Abstract
Objectives:
Decline of platelets with or without thrombocytopenia is observed in critically ill preterm newborns. Prognostic significance of platelets count in Neonatal Intensive Care Unit focused on outcome after thrombocytopenia. We aimed to estimate the changes in platelets count within the first 7 days of life in preterm newborns and its relation to final outcomes.
Methods:
Retrospectively, the platelets count during the first 7 days of life, and its association with mortality, length of stay among survivors (LOS), and later severe morbidities were determined. Appropriate regression analyses were used to examine possible relations between studied variables.
Results and Discussion:
Platelets drop that did not reach thrombocytopenia level in the first 7 days of life happened in 61.7%. Platelets count drop in the first 7 days of life was a predictor of mortality, LOS, and major morbidities such as intraventricular hemorrhage and necrotizing enterocolitis.
Conclusions:
Platelets count drop within the first 7 days of life independent of thrombocytopenia can be used to predict increased mortality, LOS, and the development of later severe morbidities in critically ill preterm neonates.
Keywords: Intraventricular hemorrhage, length of stay, neonatal infections, neonatal outcome, platelets, preterm, thrombocytopenia
Introduction
Thrombocytopenia in newborns has an overall prevalence that ranges from 1% to 5%.[1,2,3,4] It is even much higher in newborns admitted to Neonatal Intensive Care Unit (NICU) reported to be ranging from 18% to 35%.[2,5,6] From 22 weeks' gestation onward, the platelets count reaches and maintains a level above 150 × 109 /L and only 2% of term newborns have platelets counts below this level at birth, thereby a platelets count below 150 × 109 /L has been used to define thrombocytopenia.[5,6] Severe thrombocytopenia (platelets <50 × 109 /L) occurs in fewer than three per 1000 term newborns; the most important cause being alloimmune thrombocytopenia.[3,4] In contrast, approximately 70% of newborns born at a weight <1000 g has thrombocytopenia at some point during their NICU stay and up to 20% of sick preterm newborns can develop severe thrombocytopenia.[7] Many neonatal and maternal conditions are associated with thrombocytopenia. However, the most common explanations for severe thrombocytopenia were acquired varieties of consumptive thrombocytopenia, especially in septicemic preterm newborns.[2,8] Thrombocytopenia has been independently related to mortality and major morbidities as intraventricular hemorrhage (IVH), disseminated intravascular coagulopathy, and necrotizing enterocolitis (NEC).[9,10,11] On the other hand, the role of thrombocytopenia in some serious morbidities as IVH is difficult to establish, especially when IVH occurs in preterm newborns with normal platelets counts.[12]
In adults and pediatric ICU patients, the drop in platelets numbers and not only thrombocytopenia was shown to be a good predictor of clinical outcome. In critically ill adults, ≥30% drop in the absolute platelets numbers, without thrombocytopenia was associated with ventilator-related pneumonia.[13,14] In PICU, a drop in platelets counts >27% and thrombocytopenia were independently related to mortality.[15] In preterm newborns, despite that the drop in absolute platelets count, without thrombocytopenia, was suggestive of fungal more than bacterial sepsis;[16] the association of morbidity and mortality with this drop of platelets counts, especially without thrombocytopenia, is poorly understood. Platelets drop in preterm newborns can be transient, or it may progress to severe thrombocytopenia. Most studies investigating platelets count changes in preterm infants, and its prognostic significance focused on outcome in newborns with platelets <50–100 × 109 /L.[17,18] Using a cut-off value similar to adult studies (≥30% platelets drop), a single study was published in newborns that investigated the significance of platelets drop at 7 and 28 days of life in extremely preterm newborns.[19] The authors demonstrated significant associations of platelets drop with mortality and major morbidities in extremely preterm newborns. Since our local NICU population and setting might be different we aimed in this study to use a similar approach to check the reproducibility of such presumed prognostic value of platelets drop during the first 7 days of life as calculated from the initial platelets count immediately after birth, with or without later thrombocytopenia in our preterm newborns. We hypothesized that in our local setting the rate of early platelets count decline in preterm newborns could predict final NICU outcome and the severity of later thrombocytopenia.
Methods
Study design
In a retrospective cohort study design, an analysis of the medical records of all preterm newborns admitted to NICU in Ohud Hospital in Madina Monawara, Saudi Arabia from November 2012 to December 2013 was conducted. This hospital is a tertiary care health center that serves a large population area.
Patients
Inclusion criteria
The criteria for inclusion of charts reviewed in this study were newborns who: (i) Have gestational age (GA) below 32 weeks; (ii) were admitted on the 1st day of life; and (iii) survived for more than 7 days.
Exclusion criteria
Exclusion criteria included newborns who: (i) Were transferred before completing their treatment; (ii) had thrombocytopenia on admission; (iii) did not have platelets count taken on 1st day of admission (iv) had thrombocytopenia with or without decline in platelets count before day 7 of life; (v) received blood product transfusion in the first 7 days of life.
Ethical considerations
The study was approved by Ohud Hospital Review Administration and the University Research Review Board. As this is a retrospective chart review study patient approval was waived. In keeping with the guidelines of Helsinki, all collected data were anonymized.
Intervention
All preterm newborns admitted in a 1-year period were identified. Needed data were extracted by examining the hospital patient database, medical files, laboratory system, and electronic records. No direct patient intervention was done. The following data were collected: (1) Demographic and antenatal data (GA, birth weight, sex, use of antenatal steroids [completed course], and method of deliver); and (2) clinical data and outcome (intrauterine growth retardation, diagnosis on admission, mortality, morbidities such as IVH [grades 3 and 4 using head ultrasound by a radiologist], other major hemorrhage such as pulmonary hemorrhage [defined as hemorrhage requiring prompt medical intensive action as blood product transfusion and sustained medical care], NEC [presence of pneumatosis intestinalis in abdominal radiograph read by a radiologist], sepsis [blood culture proven], and length of stay [LOS] among survivors). Platelets count recorded on the 1st day of admission was taken as the base to which subsequent platelets numbers were compared. All subsequent counts in the first 7 days of life were recorded to define the lowest count in the first 7 days. Drop in the platelets counts was defined as a decrease of a ≥30% from the first day platelets count. Thrombocytopenia in the study was defined as platelets count <150 × 109 /L that was proven in two consecutive measurements.
Outcome measures
Our primary outcome measures were mortality and LOS among survivors. Secondary outcome included IVH (grade 3–4), ROP (grade 3–4), NEC, and culture proven sepsis (Gram-positive and Gram-negative fungus)
Statistical analysis
Data were analyzed using SPSS software (SPSS for Windows, version 16.0, SPSS Inc., Chicago, IL, USA). To analyze the prognostic value of platelets decline with and without thrombocytopenia included newborns were classified into four groups; Group 1: No thrombocytopenia with no platelets decline; Group 2: No thrombocytopenia with platelets decline, Group 3: Thrombocytopenia with no platelets decline, Group 4: Thrombocytopenia with platelets decline. Continuous variable were expressed as mean ± standard deviation (SD) and categorical variables as number (percentage). Normality was checked by 1-sample Kolmogorov-Smirnov test. The assumption of a normal distribution was rejected at an alpha <0.1. Descriptive analysis was performed on demographic and baseline clinical characteristics; as well as the age of platelets count nadir, and magnitude of platelets declines in the first 7 days. Comparisons between the four groups were performed using analysis of variance and Chi-square for categorical variables. Logistic regression was used to examine for the odds of mortality and morbidities in the four groups. Variables that reached a significance level of 0.15 in univariate analysis were included in logistic regression analysis, using the Group 1 (no thrombocytopenia no drop) as reference. P < 0.05 was considered as statistically significant.
Results
Of 620 neonate admitted to the hospital in the study period, 188 preterm newborns (91 males, 97 females) were included in our study. The mean (SD) GA for our sample was 28.3 (1.6) (range, 26–32) weeks; and mean (SD) birth weight was 1025 (385) (range, 610–2400) grams. Clinical characteristics of all preterm newborns involved in the study based on study group are shown in Table 1. Preterm newborns in Group 4 had significantly lower GA and birth weight than the other groups. Platelets drop occurred at an earlier age in preterm newborns, who developed thrombocytopenia. On the other hand, thrombocytopenia occurred earlier in children who demonstrated platelets drop in the first 7 days of life.
Table 1.
Comparison of patients characteristics between study groups

The overall incidence of thrombocytopenia was 31.4% in our sample with 61.7% had ≥30% drop in platelets counts before the age of 7 days. The relationship between mortality and the study groups are shown in Figure 1. The mortality rates were higher in preterm newborns with ≥30% drop in platelets count being highest in Group 4 (both thrombocytopenia and ≥30% drop in platelets count). Preterm newborns without thrombocytopenia and ≥30% drop in platelets count (Group 2) had mortality rate higher than Group 1 (no thrombocytopenia with no platelets decline) and Group 3 (thrombocytopenia without platelets drop). Significance was reached only when Group 4 was compared to Group 1 (P < 0.05).
Figure 1.
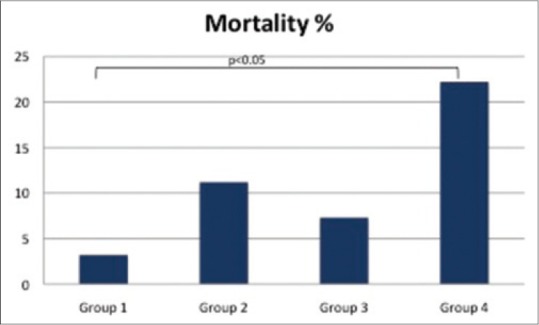
Mortality rates in study groups
Nineteen newborns died before discharge with an overall mortality rate in the study group of 10.1%. Table 2 shows the odds of mortality in various groups of the study. After controlling for other demographic and clinical factors, ≥30% drop in platelets counts was associated with significantly increased odds of mortality when it was associated with thrombocytopenia in preterm newborns. When taken together preterm newborns with ≥30% drop in platelets showed an increased mortality odds, (1.84 [0.63–5.34], P > 0.05) when compared to children with no platelets drop; however, this did not reach statistical significance. In those preterm newborns who died before discharge, mean ± SD ages of death were 33.0 (9.9), 12.7 (7.4), 20.1 (6.3), and 15.6 (7.6) days in Group 1, 2, 3, and 4, respectively.
Table 2.
Probability of death in various study groups in relation to Group 1

From the 188 studied preterm newborns, 29 (15.4%) showed evidence of grade 3 or 4 IVH, 10 (5.3%) had other major bleedings, 29 (15.4%) showed evidences suggestive of NEC, 46 (24.5%) had Gram-positive bacterial infection, 38 (20.2%) had Gram-negative bacterial infection, and 30 (16.0%) had fungal infection. The odds of having major morbidities in each study group are shown in Table 3. The odds of having IVH and NEC were significant (P < 0.05 and <0.01, respectively) in both Group 2 and Group 4 (those preterm newborns who had ≥30% drop in platelets either without or with thrombocytopenia). Preterm newborns in Group 3 (thrombocytopenia with no platelets decline) did not have IVH or NEC cases. The odds of other major hemorrhages were increased only in Group 4.
Table 3.
Drop of platelet count on day 7 and odds of morbidities

While the odds of having Gram-positive infection was significant (P < 0.05) only in preterm newborns with ≥30% drop of platelets with thrombocytopenia (Group 4), it was not significant in both Group 2 and Group 3. The odds of having Gram-negative or fungal infection were significant in preterm newborns with ≥30% drop in platelets count either without thrombocytopenia (Group 2) or with thrombocytopenia (Group 4). The odds of having Gram-negative infection were not significant in preterm newborns with only thrombocytopenia without platelets drop (Group 3) and there were no cases of fungal infection among preterm newborns in this group.
The association between LOS and platelets evaluation was shown in Figure 2. There was significant increase in the LOS in preterm newborns with ≥30% drop of platelets count either without thrombocytopenia (Group 2; P < 0.01) or with thrombocytopenia (Group 4; P < 0.01) when compared to the normal preterm newborns (Group 1) while LOS of Group 3 was not significantly different from those of normal preterm newborns (Group 1; P > 0.05). There was no significant difference in LOS between Group 2 and Group 4 (P > 0.05).
Figure 2.
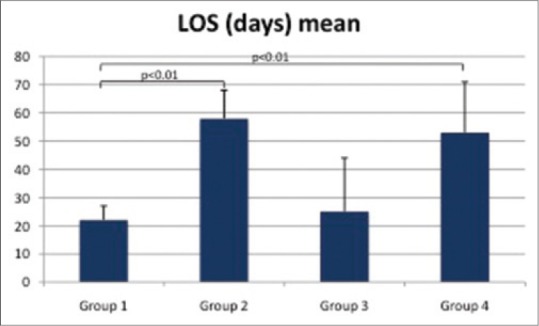
Length of stay among survivors in study groups
Discussion
Although drop in the platelets count without thrombocytopenia is a common observation in preterm newborns; usually, it neither trigger action nor used to draw conclusions on expected clinical coarse or prognosis except after reaching the threshold of thrombocytopenia or even severe thrombocytopenia.[8,20] Our study confirmed the significant association between drop of platelets with or without thrombocytopenia and poor outcomes. Our findings agree with the results of Rastogi et al., who demonstrated a significant association of mortality and major morbidities in preterm newborns below 28 weeks gestation and platelets drop in the first 7 days of life.[19] Previous studies in critically ill older children and adults have shown similar results.[13,14,15]
The rate of thrombocytopenia in our study (31.4%) was much lower than that reported previously in preterm newborns.[9] This is mostly a reflection of excluding thrombocytopenia occurring in the first 7 days of life that is mostly caused by maternal causes. Using same criteria, the incidence of thrombocytopenia in extremely preterm neonates below 28 weeks gestation was found to be 48.6%.[19] The authors believed that this incidence of thrombocytopenia in their study was more likely to be reflective of primary neonatal causes. However, other authors have published thrombocytopenia rates similar to ours when calculated for all babies admitted to NICUs.[1,21]
The platelets drop and thrombocytopenia in newborns have traditionally been attributed to a combined process; impaired platelets production and increased platelets consumption and sequestration.[22] Impaired platelet production usually results in low platelet count that is either present at birth or develops within 72 h of life.[21,23] In our study, it is reasonable to assume that the decreased production play a little role as we excluded all newborns with early thrombocytopenia. Hence, the most plausible explanation for the platelets drop and thrombocytopenia in our study is increased consumption and sequestration. It was shown that late onset thrombocytopenia in newborns is almost exclusively caused by sepsis or NEC.[24] Thrombosis and platelet activation/immobilization at sites of inflammation (as in the gut during NEC) were suggested as the processes behind platelets consumption in such conditions.[23] Such affected neonates were often profoundly sick, required intensive care, and had 10–15% mortality.[24] In our study NEC, Gram-negative bacterial and fungal infections were associated with platelets drop even in the absence of thrombocytopenia. Platelets drop occurred several days before reaching the level of thrombocytopenia and mostly before other signs of illness appeared. As such, platelets drop in the first 7 days of life represents a strong indicator of the later development of these two problems (infections and NEC). Another group found that such prognostic value of platelets drop remained valid in predicting mortality and serious morbidities at 28 days of life.[19] Many authors have expressed doubts regarding whether thrombocytopenia itself directly contributes to adverse outcome or is simply a marker of the severity of precipitating complications, which themselves carry a poor prognosis.[21,23,25] Our results would lend evidence to the latter reasoning and extend this reasoning to platelets drop not only thrombocytopenia. Indeed the early use of platelet concentrates to prevent moderate thrombocytopenia (platelets, 50–150 × 109 /L) failed to reduce hemorrhage,[26] reflecting the difficulty clinicians face to assess the clinical impact of thrombocytopenia in newborns.
As regards type of infection, our results suggest a strong correlation between platelets drop with or without later thrombocytopenia in preterm newborns and both Gram-negative bacterial and fungal infections. Gram-positive bacterial infection odds increased only in newborns that had shown thrombocytopenia after early platelets drop. Thrombocytopenia happening without prior early platelets drop did not show any association with any type of infection. These findings agree in part with the results published by Rastogi et al.,[11] who reported Gram-negative bacterial or fungal infections happening only in newborns with platelets drop with or without thrombocytopenia. In our study, we still see children with Gram-negative bacterial infection in the other two groups (no drop, no thrombocytopenia and thrombocytopenia without a drop). On the contrary to our findings, they reported significant associations between platelets drop with or without thrombocytopenia and Gram-positive bacterial infections.[19] These differences might be attributed to the differences in the local NICU environments as rates of nosocomial infections and predominant environmental pathogens; as well as clinical and demographic backgrounds of newborns included in the study. Actually, our reported rates of various types of infections are higher than those reported in other units.[19] However, although infections have been recognized as a factor that enhances platelets destruction,[27] the role of specific type of infection or organism is controversial.[17,19]
Intraventricular hemorrhage was associated with platelets drop even if no thrombocytopenia developed. There were no IVH cases in the group that developed thrombocytopenia without prior observed platelets drop. In previous works association between IVH in preterm newborns and thrombocytopenia was questioned. In patients with severe thrombocytopenia and in contrast to cutaneous and gastrointestinal hemorrhage no relationship between the lowest platelet count recorded, and the presence of IVH or pulmonary hemorrhage were found.[28] Furthermore, it was not clear how to interpret the association seen in some studies between lower platelets counts and higher prevalence of IVH. Two possible explanations have been propagated; thrombocytopenia might have caused the IVH, or it was a result of IVH through a consumptive process.[25,28] Our results illustrate that probably thrombocytopenia is not the main trigger for IVH and other factors contribute to its pathogenesis.
This study is mainly limited by the nature of its design. Being a retrospective study might have influenced several key variables validity resulting in both selection and information biases. The collection of samples in our study was part of routine investigations that were not scheduled on similar timing for all patients, which may have resulted in missing some newborns with a drop of platelets or thrombocytopenia. Furthermore, definitions of morbidities such as IVH, infections, and NEC; as well as investigating newborns for them, were largely dependent on the treating physician. Newborns with thrombocytopenia were subjected to investigations as head ultrasound, blood cultures, and abdominal X-rays more than newborns without thrombocytopenia. This might have falsely increased the association between thrombocytopenia and such morbidities.
Conclusion
Our study highlights, the need to consider not only thrombocytopenia but also platelets drop when predicting the outcome and major morbidities in preterm newborns. The early platelets drop even without the later development of thrombocytopenia is an early indicator of poor outcome and major morbidities, mainly infection. There is a need to investigate these observations in prospective study design.
Acknowledgments
The Authors would like to thank Dr. Abd Allah Naif Eldeasy and Dr. Raaef Abd Elkader Keratly, Demonstrators at the pediatric Department, Taibah University, Saudi Arabia, for their helpful role in data collection and analysis. The authors also would like to thank Bushra Al Ahmadi, Amjad Junaid, Khadijah Alshangiti, Esraa Osam, Maryam Alharbi, and Hala Aljohany, who are medical students at Taibah University, Saudi Arabia, for their helpful role in data collection and retrospective search in patients' files.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Mehta P, Vasa R, Neumann L, Karpatkin M. Thrombocytopenia in the high-risk infant. J Pediatr. 1980;97:791–4. doi: 10.1016/s0022-3476(80)80272-1. [DOI] [PubMed] [Google Scholar]
- 2.Roberts I, Stanworth S, Murray NA. Thrombocytopenia in the neonate. Blood Rev. 2008;22:173–86. doi: 10.1016/j.blre.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Chakravorty S, Murray N, Roberts I. Neonatal thrombocytopenia. Early Hum Dev. 2005;81:35–41. doi: 10.1016/j.earlhumdev.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Bussel JB, Sola-Visner M. Current approaches to the evaluation and management of the fetus and neonate with immune thrombocytopenia. Semin Perinatol. 2009;33:35–42. doi: 10.1053/j.semperi.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Sola-Visner M, Saxonhouse MA, Brown RE. Neonatal thrombocytopenia: What we do and don't know. Early Hum Dev. 2008;84:499–506. doi: 10.1016/j.earlhumdev.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Sola MC, Rimsza LM. Mechanisms underlying thrombocytopenia in the neonatal intensive care unit. Acta Paediatr Suppl. 2002;91:66–73. doi: 10.1111/j.1651-2227.2002.tb02907.x. [DOI] [PubMed] [Google Scholar]
- 7.Roberts IA, Murray NA. Neonatal thrombocytopenia: New insights into pathogenesis and implications for clinical management. Curr Opin Pediatr. 2001;13:16–21. doi: 10.1097/00008480-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Baer VL, Lambert DK, Henry E, Christensen RD. Severe thrombocytopenia in the NICU. Pediatrics. 2009;124:e1095–100. doi: 10.1542/peds.2009-0582. [DOI] [PubMed] [Google Scholar]
- 9.Christensen RD, Henry E, Wiedmeier SE, Stoddard RA, Sola-Visner MC, Lambert DK, et al. Thrombocytopenia among extremely low birth weight neonates: Data from a multihospital healthcare system. J Perinatol. 2006;26:348–53. doi: 10.1038/sj.jp.7211509. [DOI] [PubMed] [Google Scholar]
- 10.Bonifacio L, Petrova A, Nanjundaswamy S, Mehta R. Thrombocytopenia related neonatal outcome in preterms. Indian J Pediatr. 2007;74:269–74. doi: 10.1007/s12098-007-0042-x. [DOI] [PubMed] [Google Scholar]
- 11.Rastogi S, Olmez I, Bhutada A, Rastogi D. NCI classification of thrombocytopenia in extremely preterm neonates and its association with mortality and morbidity. J Perinat Med. 2011;39:65–9. doi: 10.1515/jpm.2010.122. [DOI] [PubMed] [Google Scholar]
- 12.Liu ZJ, Italiano J, Jr, Ferrer-Marin F, Gutti R, Bailey M, Poterjoy B, et al. Developmental differences in megakaryocytopoiesis are associated with up-regulated TPO signaling through mTOR and elevated GATA-1 levels in neonatal megakaryocytes. Blood. 2011;117:4106–17. doi: 10.1182/blood-2010-07-293092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor FB, Jr, Toh CH, Hoots WK, Wada H, Levi M. Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–30. [PubMed] [Google Scholar]
- 14.Oguzulgen IK, Ozis T, Gursel G. Is the fall in platelet count associated with intensive care unit acquired pneumonia? Swiss Med Wkly. 2004;134:430–4. doi: 10.4414/smw.2004.10670. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal S, Sachdev A, Gupta D, Chugh K. Platelet counts and outcome in the pediatric intensive care unit. Indian J Crit Care Med. 2008;12:102–8. doi: 10.4103/0972-5229.43678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Israels SJ, Odaibo FS, Robertson C, McMillan EM, McNicol A. Deficient thromboxane synthesis and response in platelets from premature infants. Pediatr Res. 1997;41:218–23. doi: 10.1203/00006450-199702000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Bhat MA, Bhat JI, Kawoosa MS, Ahmad SM, Ali SW. Organism-specific platelet response and factors affecting survival in thrombocytopenic very low birth weight babies with sepsis. J Perinatol. 2009;29:702–8. doi: 10.1038/jp.2009.72. [DOI] [PubMed] [Google Scholar]
- 18.Guida JD, Kunig AM, Leef KH, McKenzie SE, Paul DA. Platelet count and sepsis in very low birth weight neonates: Is there an organism-specific response? Pediatrics. 2003;111:1411–5. doi: 10.1542/peds.111.6.1411. [DOI] [PubMed] [Google Scholar]
- 19.Rastogi S, Olmez I, Bhutada A, Rastogi D. Drop in platelet counts in extremely preterm neonates and its association with clinical outcomes. J Pediatr Hematol Oncol. 2011;33:580–4. doi: 10.1097/MPH.0b013e31821e5f44. [DOI] [PubMed] [Google Scholar]
- 20.Stanworth SJ, Clarke P, Watts T, Ballard S, Choo L, Morris T, et al. Prospective, observational study of outcomes in neonates with severe thrombocytopenia. Pediatrics. 2009;124:e826–34. doi: 10.1542/peds.2009-0332. [DOI] [PubMed] [Google Scholar]
- 21.Murray NA, Roberts IA. Circulating megakaryocytes and their progenitors in early thrombocytopenia in preterm neonates. Pediatr Res. 1996;40:112–9. doi: 10.1203/00006450-199607000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Roberts I, Murray NA. Neonatal thrombocytopenia: Causes and management. Arch Dis Child Fetal Neonatal Ed. 2003;88:F359–64. doi: 10.1136/fn.88.5.F359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castle V, Andrew M, Kelton J, Giron D, Johnston M, Carter C. Frequency and mechanism of neonatal thrombocytopenia. J Pediatr. 1986;108:749–55. doi: 10.1016/s0022-3476(86)81059-9. [DOI] [PubMed] [Google Scholar]
- 24.Murray NA, Howarth LJ, McCloy MP, Letsky EA, Roberts IA. Platelet transfusion in the management of severe thrombocytopenia in neonatal intensive care unit patients. Transfus Med. 2002;12:35–41. doi: 10.1046/j.1365-3148.2002.00343.x. [DOI] [PubMed] [Google Scholar]
- 25.Andrew M, Castle V, Saigal S, Carter C, Kelton JG. Clinical impact of neonatal thrombocytopenia. J Pediatr. 1987;110:457–64. doi: 10.1016/s0022-3476(87)80517-6. [DOI] [PubMed] [Google Scholar]
- 26.Andrew M, Vegh P, Caco C, Kirpalani H, Jefferies A, Ohlsson A, et al. A randomized, controlled trial of platelet transfusions in thrombocytopenic premature infants. J Pediatr. 1993;123:285–91. doi: 10.1016/s0022-3476(05)81705-6. [DOI] [PubMed] [Google Scholar]
- 27.François B, Trimoreau F, Vignon P, Fixe P, Praloran V, Gastinne H. Thrombocytopenia in the sepsis syndrome: Role of hemophagocytosis and macrophage colony-stimulating factor. Am J Med. 1997;103:114–20. doi: 10.1016/s0002-9343(97)00136-8. [DOI] [PubMed] [Google Scholar]
- 28.Setzer ES, Webb IB, Wassenaar JW, Reeder JD, Mehta PS, Eitzman DV. Platelet dysfunction and coagulopathy in intraventricular hemorrhage in the premature infant. J Pediatr. 1982;100:599–605. doi: 10.1016/s0022-3476(82)80766-x. [DOI] [PubMed] [Google Scholar]


