Abstract
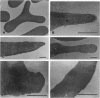
A previously uncharacterized type of sickled cell was found in venous blood of patients with sickle cell disease when blood was collected without exposure to air and fixed immediately with 1% glutaraldehyde solution equilibrated with 5% oxygen. These cells were either elongated, resembling irreversibly sickled cells (ISCs), or nonelongated, with a raisin-like shape. Both types assumed a normal discoidal shape upon full oxygenation. Since these cells exist only under partially oxygenated conditions, they are described as partially oxygenated sickled cells (POSCs). POSCs are morphologically distinct from partially deoxygenated sickled cells formed during deoxygenation by having rounded edges, while the latter have sharp edges. Transmission electron microscopy of POSCs revealed various amounts of misaligned Hb S polymers. Investigations in vitro demonstrated the formation of POSC-like cells by partial oxygenation of deoxygenated cells. Since POSCs contain intracellular fibers and sickle readily upon deoxygenation, they may have clinical and pathological significance.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C. Observations on the sickling phenomenon and on the distribution of different haemoglobin types in erythrocyte populations. Clin Sci. 1956 Nov;15(4):497–510. [PubMed] [Google Scholar]
- Asakura T. Automated method for determination of oxygen equilibrium curves of red cell suspensions under controlled buffer conditions and its clinical applications. Crit Care Med. 1979 Sep;7(9):391–395. doi: 10.1097/00003246-197909000-00008. [DOI] [PubMed] [Google Scholar]
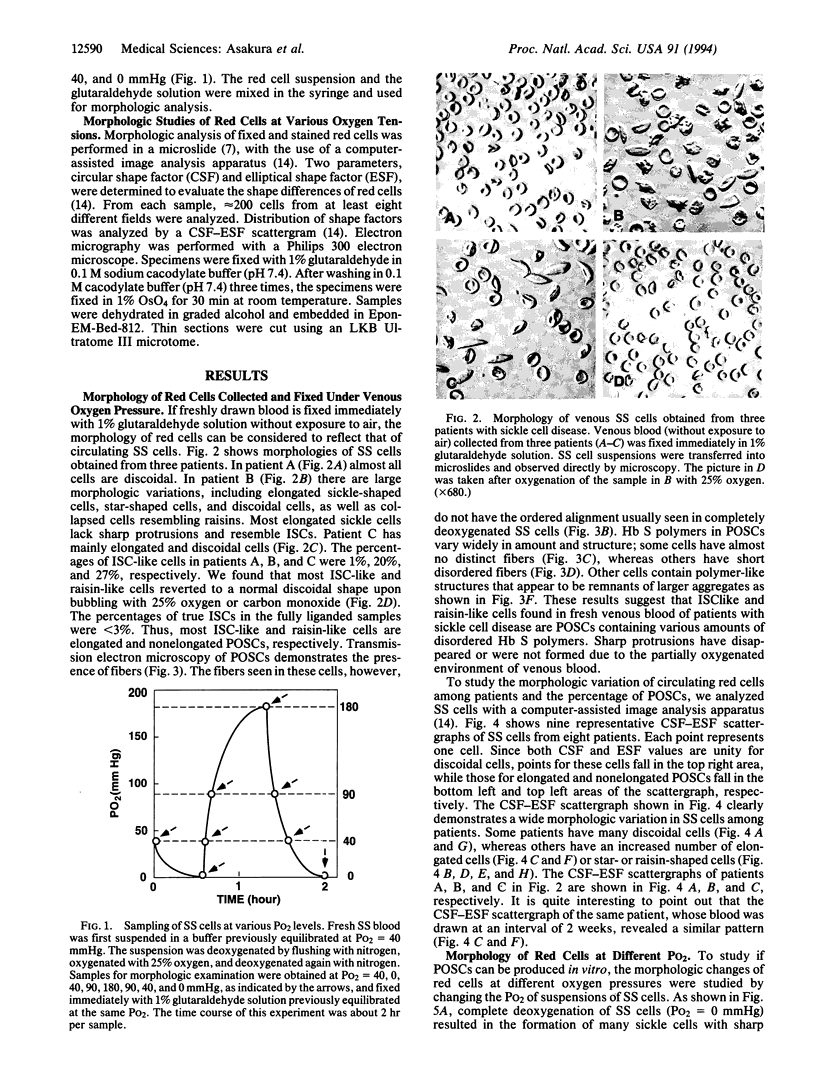
- Asakura T., Mayberry J. Relationship between morphologic characteristics of sickle cells and method of deoxygenation. J Lab Clin Med. 1984 Dec;104(6):987–994. [PubMed] [Google Scholar]
- Bertles J. F., Milner P. F. Irreversibly sickled erythrocytes: a consequence of the heterogeneous distribution of hemoglobin types in sickle-cell anemia. J Clin Invest. 1968 Aug;47(8):1731–1741. doi: 10.1172/JCI105863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briehl R. W., Guzman A. E. Fragility and structure of hemoglobin S fibers and gels and their consequences for gelation kinetics and rheology. Blood. 1994 Jan 15;83(2):573–579. [PubMed] [Google Scholar]
- Eaton W. A., Hofrichter J. Sickle cell hemoglobin polymerization. Adv Protein Chem. 1990;40:63–279. doi: 10.1016/s0065-3233(08)60287-9. [DOI] [PubMed] [Google Scholar]
- HARRIS J. W., BREWSTER H. H., HAM T. H., CASTLE W. B. Studies on the destruction of red blood cells. X. The biophysics and biology of sickle-cell disease. AMA Arch Intern Med. 1956 Feb;97(2):145–168. doi: 10.1001/archinte.1956.00250200021002. [DOI] [PubMed] [Google Scholar]
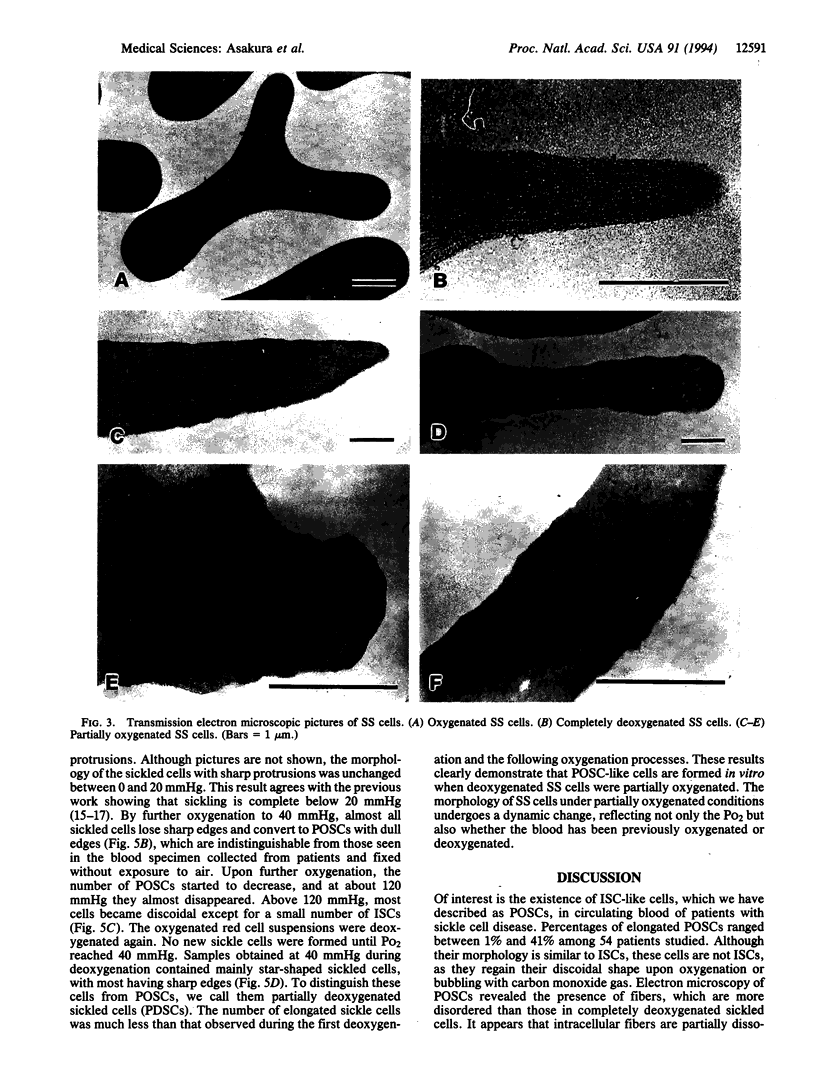
- Hahn J. A., Messer M. J., Bradley T. B. Ultrastructure of sickling and unsickling in time-lapse studies. Br J Haematol. 1976 Dec;34(4):559–565. doi: 10.1111/j.1365-2141.1976.tb03601.x. [DOI] [PubMed] [Google Scholar]
- Horiuchi K., Ballas S. K., Asakura T. The effect of deoxygenation rate on the formation of irreversibly sickled cells. Blood. 1988 Jan;71(1):46–51. [PubMed] [Google Scholar]
- Horiuchi K., Ohata J., Hirano Y., Asakura T. Morphologic studies of sickle erythrocytes by image analysis. J Lab Clin Med. 1990 May;115(5):613–620. [PubMed] [Google Scholar]
- INGRAM V. M. A specific chemical difference between the globins of normal human and sickle-cell anaemia haemoglobin. Nature. 1956 Oct 13;178(4537):792–794. doi: 10.1038/178792a0. [DOI] [PubMed] [Google Scholar]
- JENSEN W. N., RUCKNAGEL D. L., TAYLOR W. J. In vivo study of the sickle cell phenomenon. J Lab Clin Med. 1960 Dec;56:854–865. [PubMed] [Google Scholar]
- Kenny M. W., Meakin M., Worthington D. J., Stuart J. Erythrocyte deformability in sickle-cell crisis. Br J Haematol. 1981 Sep;49(1):103–109. doi: 10.1111/j.1365-2141.1981.tb07202.x. [DOI] [PubMed] [Google Scholar]
- LANGE R. D., MINNICH V., MOORE C. V. Effect of oxygen tension and of pH on the sickling and mechanical fragility of erythrocytes from patients with sickle cell anemia and the sickle cell trait. J Lab Clin Med. 1951 May;37(5):789–802. [PubMed] [Google Scholar]
- Noguchi C. T., Schechter A. N. The intracellular polymerization of sickle hemoglobin and its relevance to sickle cell disease. Blood. 1981 Dec;58(6):1057–1068. [PubMed] [Google Scholar]
- PAULING L., ITANO H. A. Sickle cell anemia a molecular disease. Science. 1949 Nov 25;110(2865):543–548. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- Rodgers G. P., Noguchi C. T., Schechter A. N. Irreversibly sickled erythrocytes in sickle cell anemia: a quantitative reappraisal. Am J Hematol. 1985 Sep;20(1):17–23. doi: 10.1002/ajh.2830200104. [DOI] [PubMed] [Google Scholar]
- SPROULE B. J., HALDEN E. R., MILLER W. F. A study of cardiopulmonary alterations in patients with sickle cell disease and its variants. J Clin Invest. 1958 Mar;37(3):486–495. doi: 10.1172/JCI103629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serjeant G. R., Serjeant B. E., Milner P. F. The irreversibly sickled cell; a determinant of haemolysis in sickle cell anaemia. Br J Haematol. 1969 Dec;17(6):527–533. doi: 10.1111/j.1365-2141.1969.tb01403.x. [DOI] [PubMed] [Google Scholar]
- Zipursky A., Chachula D. M., Brown E. J. The reversibly sickled cell. Am J Pediatr Hematol Oncol. 1993 May;15(2):219–225. doi: 10.1097/00043426-199305000-00010. [DOI] [PubMed] [Google Scholar]