Abstract
While Staphylococcus aureus accelerates human neutrophil cell death, the underlying host- and pathogen-derived mechanisms remain incompletely defined. Previous studies demonstrated that the S. aureus SaeR/S sensory system is essential for pathogen survival following neutrophil phagocytosis. Herein, we demonstrate that the SaeR/S system promoted accelerated cell death, suppressed phosphorylation of nuclear factor-κB, and reduced interleukin-8 (IL-8) production in human neutrophils. Treatment of neutrophils with recombinant IL-8 significantly reduced bacterial burden and apoptosis. Our findings demonstrate a mechanism by which S. aureus suppresses the early neutrophil-derived IL-8 response to disrupt cell fate and promote disease.
Keywords: cell fate, IL-8, neutrophils, NF-κB, pathogenesis, SaeR/S, Staphylococcus aureus
Staphylococcus aureus is a common pathogen that can infect immunocompromised as well as healthy individuals. Human polymorphonuclear leukocytes (PMNs or neutrophils) are the first line of defense against S. aureus. It follows that the ability of S. aureus to initiate infection includes survival after PMN phagocytosis and alteration of PMN cell fate [1–3]. Although it has been shown that S. aureus alters neutrophil lifespan by inducing rapid neutrophil death (referred to as programmed necrosis or necroptosis), the host and pathogen mechanisms associated with this phenotype have not been fully elucidated [2, 3]. To advance our understanding of how S. aureus influences PMN cell fate to promote disease, we investigated the role of the SaeR/S 2-component system (TCS) in PMN cell death.
The SaeR/S TCS regulates production of virulence factors that promote evasion and destruction of neutrophils [1, 4, 5]. Moreover, neutrophil components, including α-defensin, trigger activation of the SaeR/S TCS and induce a transcriptional response tailored for its environment [6]. In this study, we demonstrate that the SaeR/S TCS contributes to accelerated neutrophil cell death via decreased nuclear factor-κB (NF-κB) phosphorylation and reduced interleukin-8 (IL-8) production by human neutrophils. Furthermore, we identified IL-8 to be an essential neutrophil-derived cytokine that can prolong PMN survival and promote bacterial clearance during staphylococcal disease.
MATERIALS AND METHODS
Bacteria Cultures
USA300, USA400, and isogenic USA300ΔsaeR/S (ΔsaeR/S) and USA400ΔsaeR/S mutant strains were previously generated [4, 7] and prepared for use in human PMN assays as described in [7].
Human PMN Isolation
Human neutrophils were isolated from heparinized venous blood obtained from healthy donors [7]. Purity (less than 1% peripheral blood mononuclear cell [PBMC] contamination) and viability were assessed via flow cytometry (fluorescence-activated cell sorter [FACS], FACSCalibur, BD Biosciences). PMNs (1 × 106) were plated onto serum-coated 96- or 24-well plates and exposed to 2 × 106 (low multiplicity of infection [MOI], 2:1) or 1 × 107 (high MOI, 10:1) colony forming units (CFUs) of USA300 or ΔsaeR/S and phagocytosis was synchronized [7].
Cell Death Assays
At designated time points, PMNs exposed to USA300 or ΔsaeR/S were subjected to Annexin V (Trevigen) and propidium iodide (PI) or nuclear condensation or terminal deoxynucleotidyltransferase dUTP nick-end labeling (TUNEL) assays as described [8]. PMN lysis was quantified using lactate dehydrogenase (LDH) assays and calculated as percent lysis compared to maximum LDH release (USA300 + PMNs at 10:1 ratio) (Promega, [1]). CaspGLOW assays (eBioscience) were used to analyze PMN caspase activity via FACS per manufacturer's suggestion.
NF-κB Inactivation and IL-8 Assays
Neutrophils (1 × 106) were plated in duplicate and incubated with 0.2% dimethyl sulfoxide (DMSO) or 20 µM parthenolide (Sigma) in DMSO for 30 minutes at 37°C and 5% CO2. USA300 or ΔsaeR/S were added (low MOI) and phagocytosis was synchronized [7]. At designated time points, samples were either subjected to nuclear condensation or TUNEL assays described above or used to determine PMN bactericidal activity (CFUs enumerated as described in [7]). To determine the role of IL-8, neutrophils were treated with recombinant IL-8 (25 ng/mL, R&D) or with anti-IL-8 (1 µg/mL, R&D) and isotype (1 µg/mL, R&D) 30 minutes prior to bacterial treatment. At 6 hours postinfection, nuclear condensation of PMNs was assessed along with bacterial survival.
Intracellular Detection of Phosphorylated NF-κB p65 Subunit and IL-8
Heparinized whole blood was treated with Roswell Park Memorial Institute (RPMI) medium or RPMI medium containing 1 × 107 CFUs USA300 or ΔsaeR/S and incubated with end-over-end mixing (20 rpm, 37°C with 5% CO2) for 5, 60, and 120 minutes. Using BD Biosciences whole-blood intracellular stain kit, samples were stained with anti-CD11b (BD Biosciences) or isotype control for 15 minutes at room temperature (RT). The optimal intracellular stain with anti-NF-κB-p65(P) (eBioscience) or corresponding isotype control was achieved after a 30 minute-incubation at RT. PMNs were determined by forward scatter/side scatter profile and high CD11b expression. For IL-8 detection, at 3 and 6 hours postexposure, infected PMNs or PMNs in media were stained with anti-CD11b or isotype control, fixed, and permeabilized per manufacture's recommendation (eBioscience). Additionally, cells were incubated with anti-IL-8 or corresponding isotope control (BD biosciences) for 30 minutes and analyzed by FACS.
Cytokine Assays
Supernatants from PMNs (1 × 106) or PBMCs (1 × 104) subjected to USA300 or ΔsaeR/S (low MOI) were collected and sterile filtered at 6 and 18 hours. Procarta assays (Affymetrix) were used to detect interferon-γ (IFN-γ), IL-6, macrophage inflammatory protein-1α (MIP-1α), IL-1α, IL-1β, IL-8, vascular endothelial growth factor (VEGF), and tumor necrosis factor-α (TNF-α). The samples were read in duplicate using a Luminex cytometer (Bio-Rad). Additionally, supernatants from PMNs (1 × 106) or PBMCs (1 × 104) subjected to USA300 or ΔsaeR/S (low MOI) were collected and sterile filtered at 6 and 18 hours and assayed in duplicate using R&D Quantikine enzyme-linked immunosorbent assay (ELISA) for IL-8 and VEGF.
Study Approval
Healthy blood donors were informed and gave written consent prior to participation. All studies were conducted in accordance with a protocol approved by the Institutional Review Board for Human Subjects at Montana State University.
RESULTS AND DISCUSSION
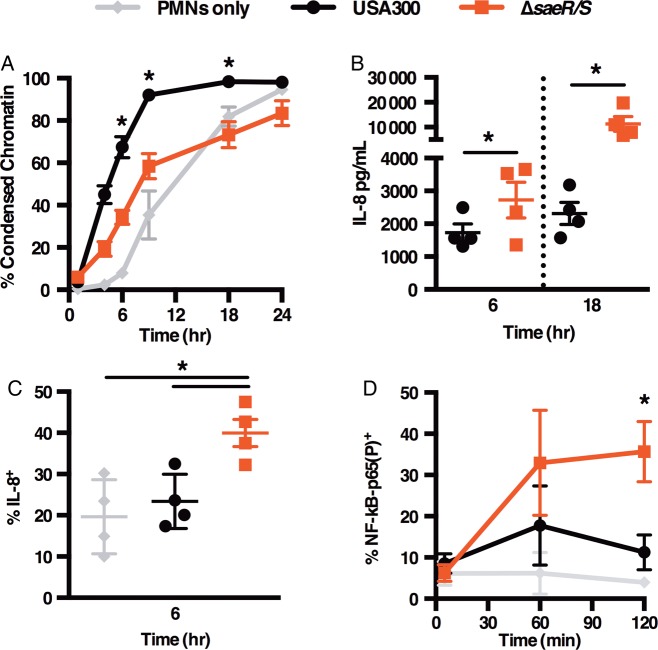
To test the hypothesis that the SaeR/S TCS influences PMN cell fate, we subjected human PMNs to USA300 or USA300ΔsaeR/S (ΔsaeR/S) strains at low MOI and measured cellular features associated with programmed cell death (Figure 1A, Supplementary Figure 1A–D). Consistent with published observations [3], USA300 promoted significant exposure of phosphatidylserine and plasma membrane damage within 6 hours of interaction (Supplementary Figure 1A and B). By 9 hours, the majority of USA300-treated cells were undergoing late apoptosis and/or necrosis, depicted by condensed nuclei (Figure 1A), fragmented DNA (Supplementary Figure 1C), and nearly complete PMN lysis (Supplementary Figure 1D). The aforementioned apoptotic features were significantly reduced in neutrophils infected with the ΔsaeR/S mutant (Figure 1A and Supplementary Figure 1A–D), demonstrating that SaeR/S contributes to accelerated neutrophil death following S. aureus phagocytosis.
Figure 1.
SaeR/S alters cell fate and suppresses IL-8 production in neutrophils. PMNs were subjected to USA300 or ΔsaeR/S at low MOI. Percent PMNs with condensed chromatin assessed via FACS at designated times (A). IL-8 release from PMNs assessed by ELISA (B) and intracellular IL-8 measured in PMNs at 6 hours postinfection via FACS (C). Whole blood was treated with USA300 or ΔsaeR/S and percent NF-κB-p65(P)+ cells from CD11bHi+ population was quantified by FACS at designated time points (D). Data shown are the means ± SEM of 4–10 experiments (A) or 3–4 experiments (B–D). *P < .05 determined by 1-way ANOVA with Tukey's posttest (A, C, and D) comparing USA300 to ΔsaeR/S groups or paired t test (B). Abbreviations: ANOVA, analysis of variance; ELISA, enzyme-linked immunosorbent assay; FACS, fluorescence-activated cell sorter; IL-8, interleukin-8; MOI, multiplicity of infection; nuclear factor-κB, NF-κB; NF-κB-p65(P), phosphorylated NF-κB p65 subunit; PMNs, polymorphonuclear leukocytes; SEM, standard error of the mean.
To determine if SaeR/S interferes with caspase activation, reported to be inhibited by USA300 [2], we measured active caspases in PMNs following interaction with USA300 and ΔsaeR/S at high MOI (Supplementary Figure 1E). The substantial decrease in caspase activity in PMNs exposed to USA300 as opposed to ΔsaeR/S demonstrated that caspase inhibition is dependent on SaeR/S-regulated factors. At low MOI, however, caspases were neither activated by USA300 nor ΔsaeR/S mutant (Supplementary Figure 1F). Given these observations, we aimed to identify additional host-derived factors that contribute to SaeR/S-mediated cell death.
Because cytokines play an essential role in modulating neutrophil lifespan [9] and because the SaeR/S system has been shown to influence cytokine levels in whole blood and murine models of infection [5], we examined the influence of SaeR/S on secreted cytokine levels in PMNs during infection with USA300 and ΔsaeR/S at 6 and 18 hours. We identified cytokines predominantly made by neutrophils by comparing cytokine profiles in purified PMNs to PBMCs representative of the contamination in the cell preparation (1 × 104 PBMCs was equivalent to <1.0% contamination in PMN preparations). Based on the cytokine survey, IFN-γ, IL-6, MIP-1α, IL-1α, IL-1β, and TNF-α were mainly produced by PBMCs (data not shown). However, IL-8 and VEGF were neutrophil-derived and, therefore, examined in subsequent experiments. At 6 and 18 hours, USA300-treated PMNs produced significantly less IL-8 than the ΔsaeR/S group (Figure 1B). VEGF levels were higher in USA300-infected PMNs than the ΔsaeR/S group at 6 hours and similar between the 2 groups at 18 hours (Supplementary Figure 2A); thus, reduced IL-8 during USA300 infection was not a result of a general protein synthesis decline.
To further demonstrate that PMNs were the source of IL-8, we measured intracellular IL-8 in PMNs by FACS. Infection with the ΔsaeR/S mutant promoted a significant increase of IL-8 expressing PMNs in contrast to PMNs only at 3 hours (Supplementary Figure 2B) and when compared to both PMNs only and USA300-treated PMNs at 6 hours postexposure (Figure 1C). This trend was also observed during USA400 and USA400ΔsaeR/S infection (Supplementary Figure 2C), confirming the SaeR/S-dependent inhibition of PMN-derived IL-8. Last, we assessed SaeR/S system's global impact on IL-8 release in whole blood and observed a significant decrease in IL-8 released in serum samples from the USA300-treated group when compared to the ΔsaeR/S-treated blood at 3 hours postinfection (Supplementary Figure 2D). VEGF levels were similar between both groups, further highlighting the specificity of SaeR/S-dependent IL-8 suppression (Supplementary Figure 2E).
IL-8 is an essential chemokine that promotes neutrophil recruitment, antimicrobial functions, and prolonged survival in vitro [9] and during infection with pathogenic microorganisms, including Anaplasma phagocytophilum, Chlamydophila pneumoniae, and Paracoccidioides brasiliensis [10, 11]. Given that USA300 does not promote de novo IL-8 transcription [3], we wanted to determine if IL-8 reduction during USA300 infection is associated with SaeR/S-dependent modulation of NF-κB activity. To test this, we employed FACS to examine phosphorylation of the phosphorylated NF-κB p65 subunit (NFκB-p65[P]) in PMNs from human blood infected with USA300 or ΔsaeR/S. At 2 hours, the percentage of PMNs with NF-κB-p65(P) was significantly reduced following interaction with USA300 when compared to ΔsaeR/S-infected cells (Figure 1D).
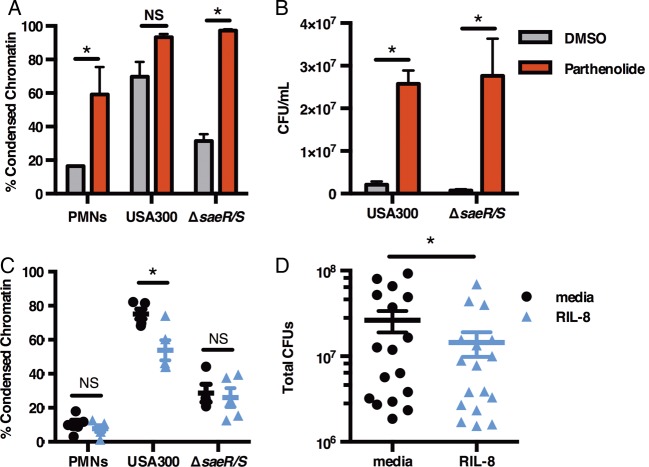
Our observation that S. aureus suppresses NF-κB is supported by published data linking NF-κB inhibition to accelerated cell death following infection with Escherichia coli, Yersinia pestis, and Salmonella typhimurium [12]. Given NF-κB's regulation of antiapoptotic factors such as myeloid cell leukemia-1 (Mcl-1) and caspase inhibitors that can extend cell lifespan [11], we wanted to determine whether functional NF-κB is necessary for neutrophil survival during infection with S. aureus. To test this, we measured nuclear condensation and DNA fragmentation in PMNs pretreated with the NF-κB inhibitor parthenolide. PMN lifespan was shortened significantly in ΔsaeR/S-infected PMNs following parthenolide pretreatment compared to untreated neutrophils infected with ΔsaeR/S (Figure 2A, confirmed by TUNEL shown in Supplementary Figure 3A). Notably, cell death of parthenolide-treated PMNs infected with USA300 did not increase significantly when compared to the USA300 group without treatment underpinning the intrinsic ability of USA300 to inhibit NF-κB in PMNs (Figure 2A). NF-κB involvement in spontaneous PMN apoptosis [13] was confirmed by PMNs that underwent accelerated death in the presence of parthenolide (Figure 2A). Using LDH assays, we showed that the observed effect of parthenolide was not due to cell poisoning (Supplementary Figure 3B). Parthenolide treatment also significantly (P < .05 determined by t test, n = 3) abolished IL-8 release by 95% in USA300-infected PMNs (DMSO 1426 ± 94 pg/mL vs parthenolide 75 ± 39 pg/mL), but did not alter VEGF production (DMSO 214 ± 52 pg/mL vs parthenolide 215 ± 21 pg/mL), further suggesting that NF-κB inhibition was specific.
Figure 2.
IL-8 promotes neutrophil survival and bacterial clearance. PMNs treated with 20 µM parthenolide or DMSO control were infected with USA300 or ΔsaeR/S at low MOI for 6 hours followed by analysis of PMNs with condensed chromatin (A) and bacterial burden (B). In C–D, PMNs were exposed to RIL-8 (25 ng/mL) and infected with USA300 or ΔsaeR/S for 6 hours followed by FACS analysis of condensed chromatin (C) or assessment of USA300 survival. D, Data shown are the means ± SEM of 3–5 separate experiments. *P < .05 determined by 1-way ANOVA with Tukey's posttest (A–C) or by paired t test (D) comparing USA300 to ΔsaeR/S groups. Abbreviations: ANOVA, analysis of variance; CFU, colony-forming unit; DMSO, dimethyl sulfoxide; FACS, fluorescence-activated cell sorter; IL-8, interleukin-8; MOI, multiplicity of infection; NS, not significant; PMNs, polymorphonuclear leukocytes; RIL-8, recombinant IL-8; SEM, standard error of the mean.
Finally, bacterial survival of both USA300 and ΔsaeR/S was significantly increased in PMNs exposed to parthenolide (Figure 2B), while parthenolide alone did not promote bacterial growth (data not shown). The observation that inhibition of NF-κB in ΔsaeR/S-treated neutrophils resulted in accelerated cell death and bacterial survival similar to USA300-treated neutrophils emphasizes the importance of the PMN response to resolution of S. aureus infection. Herein, we demonstrate that the SaeR/S TCS reduced phosphorylation of NF-κB to promote evasion of PMN killing. Our results are supported by skin infection models where lack of MyD88 protein, essential to NF-κB activation, led to larger abscess formation and S. aureus survival [14]. Also, patients with NF-κB signaling deficiencies are highly susceptible to staphylococcal infections [15], further emphasizing the importance of NF-κB-dependent inflammation during staphylococcal disease.
To determine the role of IL-8 during S. aureus–neutrophil interaction, PMNs were replenished with recombinant IL-8 (RIL-8). The percentage of USA300-treated PMNs undergoing cell death was significantly reduced when compared to PMNs exposed to USA300 without treatment (Figure 2C). IL-8 can inhibit PMN apoptosis via the PI3K/Akt pathway, leading to subsequent activation of NF-κB and antiapoptotic Mcl-1 production, needed for neutrophil survival [10]. Therefore, future studies are necessary to define the precise mechanism of how SaeR/S-derived IL-8 alters PMN cell fate. In correlation with improved neutrophil survival, RIL-8 significantly enhanced PMN bactericidal activity against USA300, demonstrated by a significant decrease in USA300 survival (Figure 2D). Finally, the importance of IL-8 in bacterial clearance was underscored by increased USA300 burden in PMNs treated with neutralizing IL-8 antibody (Supplementary Figure 3C).
Taken together, our findings identify a mechanism employed by S. aureus to evade human neutrophils by which the SaeR/S TCS disrupts PMN cell fate via suppression of NF-κB and IL-8 production to promote bacterial survival. We propose that both timing and magnitude of inflammation in PMNs play major roles in dictating the outcome of staphylococcal disease and that alteration in the innate ability of PMNs to produce IL-8 may increase susceptibility to S. aureus infections.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported by the National Institutes of Health (NIH) grants (NIH-PAR98-072, NIH-RR020185, NIH-R01 award A1090046-01), and an American Heart Association Fellowship (13PRE16850054) to O. W. Z.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Voyich JM, Vuong C, DeWald M, et al. The SaeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus. J Infect Dis 2009; 199:1698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenlee-Wacker MC, Rigby KM, Kobayashi SD, Porter AR, DeLeo FR, Nauseef WM. Phagocytosis of Staphylococcus aureus by human neutrophils prevents macrophage efferocytosis and induces programmed necrosis. J Immunol 2014; 192:4709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi SD, Braughton KR, Palazzolo-Ballance AM, et al. Rapid neutrophil destruction following phagocytosis of Staphylococcus aureus. J Innate Immun 2010; 2:560–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nygaard TK, Pallister KB, Ruzevich P, Griffith S, Vuong C, Voyich JM. SaeR binds a consensus sequence within virulence gene Ppomoters to advance USA300 pathogenesis. J Infect Dis 2010; 201:241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watkins RL, Pallister KB, Voyich JM. The SaeR/S gene regulatory system Iiduces a pro-inflammatory cytokine response during Staphylococcus aureus infection. PLOS One 2011; 6:e19939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zurek OW, Nygaard TK, Watkins RL, et al. The role of innate immunity in promoting SaeR/S-mediated virulence in Staphylococcus aureus. J Innate Immun 2014; 6:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voyich JM, Braughton KR, Sturdevant DE, et al. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol 2005; 175:3907–19. [DOI] [PubMed] [Google Scholar]
- 8.Voyich JM, DeLeo FR. Host-pathogen interactions: leukocyte phagocytosis and associated sequelae. Methods Cell Sci 2002; 24:79–90. [DOI] [PubMed] [Google Scholar]
- 9.Kettritz R, Gaido ML, Haller H, Luft FC, Jennette CJ, Falk RJ. Interleukin-8 delays spontaneous and tumor necrosis factor-alpha-mediated apoptosis of human neutrophils. Kidney Int 1998; 53:84–91. [DOI] [PubMed] [Google Scholar]
- 10.Sarkar A, Hellberg L, Bhattacharyya A, et al. Infection with Anaplasma phagocytophilum activates the phosphatidylinositol 3-Kinase/Akt and NF-kB survival pathways in neutrophil granulocytes. Infect Immun 2012; 80:1615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCracken JM, Allen LA. Regulation of human neutrophil apoptosis and lifespan in health and disease. JCD 2014; 7:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neish AS, Naumann M. Microbial-induced immunomodulation by targeting the NF-κB system. Trends Microbiol. Elsevier Ltd 2011; 19:596–605. [DOI] [PubMed] [Google Scholar]
- 13.Ward C. NF-kappa B activation is a critical regulator of human granulocyte apoptosis in vitro. J Biol Chem 1999; 274:4309–18. [DOI] [PubMed] [Google Scholar]
- 14.Miller LS, O'Connell RM, Gutierrez MA, et al. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity 2006; 24:79–91. [DOI] [PubMed] [Google Scholar]
- 15.Picard C, Casanova JL, Puel A. Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IkB deficiency. Clin Microbiol Rev 2011; 24:490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.