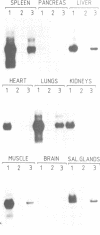
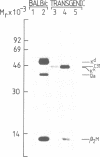
Abstract
A chimeric H-2Kd/Kk gene, called pC31, contains the extracellular alpha 1 domain of Kd origin whereas the rest of the molecule is of Kk origin. Disruption of the syngeneic alpha 1-alpha 2 structure results in a total abrogation of the function of the C31 protein as a restriction element for H-2Kd and Kk restricted T cells during virus infection. In an attempt to obtain information on the functional polymorphism of MHC class I antigens as restriction elements, we have introduced the pC31 gene into the germ line of C3H/He mice (H-2k). The pC31 gene was transcribed in all tissues examined and the expression pattern paralleled the endogenous H-2Kk gene. However, the mRNA for the transgene was approximately 10-times more abundant, which was reflected in an elevated expression of the C31 protein in transgenic splenocytes. Most of the C31 antigen was found intracellularly. The C31 antigen could condition transgenic cytotoxic T lymphocytes in a specific manner during influenza A virus infection and functioned as the restricting element during T cell lysis of the infected cells. These results suggest that entire exons may be exchanged between MHC class I genes and that this exchange can generate novel and functional restriction elements.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold B., Burgert H. G., Hamann U., Hämmerling G., Kees U., Kvist S. Cytolytic T cells recognize the two amino-terminal domains of H-2 K antigens in tandem in influenza A infected cells. Cell. 1984 Aug;38(1):79–87. doi: 10.1016/0092-8674(84)90528-2. [DOI] [PubMed] [Google Scholar]
- Arnold B., Horstmann U., Kuon W., Burgert H. G., Hämmerling G. J., Kvist S. Alloreactive cytolytic T-cell clones preferentially recognize conformational determinants on histocompatibility antigens: analysis with genetically engineered hybrid antigens. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7030–7034. doi: 10.1073/pnas.82.20.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberich C., Scangos G., Tanaka K., Jay G. Regulated expression of a murine class I gene in transgenic mice. Mol Cell Biol. 1986 Apr;6(4):1339–1342. doi: 10.1128/mcb.6.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberich C., Yoshioka T., Tanaka K., Jay G., Scangos G. Functional expression of a heterologous major histocompatibility complex class I gene in transgenic mice. Mol Cell Biol. 1987 Nov;7(11):4003–4009. doi: 10.1128/mcb.7.11.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Bluestone J. A., Pescovitz M. D., Frels W. I., Singer D. S., Hodes R. J. Cytotoxic T lymphocyte recognition of a xenogeneic major histocompatibility complex antigen expressed in transgenic mice. Eur J Immunol. 1987 Jul;17(7):1035–1041. doi: 10.1002/eji.1830170721. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coligan J. E., Kindt T. J., Uehara H., Martinko J., Nathenson S. G. Primary structure of a murine transplantation antigen. Nature. 1981 May 7;291(5810):35–39. doi: 10.1038/291035a0. [DOI] [PubMed] [Google Scholar]
- Dower S. K., Segal D. M. Interaction of monoclonal antibodies with MHC class I antigens on mouse spleen cells. II. Levels of expression of H-2K, H-2D, and H-2L in different mouse strains. J Immunol. 1985 Jan;134(1):431–435. [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Camerini-Otero R. D., Ozato K., Seidman J. G. Structure and expression of a mouse major histocompatibility antigen gene, H-2Ld. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1994–1998. doi: 10.1073/pnas.79.6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frels W. I., Bluestone J. A., Hodes R. J., Capecchi M. R., Singer D. S. Expression of a microinjected porcine class I major histocompatibility complex gene in transgenic mice. Science. 1985 May 3;228(4699):577–580. doi: 10.1126/science.3885396. [DOI] [PubMed] [Google Scholar]
- Holmes N., Parham P. Exon shuffling in vivo can generate novel HLA class I molecules. EMBO J. 1985 Nov;4(11):2849–2854. doi: 10.1002/j.1460-2075.1985.tb04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann U., Arnold B., Hämmerling G. J. Unrestricted recognition of class I neodeterminants generated by exon shuffling. Eur J Immunol. 1986 Jul;16(7):863–865. doi: 10.1002/eji.1830160725. [DOI] [PubMed] [Google Scholar]
- Hunt S. V., Fowler M. H. A repopulation assay for B and T lymphocyte stem cells employing radiation chimaeras. Cell Tissue Kinet. 1981 Jul;14(4):445–464. doi: 10.1111/j.1365-2184.1981.tb00551.x. [DOI] [PubMed] [Google Scholar]
- Jefferies W. A., Brandon M. R., Williams A. F., Hunt S. V. Analysis of lymphopoietic stem cells with a monoclonal antibody to the rat transferrin receptor. Immunology. 1985 Feb;54(2):333–341. [PMC free article] [PubMed] [Google Scholar]
- Jefferies W. A., Green J. R., Williams A. F. Authentic T helper CD4 (W3/25) antigen on rat peritoneal macrophages. J Exp Med. 1985 Jul 1;162(1):117–127. doi: 10.1084/jem.162.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kievits F., Ivanyi P., Krimpenfort P., Berns A., Ploegh H. L. HLA-restricted recognition of viral antigens in HLA transgenic mice. Nature. 1987 Oct 1;329(6138):447–449. doi: 10.1038/329447a0. [DOI] [PubMed] [Google Scholar]
- Klein J. The major histocompatibility complex of the mouse. Science. 1979 Feb 9;203(4380):516–521. doi: 10.1126/science.104386. [DOI] [PubMed] [Google Scholar]
- Kreis T. E. Microtubules containing detyrosinated tubulin are less dynamic. EMBO J. 1987 Sep;6(9):2597–2606. doi: 10.1002/j.1460-2075.1987.tb02550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvist S., Wiman K., Claesson L., Peterson P. A., Dobberstein B. Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell. 1982 May;29(1):61–69. doi: 10.1016/0092-8674(82)90090-3. [DOI] [PubMed] [Google Scholar]
- Lemke H., Hämmerling G. J., Hämmerling U. Fine specificity analysis with monoclonal antibodies of antigens controlled by the major histocompatibility complex and by the Qa/TL region in mice. Immunol Rev. 1979;47:175–206. doi: 10.1111/j.1600-065x.1979.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Lemonnier F. A., Rebai N., Le Bouteiller P. P., Malissen B., Caillol D. H., Kourilsky F. M. Epitopic analysis of detergent-solubilized HLA molecules by solid-phase radioimmunoassay. J Immunol Methods. 1982 Oct 15;54(1):9–22. doi: 10.1016/0022-1759(82)90108-9. [DOI] [PubMed] [Google Scholar]
- Maloy W. L., Coligan J. E. Primary structure of the H-2Db alloantigen. II. Additional amino acid sequence information, localization of a third site of glycosylation and evidence for K and D region specific sequences. Immunogenetics. 1982;16(1):11–22. doi: 10.1007/BF00364438. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The T cell receptor. Science. 1987 Nov 20;238(4830):1073–1079. doi: 10.1126/science.3317824. [DOI] [PubMed] [Google Scholar]
- Mellor A. L., Weiss E. H., Ramachandran K., Flavell R. A. A potential donor gene for the bm1 gene conversion event in the C57BL mouse. Nature. 1983 Dec 22;306(5945):792–795. doi: 10.1038/306792a0. [DOI] [PubMed] [Google Scholar]
- Miyata T., Yasunaga T., Yamawaki-Kataoka Y., Obata M., Honjo T. Nucleotide sequence divergence of mouse immunoglobulin gamma 1 and gamma 2b chain genes and the hypothesis of intervening sequence-mediated domain transfer. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2143–2147. doi: 10.1073/pnas.77.4.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathenson S. G., Geliebter J., Pfaffenbach G. M., Zeff R. A. Murine major histocompatibility complex class-I mutants: molecular analysis and structure-function implications. Annu Rev Immunol. 1986;4:471–502. doi: 10.1146/annurev.iy.04.040186.002351. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Ozato K., Evans G. A., Shykind B., Margulies D. H., Seidman J. G. Hybrid H-2 histocompatibility gene products assign domains recognized by alloreactive T cells. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2040–2043. doi: 10.1073/pnas.80.7.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato K., Hansen T. H., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. II. Antibodies to the H-2Ld antigen, the products of a third polymorphic locus of the mouse major histocompatibility complex. J Immunol. 1980 Dec;125(6):2473–2477. [PubMed] [Google Scholar]
- Ozato K., Mayer N. M., Sachs D. H. Monoclonal antibodies to mouse major histocompatibility complex antigens. Transplantation. 1982 Sep;34(3):113–120. doi: 10.1097/00007890-198209000-00001. [DOI] [PubMed] [Google Scholar]
- Ozato K., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981 Jan;126(1):317–321. [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Norstedt G., Gelinas R. E., Hammer R. E., Brinster R. L. Metallothionein-human GH fusion genes stimulate growth of mice. Science. 1983 Nov 18;222(4625):809–814. doi: 10.1126/science.6356363. [DOI] [PubMed] [Google Scholar]
- Parham P., Lomen C. E., Lawlor D. A., Ways J. P., Holmes N., Coppin H. L., Salter R. D., Wan A. M., Ennis P. D. Nature of polymorphism in HLA-A, -B, and -C molecules. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4005–4009. doi: 10.1073/pnas.85.11.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson D. J., Jefferies W. A., Green J. R., Brandon M. R., Corthesy P., Puklavec M., Williams A. F. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987 Dec;24(12):1281–1290. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- Pease L. R., Schulze D. H., Pfaffenbach G. M., Nathenson S. G. Spontaneous H-2 mutants provide evidence that a copy mechanism analogous to gene conversion generates polymorphism in the major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 Jan;80(1):242–246. doi: 10.1073/pnas.80.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Major histocompatibility antigens: the human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell. 1981 May;24(2):287–299. doi: 10.1016/0092-8674(81)90318-4. [DOI] [PubMed] [Google Scholar]
- Reyes A. A., Johnson M. J., Schöld M., Ito H., Ike Y., Morin C., Itakura K., Wallace R. B. Identification of an H-2Kb-related molecule by molecular cloning. Immunogenetics. 1981;14(5):383–392. doi: 10.1007/BF00373318. [DOI] [PubMed] [Google Scholar]
- Reyes A. A., Schöld M., Wallace R. B. The complete amino acid sequence of the murine transplantation antigen H-2Db as deduced by molecular cloning. Immunogenetics. 1982;16(1):1–9. doi: 10.1007/BF00364437. [DOI] [PubMed] [Google Scholar]
- Schreier P. H., Bothwell A. L., Mueller-Hill B., Baltimore D. Multiple differences between the nucleic acid sequences of the IgG2aa and IgG2ab alleles of the mouse. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4495–4499. doi: 10.1073/pnas.78.7.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze D. H., Pease L. R., Geier S. S., Reyes A. A., Sarmiento L. A., Wallace R. B., Nathenson S. G. Comparison of the cloned H-2Kbm1 variant gene with the H-2Kb gene shows a cluster of seven nucleotide differences. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2007–2011. doi: 10.1073/pnas.80.7.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schøller J., Shimonkevitz R., MacDonald H. R., Kvist S. Different structural constraints for recognition of mouse H-2Kd and -Kk antigens by alloimmune cytolytic T lymphocytes. J Exp Med. 1986 Dec 1;164(6):1823–1834. doi: 10.1084/jem.164.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Hood L. Genes of the major histocompatibility complex in mouse and man. Science. 1983 Nov 18;222(4625):727–733. doi: 10.1126/science.6356354. [DOI] [PubMed] [Google Scholar]
- Stroynowski I., Orn A., Goodenow R. S., McMillan M., Forman J., Brayton P. R., Frelinger J., Hood L. Cytotoxic T lymphocytes recognize determinants on the BALB/c-H-2Ld molecule controlled by alpha 1 and alpha 2 but not alpha 3 external domains. Immunogenetics. 1984;20(2):141–154. doi: 10.1007/BF00364486. [DOI] [PubMed] [Google Scholar]
- Sun Y. H., Goodenow R. S., Hood L. Molecular basis of the dm1 mutation in the major histocompatibility complex of the mouse: a D/L hybrid gene. J Exp Med. 1985 Nov 1;162(5):1588–1602. doi: 10.1084/jem.162.5.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Wallich R., Bulbuc N., Hämmerling G. J., Katzav S., Segal S., Feldman M. Abrogation of metastatic properties of tumour cells by de novo expression of H-2K antigens following H-2 gene transfection. Nature. 1985 May 23;315(6017):301–305. doi: 10.1038/315301a0. [DOI] [PubMed] [Google Scholar]
- Weiss E. H., Mellor A., Golden L., Fahrner K., Simpson E., Hurst J., Flavell R. A. The structure of a mutant H-2 gene suggests that the generation of polymorphism in H-2 genes may occur by gene conversion-like events. Nature. 1983 Feb 24;301(5902):671–674. doi: 10.1038/301671a0. [DOI] [PubMed] [Google Scholar]
- Weiss E., Golden L., Zakut R., Mellor A., Fahrner K., Kvist S., Flavell R. A. The DNA sequence of the H-2kb gene: evidence for gene conversion as a mechanism for the generation of polymorphism in histocompatibilty antigens. EMBO J. 1983;2(3):453–462. doi: 10.1002/j.1460-2075.1983.tb01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollett G. R., Barclay A. N., Puklavec M., Williams A. F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985 Feb;15(2):168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]