Abstract
Objective:
Hypertension and hyperlipidemia are two major risk factors for cardiovascular disease in continuous ambulatory peritoneal dialysis (CAPD) patients. This study was designed to investigate the effect of omega-3 fatty acids on blood pressure (BP) and serum lipids in CAPD patients.
Methods:
This study was a randomized double-blind clinical trial in which 90 CAPD patients were randomly assigned to either the omega-3 or the placebo group. Patients in omega-3 group received 3 g/day omega-3 for 8 weeks, whereas patients in the control group received placebo. At baseline and at the end of 8 weeks, the patients’ BP was controlled, and serum biochemistry was measured.
Findings:
Mean systolic BP decreased (–22.2 ± 14.2 mmHg) in the omega-3 group at the end of the study while in the placebo group increased (+0.5 ± 30.2 mmHg) (P < 0.0001). Mean diastolic BP of the omega-3 group decreased more (–11.95 ± 11.9 mmHg) comparing with the placebo group (–1.1 ± 17.3 mmHg) (P = 0.001). There were no significant differences between the two groups in mean changes in serum triglyceride, and total, high-density lipoprotein, and low-density lipoprotein cholesterol.
Conclusion:
The results of this study indicate that omega-3 reduced BP significantly but had no effect on lipid profile in our CAPD patients.
Keywords: Blood pressure, continuous ambulatory peritoneal dialysis, Omega-3, serum lipids
INTRODUCTION
Continuous ambulatory peritoneal dialysis (CAPD) is one of the renal replacement therapy modalities in end-stage renal disease (ESRD) patients. CAPD patients have many risk factors for cardiovascular diseases, including hypertension, hyperlipidemia, and abnormal glucose metabolism. Cardiovascular disease is a major cause of mortality in ESRD population.
Despite many improvements in care of ESRD patients, however, only 51% of dialysis patients, and 82% of those who receive a preemptive transplant, are still alive 3 years after the start of ESRD therapy – numbers that help illustrate the extreme vulnerability of these patients when compared to the general population. Among dialysis patients aged 65 years and older, for example, mortality is twice as for patients in the general population suffering diabetes, cancer, congestive heart failure, cerebro-vascular accident, or acute myocardial infarction.[1]
Cold water fish is the main source of eicossapantonoic and docosahexanoic acids the two major bioactive omega-3 fatty acids. Omega-3 fatty acids may have therapeutic beneficial effect such as decreasing triglyceride level, uremic pruritus and oxidative stress and improving dialysis access patency in dialysis patients through altering cell membrane structure and function and the synthesis of lipid mediation such as eicosanoids.[2]
Epidemiological studies in the last 40 years suggest that omega-3 fatty acids derived from fish decrease the risk of coronary heart disease, hypertension and stroke and their complications. The beneficial effect of omega-3 fatty acids include effects on lipids, blood pressure (BP), cardiac and vascular function, coagulation and immunological responses, systemic inflammations, endothelial function, reduction of pro-inflammatory responses and cardiac rhythm. Dietary omega-3 fatty acids are associated with plasma biomarker levels, reflecting lower levels of inflammation and endothelial activation in cardiovascular disease and other chronic and acute diseases, including chronic renal disease, sepsis, and acute pancreatitis.[2,3,4,5,6,7,8,9,10]
Data on the use of omega-3 as a drug is limited in ESRD patients, and only few studies in CAPD patients are available testing its beneficial effects. In this study, we aimed to evaluate the effect of omega-3 on BP and serum lipid level of our CAPD patients.
METHODS
In this randomized double-blind clinical trial, we evaluated the effect of omega-3 on BP and lipid level in CAPD patients during April and May 2012. All patients (170 patients) in two CAPD centers in two university hospitals, were evaluated for inclusion criteria as: Age 18 years old or more, at least 3 months on CAPD, has hypertension stage one according to JNC 7 or normal BP with antihypertensive treatment and has read and signed the informed consent and excluded who has history of steatorrhea, malignancy, thrombocytopenia (platelet count [Plt] <100,000), abnormal coagulation profile or need anticoagulation treatment and psychosomatic disorders. All patients dialyzed with glucose-based CAPD solutions. Dialysate glucose concentrations (1.35, 2.25, and 3.75%), number of daily dialysis exchanges, and antihypertensive medications selected according to the responsible physician decision.
This study has been conducted by Isfahan Kidney Diseases Research Center (project No. 290370) as a sub-special thesis, funded by the Vice-Chancellery for Research and Technology, Isfahan University of Medical Sciences, and approved by Ethical Committee of Isfahan University of Medical Sciences Isfahan, Iran. The study was registered by Iranian Registry of Clinical Trials (IRCT; registration number: IRCT201501112417N16).
Ninety patients were fulfilled the inclusion criteria, and randomly assigned by computer-based simple randomization in omega-3 group and placebo groups (each 45 patients). Demographic characteristics including gender, age, body mass index (BMI), and cause of renal failure, time on dialysis and CAPD characteristics including number of daily dialysis exchange, glucose concentration of dialysis solutions, antihypertensive medications, erythropoietin dosage, and urine output were recorded. Dialysis adequacy (based on Kt/V) was calculated at the baseline and after completion of the study by a standard method using PD Adequest Software, Baxter®. Biochemistry analysis including triglyceride, total cholesterol, high-density lipoprotein (HDL)/low-density lipoprotein (LDL) cholesterol, prothrombin time (PT)/international normalized ratio (INR), partial thromboplastin time (PTT), white blood cell count (WBC), Plt, hemoglobin (Hb), and serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), serum iron (SI), total iron binding capacity (TIBC), ferritin tests was measured by autoanalyzer at baseline and after 8 weeks. Patients in both groups were advised to be on low salt, high protein (1.3 g/kg) diet.
At the baseline BP was measured after 5 min rest in sitting position by digital sphygmomanometer from both arms (if possible) and recorded the higher one by CAPD nurses and repeated after 8 weeks. A 3-g fish-oil capsules (omega-3, Zahravi Pharmaceutical co. Tabriz, Iran) in which every gram contains 180 mg eicosapentaenoic acid and 120 mg docosahexaenoic acid was given to omega-3 group patients and corresponding placebo given to placebo group.
Qualitative and quantitative variables between two groups were analyzed with Chi-square test and independent sample t-test respectively. For comparing the effect of the intervention on BP and lipid profile within each group we used paired t-test. Initial test was done on multiple variables, which could affect as confounding variables on final results with partial correlation coefficient test to find out parameters which had a significant correlation in the range of <0.5. Selected variables then entered to analysis of covariance test (ANCOVA) for final analysis, with the use of PASWStatistics 18, SPSS Inc, IBM. P < 0.05 was supposed to be statistically significant.
RESULTS
All the selected patients completed the trial and included in final analysis. None of patients in both groups complaint of any major side-effects to discontinue the study. During the study period, the antihypertensive medications was not necessitated to change.
Patients’ demographic and CAPD characteristics and laboratory tests data are shown in Table 1 which showed no significant difference between two arms of the study population except for TIBC and ferritin level at the baseline.
Table 1.
Comparison of demographic, CAPD characteristics, and laboratory tests of patients at the study beginning between omega-3 and placebo groups
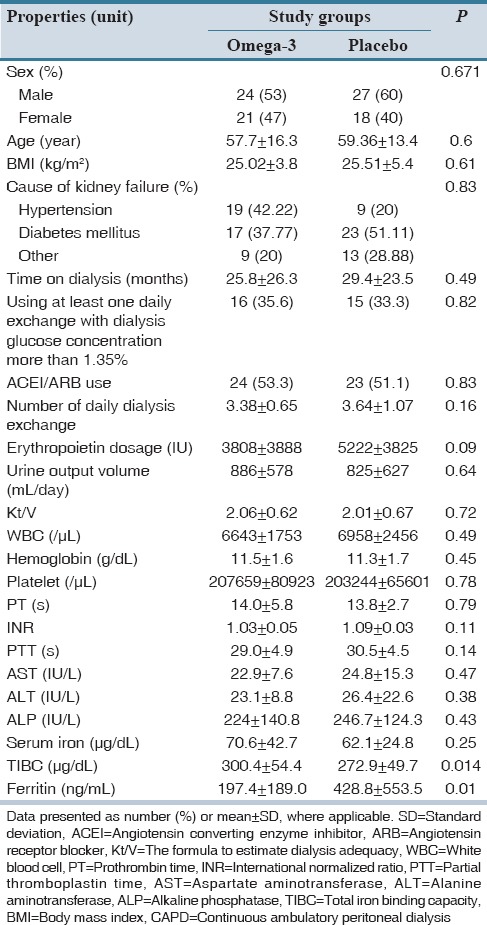
During the study period, blood WBC, Plt, Hb, level and Kt/V, and serum ALT, AST, ALP, SI, TIBC, ferritin level did not change in the both groups significantly, except for increased WBC in placebo group (6958 ± 2457 vs. 7450 ± 2613; P = 0.031).
In our study, there were no significant changes in PT-PTT but only INR level increased in omega-3 group relative to placebo (+0.04 vs.−0.03 P = 0.043).
The mean systolic and diastolic BP changes during the study between the two groups were significant. Although at the start of the study, mean systolic BP in the omega-3 treated was higher than placebo group (148.6 ± 18.3 vs. 140.2 ± 19.1, P = 0.036) the mean systolic BP decreased (−22.2 ± 14.2 mmHg) at the end of the study while in the placebo group increased (+0.5 ± 30.2 mmHg) (P < 0.0001). Furthermore, the mean diastolic BP of the omega-3 group decreased more (−11.95 ± 11.9 mmHg) at the end of study comparing with the placebo group (−1.1 ± 17.3 mmHg) (P = 0.001) [Table 2 and Figures 1 and 2].
Table 2.
Comparison of systolic and diastolic BP, triglyceride, cholesterol, LDL-cholesterol and HDLcholesterol between the omega-3 and placebo groups during the study period
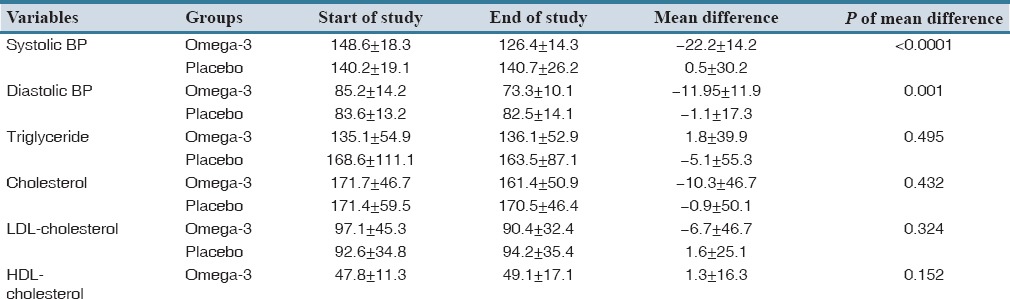
Figure 1.
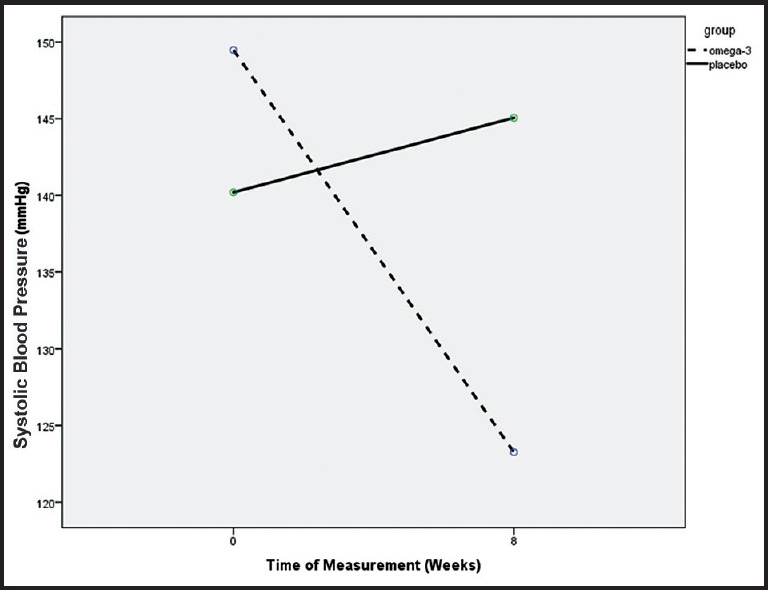
Systolic blood pressure decreased significantly in omega-3 comparing with placebo group during the study period (P < 0.0001)
Figure 2.
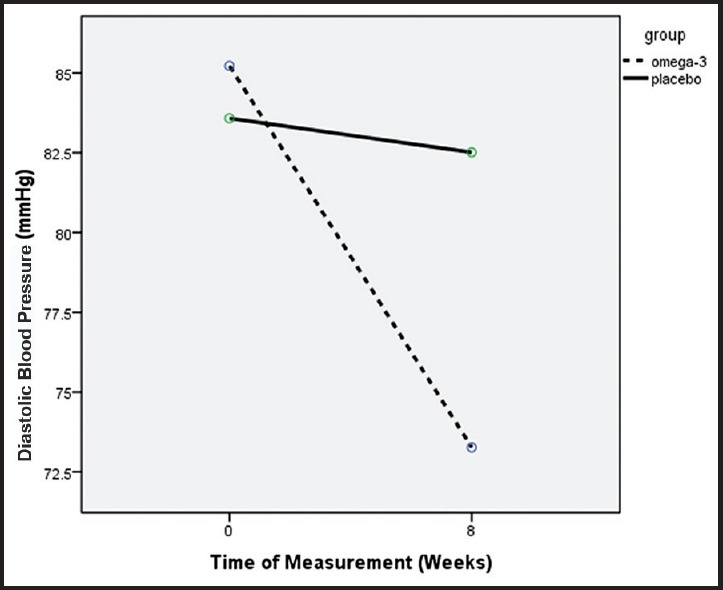
Diastolic blood pressure decreased significantly in omega-3 comparing with placebo group during the study period (P = 0.001)
With partial correlation coefficient test we analyzed correlation of multiple variables including baseline systolic and diastolic BP, age, sex, cause of kidney failure, number of daily dialysis exchange, use of at least one daily dialysis exchange with higher glucose concentration (2.25 and 3.75%), BMI, angiotensin converting enzyme inhibitor/angiotensin receptor blocker use as antihypertensive medications, initial erythropoietin dosage, WBC, Hb, Plt, Kt/V, triglyceride, cholesterol, HDL/LDL cholesterol, ALT, AST, ALP, SI, TIBC, ferritin with endpoint systolic and diastolic BP in each treatment group. For endpoint systolic BP, significant correlation in the range of < 0.5 was observed with baseline systolic BP (r = 0.30, P = 0.004), age (r = 0.15, P = 0.16), cause of kidney failure (r = 0.18, P = 0.09), use of at least one daily dialysis exchange with higher glucose concentration (2.25 and 3.75%) (r = 0.23, P = 0.027), BMI (r = 0.27, P = 0.011), initial erythropoietin dosage (r = −0.14, P = 0.18), AST (r = 0.10, P = 0.35), ALP (r = −0.11, P = 0.32), Kt/V (r = 0.11, P = 0.31), cholesterol (P = 0.13, P = 0.23), LDL cholesterol (r = 0.12, P = 0.26), SI (r = −0.14, P = 0.21). For endpoint diastolic BP, significant correlation in the range of < 0.5 was observed with baseline diastolic BP (r = 0.34, P = 0.001), number of daily dialysis exchange (r = 0.23, P = 0.32), use of at least one daily dialysis exchange with higher glucose concentration (2.25% and 3.75%) (r = 0.27, P = 0.12), BMI (r = 0.21, P = 0.051), initial erythropoietin dosage (r = −0.17, P = 0.11), WBC (r = 0.087, P = 0.42), ALP (r = −0.13, P = 0.23), Kt/V (r = 0.21, P = 0.05), triglyceride (r = −014, P = 0.18), LDL cholesterol (r = 0.11, P = 0.31), SI (r = −016, P = 0.14), TIBC (r = −0.14, P = 0.18).
In ANCOVA, endpoint systolic BP as a dependent variable, treatment groups as an independent variable and including mentioned variables correlated in the range of <0.5 covariates found that only being in omega-3 group (B = −17.516, 95% confidence interval [CI]: −26.786–−8.247, P < 0.0001) and baseline systolic BP (B = 0.334, 95% CI: 0.090–0.577, P = 0.008) had significant effect and use of at least one daily dialysis exchange with higher glucose concentration (2.25 and 3.75%) (B = 8.429, 95|% CI: −1.033–17.892, P = 0.08) had trend toward significance on final systolic BP. Also for diastolic BP, being in omega-3 group (B = −10.173, 95% CI: −15.289–−5.056, P < 0.0001) and baseline diastolic BP (B = 0.247, 95% CI: 0.063–0.432, P = 0.009) had significant effect and initial erythropoietin dosage (B = −0.001, 95|% CI: −0.008–0.001, P = 0.074) had trend toward significance on final diastolic BP.
Changes in triglyceride, cholesterol, HDL and LDL in both groups during the study period were not significant [Table 2].
DISCUSSION
The first study on the effect of omega-3 fatty acids on BP was done in 1983 by Singer et al. that observed using Mackerel diet decreased significantly systolic and diastolic BP in healthy volunteers.[11]
Cabo et al. suggested that the use of polyunsaturated fatty acids (PUFAs) may have a role to decrease BP in mild hypertensive patients before starting drug therapy.[12]
Appel et al. demonstrated that diet supplementation with a high dose of omega-3, (more than 3 g/day), can reduce to BP in patients with untreated hypertension.[8]
Vernaglione et al. reported that fish oil supplementation reduced systolic and diastolic BP in the chronic dialysis patients dramatically.[13]
Fish oil that contains omega-3 fatty acids is beneficial in the prevention of atherosclerosis and thrombosis and evidence of this has been proved in epidemiologic research among the Eskimos.[13,14]
Donnelly et al. in a double-blind cross-over study on dialysis patients (hemodialysis or peritoneal dialysis) showed receiving 12 fish oil capsules for 4 weeks didn’t reduce their BP compared with placebo group.[15] However, one patient needed increasing dry weight due to intradialytic hypotension. The cause may be due to participation of normal BP patients at the beginning of the study and short term patients’ follow-up as showed in our study that 8 weeks supplementation need to show the therapeutic effect of omega-3.
Singer's study represented that consumption of fish oil by increasing urinary sodium and decreasing plasma renin activity reduced BP.[11]
Friedman and Moe in a review article concluded that omega-3 fatty acids are effective in the immune and inflammatory responses, arteriosclerosis, vascular reactivity and BP control, cell membrane function, and gene expression. They also suggested that omega-3 supplementation may offer a host of benefits to dialysis patients.[2]
Little information about blood levels of omega-3 fatty acids are found in dialysis patients. Some studies showed that blood levels of omega-3 and omega-6 in dialysis patients are low,[16,17,18] which may be associated with some uremic signs and symptoms, such as skin problems, fragility of erythrocytes associated with anemia, lipid alterations and endocrine dysfunction.
Knapp and Fitzgerald were studied the effect of different doses of omega-3 and omega-6 in patients with essential hypertension. They showed that high doses of fish oil (MaxEPA, 50 ml) reduce BP in men with essential hypertension. In this study the vasodilatory effect of prostaglandins (PG12, PG13) was increased.[19]
Bao et al. show that a combination of omega-3 and weight loss cause significant reduction of BP and heart rate in essential hypertensive patients. They concluded that increased consumption of omega-3 fatty acid from fish may cause a reduction in heart rate through cardiac/autonomic component, and vascular effects.[20]
In the present study, both the mean systolic and diastolic BP decreased more in omega-3 group than placebo group. After controlling for confounding parameters in ANCOVA test, only use of omega-3 and baseline systolic and diastolic BP had statistically significant effect on final patients’ BP.
It is interesting that this effect was shown in much lower doses of omega-3 in comparison with previous studies. It may not only increase compliance compare with large doses of omega-3 but also decrease side-effects of the drug as none of our patients complained of any major drug-related disturbances.
Pei et al. in meta-analysis in patients with ESRD, evaluated the effect of n-3 PUFA use on plasma lipids and lipoproteins and concluded that its consumption significantly lowered the serum triglyceride levels but had no significant effect on the degrees of LDL-cholesterol, total cholesterol, and HDL-cholesterol.[21]
Some studies in different population groups have shown that omega-3 fatty acids had mainly reducing the effect on serum triglycerides and had no or nonsignificant effect on total, LDL, and HDL cholesterol levels.[22,23,24]
Unsaturated fat supplementation increased total dietary energy intake to recommended levels, had no adverse impact on blood lipids, improved nutritional status as assessed according to dry body weight, and reduced systemic inflammation as assessed according to C-reactive protein serum concentrations. Adding unsaturated fat to the diet seems to be a safe and effective way to prevent and treat malnutrition in hemodialysis patients.[25]
In our study, lower total cholesterol, and LDL-cholesterol levels was observed in omega-3 group at the end of the study, but these changes were not significant compared to the placebo group.
The omega-3 group showed a trend toward increasing in HDL-cholesterol level comparing with the placebo group.
In our study, no saturated fatty acids intake reduction was applied, so more beneficial effect on lipid profile would be found if such diet modification was requested.
During this study, no significant change was found in the LDL levels in omega-3 group. However, most studies have reported raised LDL level by omega-3.[23,24,26,27]
In these studies, rising of LDL level was maybe due to use of high doses or unpurified fish oil or omega-3 fatty acids such as MaxEPA (that contained saturated fatty acid and cholesterol) or methylated esters of omega-3 compared with our study, using purified omega-3 fatty acids.
In another study, Taziki et al. reported that daily consumption of 2 g of fish oil capsules containing 600 mg omega-3 in 33 dialysis patients for 12 weeks resulted significant increase in HDL level and decrease in triglyceride level.[28]
As can be seen from the results of omega-3 supplementation studies, it has a different impact on the patients’ lipid profile. This can be due to several factors, including the amount and how to get omega-3 and type of dietary intake.
Overall, our results indicate that omega-3 significantly reduced systolic and diastolic BP in CAPD patients and this effect may reduce cardiovascular risk factors but had no effect on lipid profile.
Our study was done in hypertensive CAPD patients and had a limitation of number of patients included and it would be better to use of ambulatory BP monitoring. It is necessary to conduct another larger multicenter randomized clinical trial to evaluate this effect of low dose omega-3 on CAPD patients’ BP and other cardiovascular risk factor and follow-up them for long period to assess the effect of this intervention on this population's cardiovascular risk reduction and overall morbidity and mortality.
AUTHORS’ CONTRIBUTION
Afsoon Emami Naini1, Nooshin Keyvandarian1, Mojgan Mortazavi1, Shahram Taheri all contributed in study design, data collection and patient care. Sayed Mohsen Hosseini contributed in study design, control of study progress, data collection and analysis. All author contributed in article writing and read and accepted the final version of the article for submission.
Acknowledgments
The findings of the research are the result of cooperation of CAPD centers in Alzahra and Noor and Aliasghar Hospitals and special thanks to our CAPD nurses: Mrs. Shirin Karimi, Homa Reyhani, Elham Yazdani, and Akram Eslami and Miss Tahereh Gholamrezapoor for laboratory support.
Footnotes
Financial support and sponsorship This study has been conducted by Isfahan Kidney Diseases Research Center (project No. 290370) as a sub-specialty thesis, funded by the Vice-Chancellery for Research and Technology, Isfahan University of Medical Sciences, and approved by Ethical Committee of Isfahan University of Medical Sciences Isfahan, Iran.
Conflict of Interest There are no conflicts of interest.
REFERENCES
- 1.US Renal Data System, USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012. p. 260. Available from: http://www.usrds.org/2012/pdf/v2_ch5_12.pdf . [Google Scholar]
- 2.Friedman A, Moe S. Review of the effects of omega-3 supplementation in dialysis patients. Clin J Am Soc Nephrol. 2006;1:182–92. doi: 10.2215/CJN.00740805. [DOI] [PubMed] [Google Scholar]
- 3.Rangel-Huerta OD, Aguilera CM, Mesa MD, Gil A. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: A systematic review of randomised clinical trials. Br J Nutr. 2012;107(Suppl 2):S159–70. doi: 10.1017/S0007114512001559. [DOI] [PubMed] [Google Scholar]
- 4.Kremer JM, Jubiz W, Michalek A, Rynes RI, Bartholomew LE, Bigaouette J, et al. Fish-oil fatty acid supplementation in active rheumatoid arthritis. A double-blinded, controlled, crossover study. Ann Intern Med. 1987;106:497–503. doi: 10.7326/0003-4819-106-4-497. [DOI] [PubMed] [Google Scholar]
- 5.Bucher HC, Hengstler P, Schindler C, Meier G. N-3 polyunsaturated fatty acids in coronary heart disease: A meta-analysis of randomized controlled trials. Am J Med. 2002;112:298–304. doi: 10.1016/s0002-9343(01)01114-7. [DOI] [PubMed] [Google Scholar]
- 6.Leaf A, Kang JX, Xiao YF, Billman GE. Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation. 2003;107:2646–52. doi: 10.1161/01.CIR.0000069566.78305.33. [DOI] [PubMed] [Google Scholar]
- 7.Harris WS. N-3 fatty acids and serum lipoproteins: Human studies. Am J Clin Nutr. 1997;65(5 Suppl):1645S–54. doi: 10.1093/ajcn/65.5.1645S. [DOI] [PubMed] [Google Scholar]
- 8.Appel LJ, Miller ER, 3rd, Seidler AJ, Whelton PK. Does supplementation of diet with ‘fish oil’ reduce blood pressure. A meta-analysis of controlled clinical trials? Arch Intern Med. 1993;153:1429–38. [PubMed] [Google Scholar]
- 9.Ho M, Maple C, Bancroft A, McLaren M, Belch JJ. The beneficial effects of omega-3 and omega-6 essential fatty acid supplementation on red blood cell rheology. Prostaglandins Leukot Essent Fatty Acids. 1999;61:13–7. doi: 10.1054/plef.1999.0066. [DOI] [PubMed] [Google Scholar]
- 10.K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 Suppl 3):S1–153. [PubMed] [Google Scholar]
- 11.Singer P, Berger I, Lück K, Taube C, Naumann E, Gödicke W. Long-term effect of mackerel diet on blood pressure, serum lipids and thromboxane formation in patients with mild essential hypertension. Atherosclerosis. 1986;62:259–65. doi: 10.1016/0021-9150(86)90100-0. [DOI] [PubMed] [Google Scholar]
- 12.Cabo J, Alonso R, Mata P. Omega-3 fatty acids and blood pressure. Br J Nutr. 2012;107(Suppl 2):S195–200. doi: 10.1017/S0007114512001584. [DOI] [PubMed] [Google Scholar]
- 13.Vernaglione L, Cristofano C, Chimienti S , Omega-3 polyunsaturated fatty acids and proxies of cardiovascular disease in hemodialysis: a prospective cohort study. J Nephrol. 2008;21:99–105. [PubMed] [Google Scholar]
- 14.Simopoulos AP. Omega-3 fatty acids and cardiovascular disease: The epidemiological evidence. Environ Health Prev Med. 2002;6:203–9. doi: 10.1007/BF02897971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnelly SM, Ali MA, Churchill DN. Effect of n-3 fatty acids from fish oil on hemostasis, blood pressure, and lipid profile of dialysis patients. J Am Soc Nephrol. 1992;2:1634–9. doi: 10.1681/ASN.V2111634. [DOI] [PubMed] [Google Scholar]
- 16.Dasgupta A, Kenny MA, Ahmad S. Abnormal fatty acid profile in chronic hemodialysis patients: Possible deficiency of essential fatty acids. Clin Physiol Biochem. 1990;8:238–43. [PubMed] [Google Scholar]
- 17.Varga Z, Kárpáti I, Paragh G, Buris L, Kakuk G. Relative abundance of some free fatty acids in plasma of uremic patients: Relationship between fatty acids, lipid parameters, and diseases. Nephron. 1997;77:417–21. doi: 10.1159/000190318. [DOI] [PubMed] [Google Scholar]
- 18.Madsen T, Christensen JH, Svensson M, Witt PM, Toft E, Schmidt EB. Marine n-3 polyunsaturated fatty acids in patients with end-stage renal failure and in subjects without kidney disease: A comparative study. J Ren Nutr. 2011;21:169–75. doi: 10.1053/j.jrn.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Knapp HR, FitzGerald GA. The antihypertensive effects of fish oil. A controlled study of polyunsaturated fatty acid supplements in essential hypertension. N Engl J Med. 1989;320:1037–43. doi: 10.1056/NEJM198904203201603. [DOI] [PubMed] [Google Scholar]
- 20.Bao DQ, Mori TA, Burke V, Puddey IB, Beilin LJ. Effects of dietary fish and weight reduction on ambulatory blood pressure in overweight hypertensives. Hypertension. 1998;32:710–7. doi: 10.1161/01.hyp.32.4.710. [DOI] [PubMed] [Google Scholar]
- 21.Pei J, Zhao Y, Huang L, Zhang X, Wu Y. The effect of n-3 polyunsaturated fatty acids on plasma lipids and lipoproteins in patients with chronic renal failure – A meta-analysis of randomized controlled trials. J Ren Nutr. 2012;22:525–32. doi: 10.1053/j.jrn.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Svensson M, Schmidt EB, Jørgensen KA, Christensen JH. The effect of n-3 fatty acids on lipids and lipoproteins in patients treated with chronic haemodialysis: a randomized placebo-controlled intervention study. Nephrol Dial Transplant. 2008;23:2918–24. doi: 10.1093/ndt/gfn180. [DOI] [PubMed] [Google Scholar]
- 23.Stark KD, Park EJ, Maines VA, Holub BJ. Effect of a fish-oil concentrate on serum lipids in postmenopausal women receiving and not receiving hormone replacement therapy in a placebo-controlled, double-blind trial. Am J Clin Nutr. 2000;72:389–94. doi: 10.1093/ajcn/72.2.389. [DOI] [PubMed] [Google Scholar]
- 24.Mori TA, Burke V, Puddey IB, Watts GF, O’Neal DN, Best JD, et al. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr. 2000;71:1085–94. doi: 10.1093/ajcn/71.5.1085. [DOI] [PubMed] [Google Scholar]
- 25.Ewers B, Riserus U, Marckmann P. Effects of unsaturated fat dietary supplements on blood lipids, and on markers of malnutrition and inflammation in hemodialysis patients. J Ren Nutr. 2009;19:401–11. doi: 10.1053/j.jrn.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Kasim-Karakas SE, Herrmann R, Almario R. Effects of omega-3 fatty acids on intravascular lipolysis of very-low-density lipoproteins in humans. Metabolism. 1995;44:1223–30. doi: 10.1016/0026-0495(95)90020-9. [DOI] [PubMed] [Google Scholar]
- 27.Tinker LF, Parks EJ, Behr SR, Schneeman BO, Davis PA. (n-3) fatty acid supplementation in moderately hypertriglyceridemic adults changes postprandial lipid and apolipoprotein B responses to a standardized test meal. J Nutr. 1999;129:1126–34. doi: 10.1093/jn/129.6.1126. [DOI] [PubMed] [Google Scholar]
- 28.Taziki O, Lessan-Pezeshki M, Akha O, Vasheghani F. The effect of low dose omega-3 on plasma lipids in hemodialysis patients. Saudi J Kidney Dis Transpl. 2007;18:571–6. [PubMed] [Google Scholar]


