Abstract
Objective:
Many hematopoietic stem cell transplantation (HSCT) patients receive vancomycin empirically during febrile neutropenia. There are several models for estimation of vancomycin pharmacokinetic parameters and calculation of initial dosing regimen accordingly. However, the performance of these methods in HSCT patients remained to be evaluated. The aim of the study was to determine which of the vancomycin population pharmacokinetic methods best fit Iranian HSCT patients.
Methods:
In order to evaluate predicted performance of seven vancomycin population pharmacokinetic models, the pharmacokinetic parameters of patients were estimated using each model's equations. Then the predicted steady-state trough vancomycin concentration was calculated based on each model's parameters and using a formula based on Sawchuk–Zaske method. The predicted steady-state trough vancomycin concentration and the real measured concentrations were compared to see which method was the most precise and least biased using mean squared error (MSE) and mean prediction error (ME) respectively.
Findings:
Forty-six patients (65% men) were included in the study. Calculated metrics showed a range of 38% under-prediction bias with Rodvold to 34% over-prediction bias with Matzke and Burton models. Birt and revised Burton methods showed no significant bias (ME [95% confidence interval (CI)]: –0.067 [–0.235–0.101] and 0.066 [–0.105–0.238]). Birt and revised Burton were not different significantly considering MSE (95% CI) of 0.385 (0.227–0.544) and 0.401 (0.255–0.546), respectively. Comparisons of precision with naive predictors revealed a delta MSE (95% CI) of –0.128 (–1.379–1.890) for Birt and 0.026 (–0.596–0.940) for revised Burton models.
Conclusion:
Although the Birt and Burton revised methods performed well, none of the studied models showed acceptable performance to be implemented as a routine method for initial dose calculation in HSCT patients. A vancomycin pharmacokinetic model specific for this high-risk subpopulation of Iranian patients should be designed and validated.
Keywords: Hematopoietic stem cell transplantation, population pharmacokinetics, predictive performance, Vancomycin
INTRODUCTION
Adequate empirical antibacterial therapy for febrile neutropenia is essential to reduce infection-related morbidity and mortality in hematopoietic stem cell transplantation (HSCT) patients.[1,2] Many HSCT patients receive vancomycin empirically during febrile neutropenia. However, the emergence and gradually increasing the prevalence of vancomycin-resistant organisms in recent years necessitates careful selection of antibiotic and dosing-regimen.[3] Determination of an optimal initial vancomycin dosing-regimen is crucial to reach recommended serum concentrations as quickly as possible because it could results in better bacterial eradication and improved outcome.[4] The observed intra and inter-patient variability in vancomycin pharmacokinetics led to the integration of vancomycin therapeutic drug monitoring and pharmacokinetic approaches into standard practice.[4,5,6] Hence, without considering vancomycin pharmacokinetic characteristics in this specific population of patients, initial empirical vancomycin treatment may not be optimal.
The most commonly used methods for determining initial dosing-regimen of vancomycin are recommended dosing by manufacturers and dosing nomograms.[7,8,9] Neglecting variations in age, weight and renal function in addition to using fixed volume of distribution (Vd) are major limitations of these methods.[10] Application of these methods as routine clinical practice for vancomycin dose calculations in high-risk patients could lead to suboptimal drug levels as were shown in previous studies included hematologic malignancy patients and in the recent one in the HSCT setting.[11,12]
Studies in patients with hematologic malignancies and neutropenic fever revealed different vancomycin pharmacokinetic parameters from other patients’ populations.[4,13,14,15] Little is known about vancomycin pharmacokinetics in HSCT patients. A recent study showed 70–80% higher mean vancomycin clearance (CL) in HSCT patients than what had been observed in adult medical and surgical patients. However, in contrary to reported higher mean Vd of vancomycin in patients with hematologic malignancy and neutropenic fever, vancomycin Vd in HSCT was similar to what was observed in other medical patients.[12]
Considering these variations, a population-specific pharmacokinetic model might be the most accurate approach. There are several models for estimation vancomycin pharmacokinetic parameters and calculation of initial dosing-regimen accordingly.[7] A number of them are population-based pharmacokinetic models that estimate patients’ parameters that are used to determine dosing-regimen.[16] Most of the methods have been designed based on one-compartmental pharmacokinetics and of these the Birt and Chandler,[10] Matzke et al.,[17] Burton et al.,[18] and Rodvold et al.[19] are more commonly cited. However, these methods may do their best in their own patients or similar population.[20] The performance of these models in specific population of patients, such as patients undergoing HSCT, needs to be evaluated. It is not clear which vancomycin population-based pharmacokinetic method could precisely determine initial dosing-regimen to achieve target trough concentrations in HSCT patients.
Therefore, we performed further analysis on data from our recent study,[12] to determine which of the vancomycin population pharmacokinetic methods best fit our HSCT patients.
METHODS
In order to evaluate the predictive performance of population pharmacokinetic methods, we performed further calculations and analysis on the gathered data from patients included in our recent study. The study was conducted at Hematology-Oncology and Stem Cell Transplant Research Center, Tehran University of Medical Sciences, between December 2012 and April 2013.[12] The protocol was reviewed and approved by the Institutional Review Board and Ethics Committee (Number: 92-02-36-22903).
Adult patients who were treated with vancomycin and received at least three consecutive doses by intermittent intravenous infusion were included. Patients, for whom vancomycin was discontinued before third dose, were excluded. We collected blood samples within 30 min prior to the administration of the fourth dose and sent to the laboratory within 2 h of collection. Fluorescence polarization immunoassay (Cobas Integra 400 system from Roche Diagnostics, Switzerland) was used to measure serum concentrations of vancomycin in steady state trough samples. The lower quantitation limit of this assay was 0.74 μg/mL, and the coefficients of variation % were 3.0% at 8.70 μg/mL, 2.2 at 26.3 μg/mL, and 3.3% at 54.6 μg/mL.
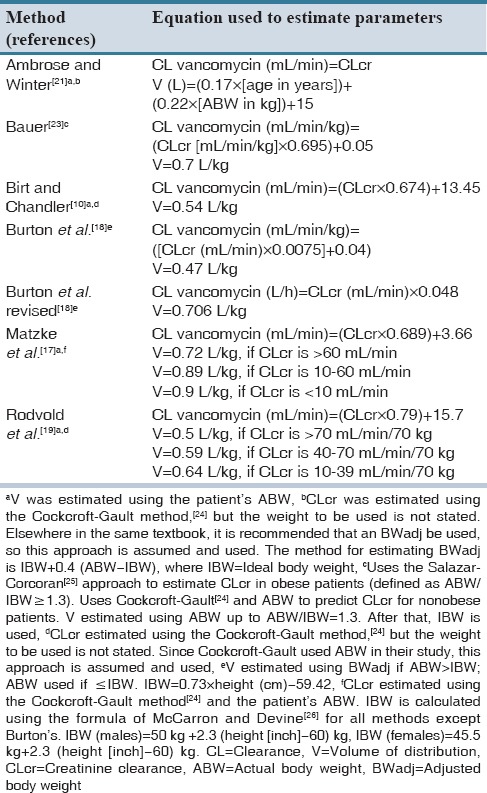
Data including patient weight, height, sex, age, daily serum creatinine, vancomycin dose, administration time, duration of infusion, and sampling date and time were registered. Among one-compartmental population pharmacokinetic models of vancomycin, seven more cited methods, including Ambrose and Winter,[21,22] Bauer,[23] Birt and Chandler,[10] Burton et al.,[18] revised Burton et al.,[18] Matzke et al.,[17] and Rodvold et al.[19] were selected. The patient information and dosing data were entered into an Excel spreadsheet (Microsoft, Redmond, WA) to estimate the relevant parameters in according to each method. The predicted vancomycin trough concentrations for every patient were calculated using estimated pharmacokinetic parameters based on each method [Table 1], dosing data and the following equation that is based on Sawchuk–Zaske method:[16,23]
Table 1.
Equations of vancomycin population pharmacokinetic models
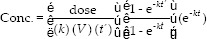
Where V is the Vd, k is the elimination rate constant, t’ is the duration of infusion, τ is the dosage interval used, and t is the time when the concentration was measured after the end of the infusion.
The predicted concentrations and the real concentrations were then compared to see which method was the most precise and least biased using the mean squared error (MSE) and mean prediction error (ME), respectively.[27] Precision was determined by the MSE and a smaller MSE indicated that predicted and measured concentrations were matched more precisely. ME was used to determine bias that shows the tendency to overestimate or underestimate the measured concentrations. The 95% confidence interval (95% confidence interval [CI]) of the mean of MSE and ME was used to determine statistical significance. In order to compare the precision of those methods that revealed no bias with a naive predictor (mean of the true values), we calculated delta MSE.[24]
RESULTS
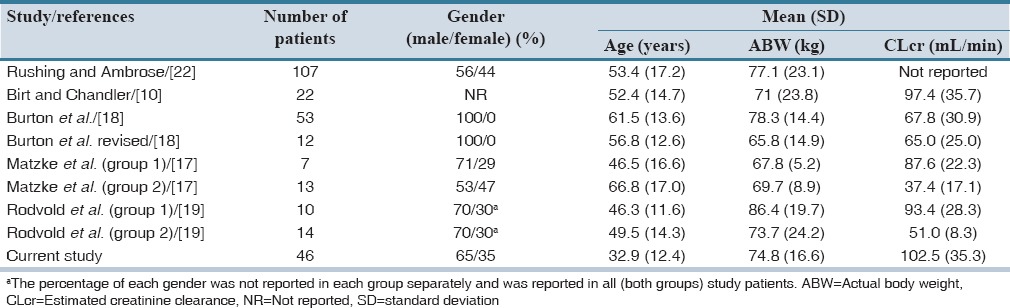
Forty-six patients were included in the study of them 65% were men. The baseline and clinical characteristics of patients are summarized in Table 2. Among study patients, 28.2% (13 patients) were more than 30% above their ideal body weight. Median (interquartile range) steady-state trough serum concentration of vancomycin was 9.3 (6.4) μg/mL. The results of predictive performance metrics, MSE, and ME are presented in Table 3. Calculated metrics showed a range of 38% under-prediction bias with Rodvold to 34% over-prediction bias with Matzke and Burton. With respect to ME, Birt and revised Burton methods showed no significant bias. They demonstrated biases of −0.067 and 0.066, respectively that are close to zero. All of the methods showed relatively poor precision. Birt and revised Burton were not different significantly considering MSE. Comparisons of precision with naive predictors revealed a delta MSE (95% CI) of −0.128 (−1.379–1.890) for Birt and 0.026 (−0.596–0.940) for revised Burton. A summary of the percentage of predictions based on each method that were within 2.5 and 5 mg/L and 25% and 50% of the measured concentrations is described in Table 4. The Birt and revised Burton methods predicted trough concentrations within 25% of the measured values in 35% and 30% of the cases, respectively. The characteristics of patients (age, body weight, estimated creatinine CL) included in the study based upon every mentioned population method was developed are summarized in Table 5.
Table 2.
Patients’ characteristics
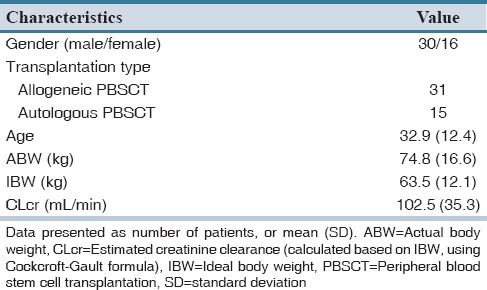
Table 3.
Predictive performance of the seven selected models of vancomycin pharmacokinetic
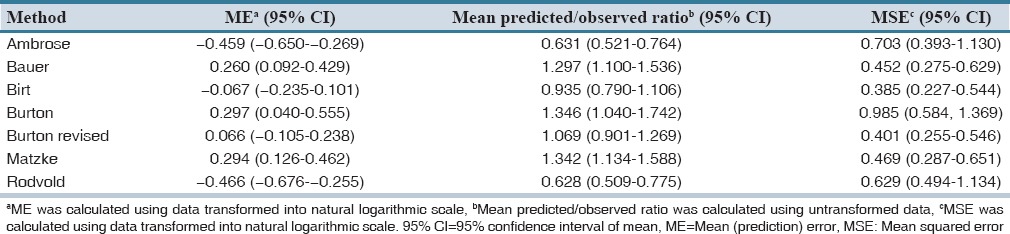
Table 4.
Predicted concentrations relative to measured concentrations of vancomycin
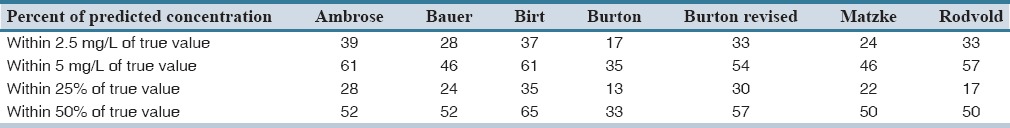
Table 5.
Characteristics of patients included in the study of each model and the current study
DISCUSSION
Given the substantial intra and inter-patients variability in vancomycin pharmacokinetic parameters, it is necessary to develop population-specific methods. Various population-based methods have been developed and used to estimate vancomycin pharmacokinetic parameters and improve initial dose calculation. However, these methods might not perform well when being used in a different population of patients.[20]
Considering the predictive performance of studied methods, we observed a wide variation. Among selected models, Birt and revised Burton had no significant bias in the prediction of trough concentrations. Although the Birt method demonstrated better precision than the revised Burton, the difference was not significant. Moreover, none of them revealed a significant difference in precision when compared with the naive predictor.
To the extent of our knowledge, there is no report regarding the predictive performance of these seven population pharmacokinetic models in HSCT and/or hematological malignancy patients. In a study performed by Murphy et al. in the United States, the predictability of these methods was evaluated in hospitalized patients.[16] Based on the study results, they judged that Matzke method had the least bias and acceptable precision. The Birt and Burton revised models showed significant bias and very poor precision in this study. The mean (standard deviation) of the patients’ age, actual body weight, and estimated creatinine CL in the mentioned study were 62 (18) years, 85.3 (26.2) kg, and 72.5 (39.6) mL/min, respectively. In addition, 61% and 31% of their patients were 20% and 50% above their ideal body weight, respectively.[16]
On the contrary, the Matzke method had a significant over-prediction bias in our study. In comparison, our study patients were younger and had lower weight and better creatinine CL that may correspond to the different observations. Another source for observed dissimilarities might be the different proportions of male and female patients. In the study by Murphy et al., nearly half of the patients were male but this proportion was 65% in our study.[16] In our study, the selected methods predicted trough concentrations within 25% of the measured ones in 13–35% of cases and Birt and Burton revised, performed more accurately than others. In the study performed by Murphy et al., predicted levels by the methods were within 25% of the measured concentrations in 7.9–31.2% of cases that is relatively similar to our findings.[16]
In a study by Buelga et al., a population pharmacokinetic model was developed and validated in hematological malignancy patients in Spain.[11] The study revealed significant effects of age, body weight, serum creatinine, and gender on pharmacokinetic parameters of vancomycin in these patients. Similarly, we found a significant relationship between creatinine CL and vancomycin CL in our previous study.[12] Considering creatinine CL and actual body weight of the patients [Table 5], Birt and Chandler study seemed to be relatively similar to the current study.[10] Birt and Chandler study patients had the most similar creatinine CL to our study patients. Difference in methods of serum creatinine measurement among studies might results in different creatinine CL estimations, which affect the pharmacokinetic parameters. However, either Birt or Burton et al. revised study did not mention the methods of measuring serum creatinine.[10,18]
The Birt and Chandler study suggested 0.54 L/kg using actual body weight for calculating Vd of vancomycin. We found a mean Vd of 0.6 L/kg for vancomycin in our previous pharmacokinetic analysis in these HSCT patients.[12] Except for actual body weight, the characteristics of patients included in the Burton et al. revised study were not similar to our study patients [Table 5].[18] Similarity of patients’ body weight and comparable vancomycin Vd may correspond to the better predictive performance of Birt model in our patients.
Due to limitations of sampling in clinical setting only trough samples were taken and one-compartmental models used. However, two-compartmental models would be more accurate in describing vancomycin pharmacokinetics.[28] Another major limitation of the study was small number of HSCT patients.
In conclusion, although the Birt and Burton revised methods had no bias and better precision, none of the seven studied models performed well in prediction trough concentrations of vancomycin and was reliable enough to be implemented as a routine method for initial dose calculation in HSCT patients. A model should be designed specifically for HSCT patients and be compared with methods that were developed in similar patients considering both pharmacokinetic predictive performance and clinical outcomes.
AUTHORS’ CONTRIBUTION
MTG designed study, gathered data, participated in pharmacokinetics calculations and drafted the manuscript; SR participated in pharmacokinetic calculations and manuscript drafting and revision; KG supervised the study and MH designed study and revised the manuscript.
Footnotes
Financial support and sponsorship Nil.
Conflict of Interest: There are no conflicts of interest.
REFERENCES
- 1.Paul M, Borok S, Fraser A, Vidal L, Leibovici L. Empirical antibiotics against Gram-positive infections for febrile neutropenia: Systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2005;55:436–44. doi: 10.1093/jac/dki028. [DOI] [PubMed] [Google Scholar]
- 2.de Naurois J, Novitzky-Basso I, Gill MJ, Marti FM, Cullen MH, Roila F, et al. Management of febrile neutropenia: ESMO Clinical Practice Guidelines. Ann Oncol. 2010;21(Suppl 5):v252–6. doi: 10.1093/annonc/mdq196. [DOI] [PubMed] [Google Scholar]
- 3.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2011;52:e56–93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 4.Jarkowski A, 3rd, Forrest A, Sweeney RP, Tan W, Segal BH, Almyroudis N, et al. Characterization of vancomycin pharmacokinetics in the adult acute myeloid leukemia population. J Oncol Pharm Pract. 2012;18:91–6. doi: 10.1177/1078155211402107. [DOI] [PubMed] [Google Scholar]
- 5.Al-Kofide H, Zaghloul I, Al-Naim L. Pharmacokinetics of vancomycin in adult cancer patients. J Oncol Pharm Pract. 2010;16:245–50. doi: 10.1177/1078155209355847. [DOI] [PubMed] [Google Scholar]
- 6.Teramachi H, Matsushita R, Tsuji A. Influence of malignancy on the pharmacokinetics of vancomycin hydrochloride in Japanese MRSA patients after dosage adjustment with the Bayesian method. Jpn J Chemother. 2005;53:357–63. [Google Scholar]
- 7.Lee E, Winter ME, Boro MS. Comparing two predictive methods for determining serum vancomycin concentrations at a Veterans Affairs Medical Center. Am J Health Syst Pharm. 2006;63:1872–5. doi: 10.2146/ajhp060138. [DOI] [PubMed] [Google Scholar]
- 8.Lake KD, Peterson CD. A simplified dosing method for initiating vancomycin therapy. Pharmacotherapy. 1985;5:340–4. doi: 10.1002/j.1875-9114.1985.tb03441.x. [DOI] [PubMed] [Google Scholar]
- 9.Elshaug EK, Manning L, Rawlins MD, Ridley BL, Ingram PR. Potential utility for nomogram-based vancomycin dosing. Int J Infect Dis. 2013;17:e355–6. doi: 10.1016/j.ijid.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Birt JK, Chandler MH. Using clinical data to determine vancomycin dosing parameters. Ther Drug Monit. 1990;12:206–9. doi: 10.1097/00007691-199003000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Buelga DS, del Mar Fernandez de Gatta M, Herrera EV, Dominguez-Gil A, García MJ. Population pharmacokinetic analysis of vancomycin in patients with hematological malignancies. Antimicrob Agents Chemother. 2005;49:4934–41. doi: 10.1128/AAC.49.12.4934-4941.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghehi MT, Rezaee S, Hayatshahi A, Hadjibabaie M, Gholami K, Javadi M, et al. Vancomycin Pharmacokinetic Parameters in Patients Undergoing Hematopoietic Stem Cell Transplantation (HSCT) Int J Hematol Oncol Stem Cell Res. 2013;7:1–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Lortholary O, Lefort A, Tod M, Chomat AM, Darras-Joly C, Cordonnier C, et al. Pharmacodynamics and pharmacokinetics of antibacterial drugs in the management of febrile neutropenia. Lancet Infect Dis. 2008;8:612–20. doi: 10.1016/S1473-3099(08)70228-7. [DOI] [PubMed] [Google Scholar]
- 14.Omote S, Yano Y, Hashida T, Masuda S, Yano I, Katsura T, et al. A retrospective analysis of vancomycin pharmacokinetics in Japanese cancer and non-cancer patients based on routine trough monitoring data. Biol Pharm Bull. 2009;32:99–104. doi: 10.1248/bpb.32.99. [DOI] [PubMed] [Google Scholar]
- 15.Le Normand Y, Milpied N, Kergueris MF, Harousseau JL. Pharmacokinetic parameters of vancomycin for therapeutic regimens in neutropenic adult patients. Int J Biomed Comput. 1994;36:121–5. doi: 10.1016/0020-7101(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 16.Murphy JE, Gillespie DE, Bateman CV. Predictability of vancomycin trough concentrations using seven approaches for estimating pharmacokinetic parameters. Am J Health Syst Pharm. 2006;63:2365–70. doi: 10.2146/ajhp060047. [DOI] [PubMed] [Google Scholar]
- 17.Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984;25:433–7. doi: 10.1128/aac.25.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burton ME, Gentle DL, Vasko MR. Evaluation of a Bayesian method for predicting vancomycin dosing. Ann Pharmacother. 1989;23:294–300. doi: 10.1177/106002808902300404. [DOI] [PubMed] [Google Scholar]
- 19.Rodvold KA, Blum RA, Fischer JH, Zokufa HZ, Rotschafer JC, Crossley KB, et al. Vancomycin pharmacokinetics in patients with various degrees of renal function. Antimicrob Agents Chemother. 1988;32:848–52. doi: 10.1128/aac.32.6.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sánchez JL, Dominguez AR, Lane JR, Anderson PO, Capparelli EV, Cornejo-Bravo JM. Population pharmacokinetics of vancomycin in adult and geriatric patients: Comparison of eleven approaches. Int J Clin Pharmacol Ther. 2010;48:525–33. doi: 10.5414/cpp48525. [DOI] [PubMed] [Google Scholar]
- 21.Ambrose PJ, Winter ME. Vancomycin. In: Winter ME, editor. Basic Clinical Pharmacokinetics. 4th ed. Philadelphia: Wolters Kluwer/Lippincott Williams and Wilkins; 2004. pp. 451–76. [Google Scholar]
- 22.Rushing TA, Ambrose PJ. Clinical application and evaluation of vancomycin dosing in adults. J Pharm Technol. 2001;17:33–8. [Google Scholar]
- 23.Bauer L. Applied Clinical Pharmacokinetics. 2nd ed. New York: McGraw-Hill; 2008. [Google Scholar]
- 24.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 25.Salazar DE, Corcoran GB. Predicting creatinine clearance and renal drug clearance in obese patients from estimated fat-free body mass. Am J Med. 1988;84:1053–60. doi: 10.1016/0002-9343(88)90310-5. [DOI] [PubMed] [Google Scholar]
- 26.McCarron MM, Devine BJ. Clinical pharmacy: Case studies case number 25 gentamicin therapy. Ann Pharmacother. 1974;8:650–5. [Google Scholar]
- 27.Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9:503–12. doi: 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]
- 28.Marsot A, Boulamery A, Bruguerolle B, Simon N. Vancomycin: A review of population pharmacokinetic analyses. Clin Pharmacokinet. 2012;51:1–13. doi: 10.2165/11596390-000000000-00000. [DOI] [PubMed] [Google Scholar]


