiol Clin. 2013;31:595–598. doi: 10.1016/j.eimc.2013.03.014. 10.1016/j.eimc.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 86.Bigaillon C, Tesse S, Lagathu G, Nicand E. Use of hepatitis E IgG avidity for diagnosis of hepatitis E infection. J Virol Methods. 2009;164:127–130. doi: 10.1016/j.jviromet.2009.11.028. 10.1016/j.jviromet.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 87.Legrand-Abravanel F, Thevenet I, Mansuy JM, Saune K, Vischi F, Peron JM, et al. Good performance of immunoglobulin M assays in diagnosing genotype 3 hepatitis E virus infections. Clin Vaccine Immunol. 2009;16:772–774. doi: 10.1128/CVI.00438-08. 10.1128/CVI.00438-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peron JM, Danjoux M, Kamar N, Missoury R, Poirson H, Vinel JP, et al. Liver histology in patients with sporadic acute hepatitis E: a study of 11 patients from south-west France. Virchows Arch. 2007;450:405–410. doi: 10.1007/s00428-007-0382-y. [DOI] [PubMed] [Google Scholar]
- 89.Malcolm P, Dalton H, Hussaini HS, Mathew J. The histology of acute autochthonous hepatitis E virus infection. Histopathology. 2007;51:190–194. doi: 10.1111/j.1365-2559.2007.02756.x. 10.1111/j.1365-2559.2007.02756.x. [DOI] [PubMed] [Google Scholar]
- 90.Lockwood GL, Fernandez-Barredo S, Bendall R, Banks M, Ijaz S, Dalton HR. Hepatitis E autochthonous infection in chronic liver disease. Eur J Gastroenterol Hepatol. 2008;20:800–803. doi: 10.1097/MEG.0b013e3282f1cbff. 10.1097/MEG.0b013e3282f1cbff. [DOI] [PubMed] [Google Scholar]
- 91.Haagsma EB, van den Berg AP, Porte RJ, Benne CA, Vennema H, Reimerink JH, et al. Chronic hepatitis E virus infection in liver transplant recipients. Liver Transpl. 2008;14:547–553. doi: 10.1002/lt.21480. 10.1002/lt.21480. [DOI] [PubMed] [Google Scholar]
- 92.Shinde NR, Patil TB, Deshpande AS, Gulhane RV, Patil MB, Bansod YV. Clinical Profile, Maternal and Fetal Outcomes of Acute Hepatitis E in Pregnancy. Ann Med Health Sci Res. 2014;4:S133–S139. doi: 10.4103/2141-9248.138033. 10.4103/2141-9248.138033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Navaneethan U. Seroprevalence of hepatitis E infection in pregnancy-More questions than answers. Indian J Med Res. 2009;130:677–679. [PubMed] [Google Scholar]
- 94.Gurley ES, Halder AK, Streatfield PK, Sazzad HM, Huda TM, Hossain MJ, et al. Estimating the burden of maternal and neonatal deaths associated with jaundice in Bangladesh: Possible role of hepatitis E infection. Am J Public Health. 2012;102:2248–2254. doi: 10.2105/AJPH.2012.300749. 10.2105/AJPH.2012.300749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Navaneethan U, Al Mohajer M, Shata MT. Hepatitis E and pregnancy: Understanding the pathogenesis. Liver Int. 2008;28:1190–1199. doi: 10.1111/j.1478-3231.2008.01840.x. 10.1111/j.1478-3231.2008.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mufti AR, Reau N. Liver disease in pregnancy. Clin Liver Dis. 2012;16:247–269. doi: 10.1016/j.cld.2012.03.011. 10.1016/j.cld.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 97.Abraham P. Viral hepatitis in India. Clin Lab Med. 2012;32:159–174. doi: 10.1016/j.cll.2012.03.003. 10.1016/j.cll.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 98.Devhare PB, Chatterjee SN, Arankalle VA, Lole KS. Analysis of antiviral response in human epithelial cells infected with hepatitis E virus. PLoS One. 2013;8:e63793. doi: 10.1371/journal.pone.0063793. 10.1371/journal.pone.0063793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tripathy AS, Das R, Rathod SB, Arankalle VA. Cytokine profiles, CTL response and T cell frequencies in the peripheral blood of acute patients and individuals recovered from hepatitis E infection. PLoS One. 2012;7:e31822. doi: 10.1371/journal.pone.0031822. 10.1371/journal.pone.0031822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Srivastava R, Aggarwal R, Jameel S, Puri P, Gupta VK, Ramesh VS, et al. Cellular immune responses in acute hepatitis E virus infection to the viral open reading frame 2 protein. Viral Immunol. 2007;20:56–65. doi: 10.1089/vim.2006.0053. 10.1089/vim.2006.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bose PD, Das BC, Hazam RK, Kumar A, Medhi S, Kar P. Evidence of extrahepatic replication of hepatitis E virus in human placenta. J Gen Virol. 2014;95:1266–1271. doi: 10.1099/vir.0.063602-0. 10.1099/vir.0.063602-0. [DOI] [PubMed] [Google Scholar]
- 102.Xia J, Liu L, Wang L, Zhang Y, Zeng H, Liu P, et al. Experimental infection of pregnant rabbits with hepatitis E virus demonstrating high mortality and vertical transmission. J Viral Hepat. 2015 doi: 10.1111/jvh.12406. 10.1111/jvh.12406. [DOI] [PubMed] [Google Scholar]
- 103.Inoue J, Takahashi M, Yazaki Y, Tsuda F, Okamoto H. Development and validation of an improved RT-PCR assay with nested universal primers for detection of hepatitis E virus strains with significant sequence divergence. J Virol Methods. 2006;2:325–333. doi: 10.1016/j.jviromet.2006.07.004. 10.1016/j.jviromet.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 104.Jothikumar N, Cromeans TL, Robertson BH, Meng XJ, Hill VR. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J Virol Methods. 2006;131:65–71. doi: 10.1016/j.jviromet.2005.07.004. 10.1016/j.jviromet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 105.Barnaud E, Rogée S, Garry P, Rose N, Pavio N. Thermal inactivation of infectious hepatitis E virus in experimentally contaminated food. Appl Environ Microbiol. 2012;78:5153–5159. doi: 10.1128/AEM.00436-12. 10.1128/AEM.00436-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bouwknegt M, Engel B, Herremans MM, Widdowson MA, Worm HC, Koopmans MP, et al. Bayesian estimation of hepatitis E virus seroprevalence for populations with different exposure levels to swine in the Netherlands. Epidemiol Infect. 2008;136:567–576. doi: 10.1017/S0950268807008941. 10.1017/S0950268807008941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kasorndorkbua C, Thacker BJ, Halbur PG, Guenette DK, Buitenwerf RM, Royer RL, et al. Experimental infection of pregnant gilts with swine hepatitis E virus. Can J Vet Res. 2003;67:303–306. [PMC free article] [PubMed] [Google Scholar]
- 108.Hakze-van der Honing RW, van Coillie E, Antonis AF, van der Poel WH. First isolation of hepatitis E virus genotype 4 in Europe through swine surveillance in the Netherlands and Belgium. PLoS One. 2011;6:e22673. doi: 10.1371/journal.pone.0022673. 10.1371/journal.pone.0022673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. http://apps.who.int/iris/bitstream/10665/129448/1/9789241507608_eng.pdf?ua=1, accessed March 2015.
- 110.Purcell RH, Nguyen H, Shapiro M, Engle RE, Govindarajan S, Blackwelder WC, et al. Pre-clinical immunogenicity and efficacy trial of a recombinant hepatitis E vaccine. Vaccine. 2003;21:2607–2615. doi: 10.1016/s0264-410x(03)00100-2. 10.1016/S0264-410X(03)00100-2. [DOI] [PubMed] [Google Scholar]
- 111.Goel A, Aggarwal R. Prevention of hepatitis E: another step forward. Future Microbiol. 2011;6:23–27. doi: 10.2217/fmb.10.151. 10.2217/fmb.10.151. [DOI] [PubMed] [Google Scholar]
- 112.Kamili S, Spelbring J, Carson D, Krawczynski K. Protective efficacy of hepatitis E virus DNA vaccine administered by gene gun in the cynomolgus macaque model of infection. J Infect Dis. 2004;189:258–264. doi: 10.1086/380801. 10.1086/380801. [DOI] [PubMed] [Google Scholar]
- 113.Shrestha MP, Scott RM, Joshi DM, Mammen MP, Jr, Thapa GB, Thapa N, et al. Safety and efficacy of a recombinant hepatitis E vaccine. N Engl J Med. 2007;356:895–903. doi: 10.1056/NEJMoa061847. 10.1056/NEJMoa061847. [DOI] [PubMed] [Google Scholar]
- 114.Li SW, Zhang J, Li YM, Ou SH, Huang GY, He ZQ, et al. A bacterially expressed particulate hepatitis E vaccine: antigenicity, immunogenicity and protectivity on primates. Vaccine. 2005;23:2893–2901. doi: 10.1016/j.vaccine.2004.11.064. 10.1016/j.vaccine.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 115.Zhang J, Liu CB, Li RC, Li YM, Zheng YJ, Li YP, et al. Randomized-controlled phase II clinical trial of a bacterially expressed recombinant hepatitis E vaccine. Vaccine. 2009;27:1869–1874. doi: 10.1016/j.vaccine.2008.12.061. 10.1016/j.vaccine.2008.12.061. [DOI] [PubMed] [Google Scholar]
- 116.Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376:895–902. doi: 10.1016/S0140-6736(10)61030-6. 10.1016/S0140-6736(10)61030-6. [DOI] [PubMed] [Google Scholar]
- 117.Park SB. Hepatitis E vaccine debuts. Nature. 2012;7422:21–22. doi: 10.1038/491021a. 10.1038/491021a. [DOI] [PubMed] [Google Scholar]
- 118.Li SW, Zhao Q, Wu T, Chen S, Zhang J, Xia NS. The development of a recombinant hepatitis E vaccine HEV 239. Hum Vaccin Immunother. 2015;11:908–914. doi: 10.1080/21645515.2015.1008870. 10.1080/21645515.2015.1008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang J, Zhang XF, Huang SJ, Wu T, Hu YM, Wang ZZ, et al. Long-term efficacy of a hepatitis E vaccine. N Eng J Med. 2015;372:914–922. doi: 10.1056/NEJMoa1406011. 10.1056/NEJMoa1406011. [DOI] [PubMed] [Google Scholar]