Abstract
Objective:
Considering the inability of neonates to swallow oral drugs in the form of solid tablets, the lack of appropriate dosage forms for infants, and the necessity to prepare some pills for neonates, the current study investigated dosage accuracy in drugs for neonates prepared from tablets by analyzing the concentrations of final products.
Methods:
Captopril and spironolactone, oral dosage forms that are not suitable for infants, were chosen as the drug model for this study. Demographic characteristics of nurses providing medications and tablet preparation methods were documented in a random observational method. To determine concentrations of final solutions, 120 drug samples (60 captopril and 60 spironolactone samples) prepared by Neonatal Intensive Care Unit nurses of the Children Cure and Health Hospital of Tabriz University of Medical Sciences were analyzed using high performance liquid chromatography (HPLC) and spectrophotometry.
Findings:
There was a significant error rate in the concentration of captopril in prepared solutions compared with the ordered dosage. No differences were observed in the demographic characteristics of the nurses and the method of preparation between the two drugs. The only difference related to the preparation technique was that in most cases (70.8%), one whole spironolactone tablet was used, whereas in around 50% of samples in captopril group, half or a quarter of one captopril tablet was utilized for the intended dosage (P = 0.009).
Conclusion:
This research suggests that the use of a whole tablet instead of a divided tablet in the manual preparation of medication dosage forms for neonates is the most appropriate approach.
Keywords: Dosage accuracy, medication error, neonatal intensive care, preparation
INTRODUCTION
Today, patient safety is a key concept in healthcare service supply systems, and it is considered a factor in patient survival.[1] Healthcare services are inevitably associated with some risks to patient safety and threats to patient health. Medication error is one relevant example of such a risk, and it threatens healthcare services supply systems.[2]
Medical errors are particularly frequent in Neonatal Intensive Care Units (NICUs),[3] increasing morbidity and mortality of newborns.[4] Neonates are vulnerable to dose errors because of their rapidly changing body surface area and weight, their fast-developing system of drug absorption, metabolism, and excretion, and because they are incapable of communicating with their drug provider; thus, giving drugs to neonates is a vitally important issue.[5,6] Given the high development costs of drugs for neonates (because of the small market size and limited return on investment) and the greater responsibility in producing drugs for this age group,[7,8] most of the drugs available for neonates are in doses and units that have been produced for children and adults. This necessitates calculation and dilution of drugs, forming a potential risk for further errors.[5] In a study by Kunac and Reith most medication errors in NICU occur during drug preparation.[9] Campino et al. found that 37.9% of errors occur during the preparation of intravenous medications.[10] Nurses play a key role in the drug preparation and delivery process.[11,12] Errors associated with medication delivery are usually committed by nurses themselves, and since medication is administered directly to the patients by nurses, there is no way to avoid this type of error; therefore, it is important to prevent it.
Various studies have been conducted on the type and extent of medication dosage errors in children and NICU wards.[2,5,12] Surveys are taken frequently to study the concentration of prepared medications in neonate units.[13,14] Nevertheless, all of these studies have focused on injection medications. According to a survey conducted by the authors of this study, no similar study exists on the preparation method and accuracy of medications for neonates. Considering the extensive use of captopril and spironolactone, these two drugs were chosen as models to conduct an experiment in order to evaluate the dosage preparation technique for infants and the accuracy of the final product prepared by nurses at the infant and NICU units at Tabriz Children's Hospital.
METHODS
This research study was approved by the Tabriz University Committee of Medical Ethics (No. 5.4.9592-January 13, 2013), as well as we carried out a prospective cross-sectional study from March to November 2014 on infants admitted in the neonate ward (23 beds) and the NICU (18 beds) of the Pediatric Teaching/University Hospital (236 beds) of Tabriz that the main referring hospital for children in North West of Iran. Data were collected through a two-part questionnaire, the first part of which determined the demographics of nurses preparing the drugs and taking care of the hospitalized neonates and the second part was related to specifications, medicine dosing technique, and documentation of drug analyses results. Validity of the codified questionnaire was examined before conducting the study and necessary adjustments were applied. A panel of health care professionals with extensive knowledge, understanding, and experience in infant caring reviewed the questionnaire for content validity (11 professionals including seven professors of nursing and midwifery, two neonatology specialist professors, and two professors of the pharmacy faculty) before the study and then their comments were applied in the questionnaire when necessary. We considered 35 nurses who have been employed for at least 6 months at the hospital, and was obtained informed consent from them as inclusion criteria. Participants in the study were free for withdraw at any stage of the investigation but none of them refused to take part in the study.
There were no standards/protocols provided to the nurses to ensure consistency among preparation of the two medications, so everybody prepared drug individually. Convenience sampling method was used in this study for obtaining samples from selective two perpetrated drugs (captopril and spironolactone). At first, after acquiring permission from the hospital, one of the researchers checked neonatal drug order prescribed by physicians in random days and then referred to the ward during the time of two drugs administration. After finishing preparation process of drug, the entire contents of the prepared drug was poured into a glass test tube with a cork top. The tube was marked with a corresponding code and then refrigerated in −20°C till concentration measurement. Finally, the nurse was asked to complete the related questionnaire.
Preparation technique of drugs by nurses
The most commonly used method was grinding the drug inside the syringe (the nurse removes the plunger from the syringe to which the needle is connected. She puts the tablet inside the barrel and returns the plunger into the barrel. By pushing the plunger to the end, she then crushes the tablet and drags the solvent by pulling the plunger back). In 100% of cases, no one had used pounder for grinding. Moreover, sterilized distilled water was used as the solvent in all cases.
Sampling
Samples were collected over the course of 2 months and refrigerated in −20°C until the time of laboratory analysis, which was carried out immediately upon completion of sample collection. All medication samples prepared (n = 120) by 45 nurses, were collected with his/her permission in the morning, evening, and night shifts. Drugs were analyzed by a researcher from the Drug Applied Research Center of Tabriz University of Medical Sciences.
Quantitative determination of spironolactone by ultraviolet spectrophotometer
Initially, a 10 μg/ml of spironolactone (pure compound provided from the PARSDAROU CO., Tehran, Iran) was prepared with ethanol (99%, Merck, Germany) as its solvent. Its absorption was scanned at 200–400 nm wavelengths, and the maximum absorption wavelength was found to be 240 nm. Afterward, at least 6 certain concentrations were prepared and their ultraviolet (UV) (Shimadzu UV 160, Japan) absorption rates were calculated. Consequently, the calibration diagram was obtained by drawing concentrations versus absorption curve.
Afterward, the volume of each sample was determined using a pipette. The contents were transferred into a falcon tube and diluted with ethanol 10-fold to 1000-fold, depending on the sample's absorption. Diluted solutions were first put into a mixer (IKA-Werk-Germany) for 10 min, and as soon as good solutions were obtained, they were transferred to a centrifuge. The solution staying on the top was then transferred into a UV cell, and the absorption rate of the drug concentration was determined at 240 nm. Finally, by employing the calibration curve of the absorption rates, the quantity of medication was determined.
Quantitative determination of captopril by high-performance liquid chromatography (HPLC)
An HPLC system (Knauer, Germany) with a sensitive variable wavelength UV spectrophotometric detector, set at 220 nm, was used to assay captopril. A Eurospher column (C18, 5 μm, 4.6 × 150 mm) and a mobile phase consisted of methanol and double distilled water containing phosphoric acid (33:67, v/v %) at flow rate of 1.5 ml/min, were used for separation. The mobile phase was filtered through a 0.45 μm cellulose acetate filter under vacuum and degassed by ultrasonication. A total of 20 μl of sample was injected into the HPLC column. The retention time was 2.4 min and the calibration curve was linear in the range of 10–200 μg/ml (r2 > 0.997). No interference from the tablet formulation components was observed. The samples were also found to be stable during the study period.
Statistical analysis
Chi-square and student's t-tests were utilized to compare qualitative and quantitative data, respectively, in both groups. The difference between expected and reported dosages was calculated. Accuracy in the prepared dose was considered equivalent to a lack of significant difference between the reported dose and expected dose. It means that the average difference between each data value and the hypothesized test value should be close to 0. For statistical comparison, the difference was determined as 0. Dose difference was analyzed by one-sample t-test, and the Spearman test was employed to study the meaningful relationship between the presence or lack of error in the preparation of drugs and the demographic parameters of the nurses. In this test, a validity level of P < 0.05 was considered significant. Due to the huge differences between reported data from the laboratory perhaps because of presence of measurement error in two of the reported cases, their corresponding samples were discarded from the data pool, and the analysis was performed using 118 samples (59 samples of spironolactone and 59 samples of captopril). Data for this research were analyzed using? SPSS 13 software (SPSS Inc., Chicago, USA).
RESULTS
The average ordered drug doses and laboratory-reported drug dosages for both spironolactone and captopril are presented in Table 1. The average ordered drug doses for spironolactone and captopril were 1.68 ± 0.58 mg and 1.12 ± 0.402 mg, respectively. Furthermore, the laboratory-reported dosages for spironolactone and captopril were 1.45 ± 1.56 mg and 0.77 ± 0.52 mg, respectively [Table 1].
Table 1.
Differences of final prepared doses from the ordered doses of two evaluated drugs
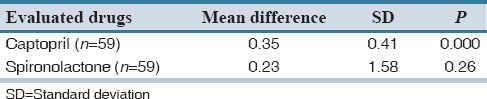
Table 1 showed that the difference between the final prepared dosage (laboratory-reported dosages) and the expected (ordered) doses of two evaluated drugs is not statistically significant for spironolactone (P = 0.26), whereas this difference is significant in the case of captopril (P = 0.001). The above result reveals that error is committed in the medication of captopril.
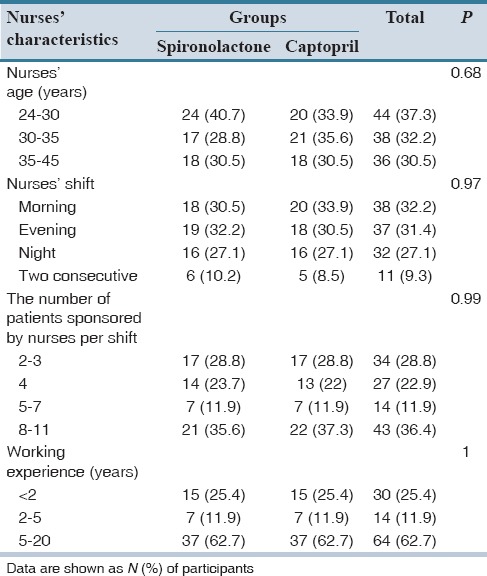
Demographic characteristics of the nurses are given in Table 2. All of the studied nurses were females with a BA degree in nursing, and all worked on a rotating shift basis. Most of the nurses (62.7%) had more than 5 years’ experience. The results shown in Table 2 indicate that there are no significant differences between the two groups of spironolactone and captopril in view of demographic characteristics of nurses, including age of the nurse, number of patients per shift as marker of workload, and work experience.
Table 2.
Demographic and social characteristics of nurses, based on the type of the prepared drug
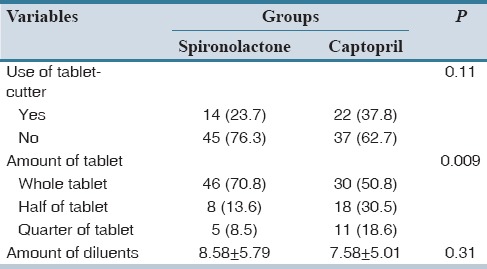
Data on the effective variables relevant to the preparation method of spironolactone and captopril are presented in Table 3. Majority of the nurses (76.3% and 62.7% in spironolactone and captopril group, respectively) did not use tablet-cutter. Average amount of diluents added (ml) were 8.6 cc for spironolactone and 7.6 cc for captopril (P = 0.31). The approach of nurses in dividing or using whole tablet for dosing preparation had a significant effect on dosing accuracy. About 70.8% and 13.5% of nurses used whole and half tablet of spironolactone, respectively, but for captopril 50.8% and 30.5% of whole and half amount of tablet was used, respectively, for drug dosing preparation (P = 0.009).
Table 3.
Items considered in preparation method with respect to the type of drug
DISCUSSION
The current methodology was adopted because routine studies on the analysis of medication errors and the accuracy of prepared medications were not feasible due to their incompetence in measuring the concentration of the finally prepared medication. In the current study, to investigate the preparation accuracy of the ordered dose in NICUs, the prepared drug concentrations were laboratory-analyzed, the results of which revealed a significant rate of error in preparing one of the studied medications.
Our findings are consistent with earlier studies that have highlighted the most frequent medication errors identified as errors committed during drug preparation. In a study by Kunac and Reith, the most frequent medication errors were identified as errors committed during drug preparation.[9] Campino et al. reported a 37.9% rate of error in the preparation of intravenously injected medications.[10]
Interestingly, this study showed that drug administration in the form of a whole tablet compared with dividing the tablet for dosage preparation significantly increased dose accuracy. This finding raises the debate of content uniformity of medication forms. It seems that the use of nondivided tablets for the preparation of the desired dosage for a neonate may eliminate the chance that variations in tablet content uniformity might affect the final prepared dosage.
In a study by van Riet-Nales et al., it was demonstrated that splitting the tablet caused significant inaccuracies in dosages delivered to patients.[15]
Content uniformity is specifically important from an industrial pharmaceutics point of view, and several protocols have been codified to control it.[16] As smaller doses are selected from main medication forms to prepare a given dose for neonates (e.g., 1 mg from 25 g spironolactone tablet), a little variation in the effective dose content of the divided tablet may cause a significant difference in the dose prepared for infants. Based on our results, in practice, it may be suggested that the need to split a tablet should be discouraged as much as possible.
It appears that medication preparation methods have significant effects on the accuracy of prepared dosages.[14] The current study attempted to determine which dosage preparation methods can lead to medication errors. Workforce related errors, such as amount of experience, work shift, and high work pressure of the nurses preparing the doses were rejected in the statistical analysis as operative parameters. To investigate the validity of morphine infusion preparation in the NICU, Parshuram et al. measured the morphine concentration of the prepared dosage and demonstrated that about two-thirds of the products were beyond the standard.[13] In this study, no significant difference in dosage preparation error rate was noted between day and night shifts. Similar to our study results that revealed no significant effect attributable to work experience, Parshuram et al. found no effect attributable to experience or to time and location of the error produced in the preparation of infusion medications.[13]
Despite the researchers’ preliminary belief that spironolactone had a greater likelihood for dosage preparation errors because of its limited solubility in water and the impossibility of preparing homogeneous stock solution, during statistical analysis, captopril demonstrated more errors. In this regard, the type, volume, and physicochemical characteristics of the solvent cannot be classified among the reasons behind dose preparation errors. These results may indicate that using or not using the tablet-cutter and the quantity of solvent utilized for thinning have no meaningful effects on the dosage accuracy of spironolactone and captopril.
Dehmel et al. compared the reliability of medication concentrations prepared in the central pharmacy of the hospital through mechanized pharmaceutical procedures to dose preparation by routine nursing method in ICUs. Concentration errors in three medications, namely amiodarone, noradrenaline, and hydrocortisone were investigated by measuring the drug contents in the finally prepared medication. The researchers showed that the solutions prepared in the central pharmacy involved less concentration errors.[17] Dosage preparation for neonates from commercially available adult dosage forms in pharmacy under pharmacist observation and using pharmaceutical techniques seems to be appropriate approaches, in order to reduce medication error.
Attendance of researcher and collection of the medication prepared by the nurse may affect nursing performance in dosage preparation. We just selected two drugs as models for our study then its extrapolation to other oral dosage forms may be questionable.
This research suggests that using a whole tablet instead of a divided tablet in manually preparing medication dosage forms for neonates is a more appropriate approach.
AUTHORS’ CONTRIBUTION
SV, drafted the manuscript, and participated in acquisition of data and data analysis. HH, participated in drafting the manuscript and Lab analysis. HH, revised manuscript critically and has given final approval of version to publish. MA, participated in drafting the manuscript. MR, participated in Acquisition of data. All authors read and approved the final manuscript. The authors alone are responsible for the content and writing of the paper
Footnotes
Financial support and sponsorship Nil.
Conflict of Interest There are no conflicts of interest.
REFERENCES
- 1.Benjamin DM. Reducing medication errors and increasing patient safety: Case studies in clinical pharmacology. J Clin Pharmacol. 2003;43:768–83. [PubMed] [Google Scholar]
- 2.Jain S, Basu S, Parmar VR. Medication errors in neonates admitted in intensive care unit and emergency department. Indian J Med Sci. 2009;63:145–51. [PubMed] [Google Scholar]
- 3.Chedoe I, Molendijk HA, Dittrich ST, Jansman FG, Harting JW, Brouwers JR, et al. Incidence and nature of medication errors in neonatal intensive care with strategies to improve safety: A review of the current literature. Drug Saf. 2007;30:503–13. doi: 10.2165/00002018-200730060-00004. [DOI] [PubMed] [Google Scholar]
- 4.Suresh G, Horbar JD, Plsek P, Gray J, Edwards WH, Shiono PH, et al. Voluntary anonymous reporting of medical errors for neonatal intensive care. Pediatrics. 2004;113:1609–18. doi: 10.1542/peds.113.6.1609. [DOI] [PubMed] [Google Scholar]
- 5.Stavroudis TA, Miller MR, Lehmann CU. Medication errors in neonates. Clin Perinatol. 2008;35:141–61, ix. doi: 10.1016/j.clp.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Ligi I, Arnaud F, Jouve E, Tardieu S, Sambuc R, Simeoni U. Iatrogenic events in admitted neonates: A prospective cohort study. Lancet. 2008;371:404–10. doi: 10.1016/S0140-6736(08)60204-4. [DOI] [PubMed] [Google Scholar]
- 7.Patel VP, Chavda BG, Katira RM. Extemporaneous dosage form for oral liquid. Pharmacophore. 2011;2:86–103. [Google Scholar]
- 8.Freed AL, Silbering SB, Kolodsick KJ, Rossi DT, Mahjour M, Kingsmill CA. The development and stability assessment of extemporaneous pediatric formulations of Accupril. Int J Pharm. 2005;304:135–44. doi: 10.1016/j.ijpharm.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Kunac DL, Reith DM. Identification of priorities for medication safety in neonatal intensive care. Drug Saf. 2005;28:251–61. doi: 10.2165/00002018-200528030-00006. [DOI] [PubMed] [Google Scholar]
- 10.Campino A, Santesteban E, Garcia M, Rueda M, Valls-I-Soler A. Intravenous drug preparation errors in a Neonatal Intensive Care Unit. A potential source of adverse events. An Pediatr (Barc) 2013;79:21–5. doi: 10.1016/j.anpedi.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Ross LM, Wallace J, Paton JY. Medication errors in a paediatric teaching hospital in the UK: Five years operational experience. Arch Dis Child. 2000;83:492–7. doi: 10.1136/adc.83.6.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raju TN, Kecskes S, Thornton JP, Perry M, Feldman S. Medication errors in neonatal and paediatric intensive-care units. Lancet. 1989;2:374–6. doi: 10.1016/s0140-6736(89)90548-5. [DOI] [PubMed] [Google Scholar]
- 13.Parshuram CS, Ng GY, Ho TK, Klein J, Moore AM, Bohn D, et al. Discrepancies between ordered and delivered concentrations of opiate infusions in critical care. Crit Care Med. 2003;31:2483–7. doi: 10.1097/01.CCM.0000089638.83803.B2. [DOI] [PubMed] [Google Scholar]
- 14.Parshuram CS, To T, Seto W, Trope A, Koren G, Laupacis A. Systematic evaluation of errors occurring during the preparation of intravenous medication. CMAJ. 2008;178:42–8. doi: 10.1503/cmaj.061743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Riet-Nales DA, Doeve ME, Nicia AE, Teerenstra S, Notenboom K, Hekster YA, et al. The accuracy, precision and sustainability of different techniques for tablet subdivision: Breaking by hand and the use of tablet splitters or a kitchen knife. Int J Pharm. 2014;466:44–51. doi: 10.1016/j.ijpharm.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 16.Center for Drug Evaluation and Research (CDER) Tablet Scoring: Nomenclature, Labeling, and Data for Evaluation. CMC; 2013. [Last accessed on Feb 2015]. U.S. Department of Health and Human Services Food and Drug Administration Center for Evaluation and Research. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm269921 . [Google Scholar]
- 17.Dehmel C, Braune SA, Kreymann G, Baehr M, Langebrake C, Hilgarth H, et al. Do centrally pre-prepared solutions achieve more reliable drug concentrations than solutions prepared on the ward? Intensive Care Med. 2011;37:1311–6. doi: 10.1007/s00134-011-2230-4. [DOI] [PubMed] [Google Scholar]


