Abstract
Vaginal shedding of cytomegalovirus (CMV) DNA was determined longitudinally among 96 women coinfected with human immunodeficiency virus (HIV), herpes simplex virus 2, and CMV starting antiretroviral therapy (ART) during a placebo-controlled trial of HSV-2 suppression with acyclovir in Rakai, Uganda. Vaginal CMV was detected in 75 of 96 women (78.0%) and 379 of 1080 individual visits (35.1%). ART status, higher HIV RNA viral load before ART initiation, and younger age were significantly associated with increased frequency of CMV shedding (P < .01). Compared to pre-ART, CMV shedding peaked from month 2 to month 4 after ART initiation, suggesting possible immune reconstitution inflammatory syndrome. Further studies need to determine the clinical significance of asymptomatic CMV shedding.
Keywords: acyclovir, antiretroviral therapy (ART), cytomegalovirus (CMV), human immunodeficiency virus (HIV), immune reconstitution inflammatory syndrome (IRIS), reactivation, Uganda
Asymptomatic cytomegalovirus (CMV) replication occurs frequently in the genital tract of human immunodeficiency virus (HIV)–infected men and is associated with increased genital shedding of HIV RNA and HIV transmission [1], upregulation of CCR5 expression [2], increased markers of T-cell activation, proliferation and exhaustion, as well as greater levels of HIV DNA [3]. Fewer data are available about the frequency of CMV shedding in the genital tract of HIV-infected women, or about factors affecting vaginal CMV shedding. Two studies of HIV-infected women conducted in the United States with partial uptake of antiretroviral therapy (ART) found that CMV DNA was detected infrequently (3%–7%) in cervicovaginal lavage [4, 5]. In a study of HIV-infected Kenyan women not on ART, CMV was detected in 59% of provider-collected cervical swabs [6]. Studies of HIV-uninfected women from India and Italy detected CMV in 26% and 66% of provider-collected cervical swabs, respectively [7, 8]. These data suggest variability and regional differences in CMV shedding from female genital sites.
Several clinical trials have investigated herpes simplex virus type 2 (HSV-2) genital shedding and its association with HIV-1. For example, treatment of HIV/HSV-2–coinfected individuals with acyclovir has been shown to slow HIV disease progression [9]. However, randomized trials with acyclovir did not significantly decrease HIV transmission or acquisition despite reducing levels of HIV RNA in blood and genital secretions [10]. A randomized trial of HSV-2 suppression in Rakai, Uganda, showed a significant increase in HSV-2 shedding and genital ulcer disease after initiation of ART in a cohort of chronically HIV-infected women, possibly as part of immune reconstitution inflammatory syndrome (IRIS) [9, 11]. In this study, we analyzed the prevalence, quantity, and factors associated with CMV vaginal shedding among women seropositive for HIV, HSV-2, and CMV from the HSV-2 suppression trial in Uganda.
METHODS
Study Participant Characteristics
HIV- and HSV-2–coinfected individuals aged 18 years or older with a CD4 cell count between 300 and 400 cells/µL were enrolled in a double-blind, randomized, placebo-controlled trial of HSV-2 suppression with acyclovir to assess HIV disease progression in Rakai, Uganda [9]. Details of the trial have been published previously [9]. Briefly, individuals with AIDS-defining illnesses or receiving ART at baseline were excluded. Participants provided written informed consent and were randomly assigned to receive placebo or 400 mg acyclovir twice daily for 24 months. The primary outcome was HIV disease progression to a CD4 cell count <250 cells/µL or World Health Organization stage IV disease, at which time ART was initiated.
Participants were followed monthly for drug refill and adverse event review. After ART initiation, the subset of enrollees who reached a study endpoint was followed for up to an additional 22 months. Women in the study provided monthly self-collected vaginal swabs, and those women who provided swabs within 6 months pre- and post-ART initiation were included in this CMV shedding study (N = 96) [11].
The trial was approved by the Uganda National Council for Science and Technology (Kampala, Uganda), Uganda Virus Research Institute Science and Ethics Committee, and the National Institute of Allergy and Infectious Diseases Intramural Institutional Review Board. The trial was registered with Clinical.Trials.gov (NCT00405821).
CMV, HSV-2, HIV, and CD4 Cell Count Testing
HIV-1 serostatus was determined using 2 enzyme immunoassays (EIAs) (Vironostika HIV-1, Organon Teknika, Charlotte, NC; and Cambridge Biotech, Worcester, Massachusetts), with Western blot confirmation of discordant EIAs (HIV-1 WB Bio-Merieux-Vitek, St Louis, Missouri). HSV-2 serostatus was determined using Focus HerpeSelect-2 EIA (Focus Technologies, Cypress, California), with an optical density cut-off of 3.4 to improve specificity [11]. All women were screened for CMV serostatus at baseline (Genway Biotech, San Diego, California).
CD4 cell count testing was performed using a FACSCalibur (Becton Dickenson, Franklin Lakes, New Jersey), and HIV viral load testing was done using the Roche Monitor v1.5 assay (Roche Diagnostics, Indianapolis, Indiana).
A real-time quantitative polymerase chain reaction (PCR) assay was used to detect HSV-2 DNA [11] and CMV DNA [1] in vaginal swabs in duplicate. CMV viral quantities were averaged for further analysis.
Statistical Analysis
Differences between groups were assessed by χ2 and Wilcoxon–Mann–Whitney tests for categorical and continuous variables, respectively. All P values were 2-sided. The primary outcomes were detection of CMV DNA shedding pre- and post-ART initiation. The intensity of CMV shedding (copies/100 µL elution volume) was analyzed as a secondary outcome. Presence of detectable CMV DNA shedding was assessed longitudinally such that each of the 96 female participants could contribute multiple outcomes before and after ART initiation. Pre-ART CD4 cell counts and viral loads were based on the last available measurement prior to ART initiation.
Univariate and multivariate modified Poisson regression models with generalized estimating equations and robust variance were used to estimate prevalence risk ratios (PRRs) and 95% confidence intervals (CIs). The correlation of repeated outcomes within individuals over time was explored using a lorelogram [12]. Primary analyses were conducted using an exchangeable correlation structure; however, sensitivity analyses using first-order autoregressive (AR1) and unstructured correlation structures were also conducted [12]. A sensitivity analysis using the lower limit of quantification of the CMV PCR assay of 60 copies/elution was performed. Multivariate models included adjustment for age, acyclovir treatment, ART status, pre-ART HIV RNA viral load and presence of HSV shedding the month before. Age, HIV viral load CD4 counts, detectable HSV-2 shedding at the same time point, and detectable HSV-2 shedding the month prior covariates were analyzed as categorical variables. Analyses were conducted in STATA Version 11.0 software.
RESULTS
Study Participant Characteristics
All women (n = 96) were CMV seropositive at baseline and contributed a total of 1080 monthly swabs out of a possible 1152 study visits (93.8%). A total of 43 (44.8%) women were randomly assigned to receive acyclovir and 53 (55.2%) received placebo (Supplementary Table 1). The median age was 34.5 (interquartile range [IQR] = 30.5–44.0) years. The majority of women (84.4%) received an ART regimen, including zidovudine (AZT)/lamivudine (3TC)/nevirapine (NVP). Prior to ART initiation, the median CD4 cell count was 217 cells/µL (IQR = 178–237), and the median viral load was 86 700 viral copies/mL (IQR = 17 600–247 700).
CMV Shedding
Shedding of CMV was detected in at least 1 study visit among 75 of the 96 women (78.0%) and at 379 of the 1080 monthly visits (35.1%). Prior to ART initiation, CMV shedding was detected in 59% of the women (57/96) and in 157 of 520 monthly visits (30.2%). After ART initiation, CMV was detected in 69% of women (66/96), and in 222 of 560 monthly study visits (39.6%), which was significantly higher than prior to ART initiation (PRR = 1.34, 95% CI, 1.1–1.6; P = .001) (Table 1). Detection of CMV shedding at a monthly visit after ART initiation was 3.4 times more common among women with detectable shedding prior to ART initiation compared to women who had no detectable CMV shedding prior to ART initiation (PRR = 3.41, 95% CI, 2.1–5.4; P < .001) (Table 1).
Table 1.
Prevalence Rate Ratio of CMV Shedding Estimated by Modified Poisson Regression
| Variable | Number of Shedding/Total Samples (%) | Univariate PRR (95% CI) | P Value | Adjusted PRR* (95% CI) | P Value |
|---|---|---|---|---|---|
| ART status | (n = 1080) | ||||
| Pre | 157/520 | (ref) | (ref) | ||
| Post | 222/560 | 1.34 (1.13–1.59) | .001 | 1.34 (1.13–1.59) | .001 |
| Acyclovir status | |||||
| Placebo | 211/587 | (ref) | (ref) | ||
| Acyclovir arm | 168/493 | 0.92 (.63–1.34) | .67 | 1.02 (.71–1.49) | .90 |
| Pre-ART HIV viral load | |||||
| <10 000 | 37/167 | (ref) | (ref) | ||
| 10 000–100 000 | 98/385 | 1.12 (.60–2.08) | .73 | 1.00 (.52–1.91) | 1.00 |
| 100 000–1 000 000 | 209/457 | 2.00 (1.19–3.38) | .009 | 1.84 (1.09–3.11) | .02 |
| >1 000 000 | 35/71 | 2.17 (1.05–4.46) | .036 | 2.34 (1.34–4.11) | .003 |
| Age | |||||
| 20–29 | 117/260 | (ref) | (ref) | ||
| 30–39 | 176/468 | 0.84 (.56–1.27) | .42 | 0.80 (.55–1.18) | .26 |
| 40–49 | 84/263 | 0.71 (.44–1.15) | .16 | 0.75 (.48–1.19) | .22 |
| ≥50 | 2/89 | 0.05 (.01–.18) | <.001 | 0.05 (.01–.18) | <.001 |
| Pre-ART CD4 count | |||||
| <200 | 206/632 | (ref) | … | … | |
| ≥200 | 173/448 | 1.16 (.81–1.68) | .42 | … | … |
| HSV shedding status | |||||
| No | 325/965 | (ref) | … | … | |
| Yes | 54/115 | 0.99 (.75–1.30) | .93 | … | … |
| HSV shedding month priora | |||||
| No | 289/874 | (ref) | (ref) | ||
| Yes | 60/110 | 1.20 (1.00–1.44) | .05 | 1.11 (.95–1.31) | .18 |
| Woman's CMV shedding status prior to ARTb | |||||
| No | 38/229 | (ref) | … | … | |
| Yes | 184/331 | 3.41 (2.15–5.43) | <.001 | … | … |
Significance based on Wald statistic.
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; CMV, cytomegalovirus; HIV, human immunodeficiency virus; HSV, herpes simplex virus-2; PRR, prevalence risk ratio; ref, reference.
a HSV shedding in the month prior was calculated using less samples (n = 984) because the first sample was removed due to lack of having HSV-2 shedding data from the prior month.
b A woman's CMV shedding status prior to ART and was not included in the adjusted model because it only included samples that were collected after ART initiation (n = 560).
In univariate analysis, ART status, younger age, higher baseline HIV viral load, and presence of HSV-2 shedding the month prior, but not during the same month, were associated with detectable CMV shedding (Table 1). CD4 count prior to ART initiation, study arm, and HSV-2 shedding during the same month were not associated with CMV shedding (Table 1). ART status remained independently associated with increased CMV shedding in a multivariate analysis adjustment (adjusted PRR = 1.34, 95% CI, 1.1–1.6; P = .001) (Table 1). Higher baseline HIV RNA viral load and younger age also remained significantly associated with CMV shedding after adjustment (Table 1). HSV-2 shedding the month prior was not significantly associated with CMV shedding after adjustment (Table 1). Pre-ART CMV shedding was not included in the adjusted model. In sensitivity analyses, unstructured and AR1 correlation models yielded similar results to the primary analysis (data not shown).
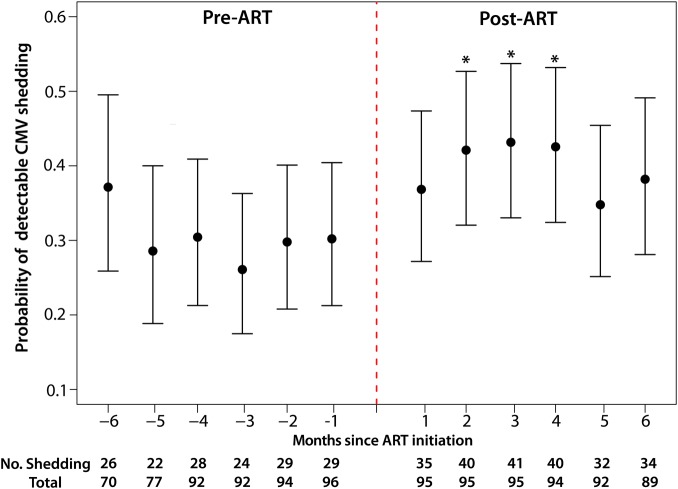
Relative to all monthly visits prior to ART initiation, probability of CMV DNA shedding after ART initiation peaked at month 2 (PRR = 1.42, 95% CI, 1.1–1.8; P = .003), and remained significantly elevated during months 3 (PRR = 1.46, 95% CI, 1.2–1.8; P = .001) and 4 (PRR = 1.46, 95% CI, 1.2–1.8; P = .001) (Figure 1). Among women with detectable CMV shedding, the median log10 CMV viral load did not differ between study visits, independent of ART status (Supplementary Figure 1). To determine if the difference in CMV shedding between pre- and post-ART was the consequence of modest increases in CMV DNA near the limit of detection, a sensitivity analysis was performed using 60 CMV DNA copies as the lower limit of quantification. In this repeated analysis, 67% of women presented CMV shedding (compared to 78% in the original analysis), and the findings were similar to the original analysis. Specifically, age and plasma HIV RNA levels were independently associated with increased CMV shedding, and there was a significant increase in the frequency of CMV shedding after ART initiation.
Figure 1.
The probability of having a detectable CMV DNA in vaginal swab by monthly study visit is shown. ART initiation is indicated with red dashed line. Prevalence of CMV DNA shedding significantly increased after initiation of ART. Months post-ART initiation with significantly higher shedding compared to all pre-ART visits are indicated with an asterisk (*). Abbreviations: ART, antiretroviral therapy; CMV, cytomegalovirus.
As part of the study protocol, the women were asked to estimate the date of their last menstruation. These data were available for 892 of the 1080 samples. For these samples, the median time from last menses was 19 days (IQR, 9–45 days) and this was not different between pre- and post-ART. Additionally, the time from menses was not predictive of CMV shedding (P = .86).
DISCUSSION
In this study of women coinfected with HIV, HSV-2, and CMV, it was observed that 78% of the 96 participants experienced CMV shedding at any time point during follow-up. These rates are considerably higher than previous reports in either HIV-infected or -uninfected individuals, most likely because previous studies were cross-sectional, while this study was longitudinal and relatively larger [4, 6–8]. Similar to what was observed for HSV-2 shedding, CMV shedding was most common among the youngest women (20–29 years) and among women with high baseline HIV RNA viral loads (>100 000 HIV RNA copies/mL) [11]. Also similar to previous reports of HSV shedding in this same cohort of women [11], there was a significant increase in CMV DNA vaginal shedding after starting ART. However, CMV DNA was detected in vaginal swabs more frequently than HSV-2 DNA in both pre-ART (30.2% vs 5.2%) and post-ART (39.6% vs 10.4%) sampling, and the detection of CMV DNA was not associated with the detection of HSV-2 DNA in the same vaginal swab. Interestingly, peak CMV vaginal shedding (months 2–4 post-ART) occurred roughly 1 month after peak HSV-2 shedding (months 1–3 post-ART), and detectable CMV shedding was positively associated with HSV-2 shedding the month prior. This association was not significant after adjusting for other significant factors. Although these associations between HSV-2 and CMV reactivation are interesting, it remains unclear what virologic and immunologic pathways are driving these associations.
This study has important limitations. First, this study was nested within a randomized trial of HIV and HSV-2 coinfected women, and included only women who started ART. This may have led to selection bias of women with a worse disease course and possibly increased CMV shedding, limiting generalizability. Additionally, blood samples were only collected every 6 months, making it impossible to accurately examine the association between time to viral suppression and vaginal CMV DNA shedding. Finally, the small study population and the relatively short follow-up time may have limited our ability to detect more subtle factors associated with CMV shedding.
The observed increased CMV shedding after ART initiation might be due to IRIS, which is typically defined by symptoms of an inflammatory process after initiation of ART [13], and has been previously documented with several viruses of the herpes family, such as HSV-2, varicella zoster virus, and Kaposi's sarcoma-associated herpes virus [13]. Also, the finding that increased CMV shedding was highest within 3 of the first 4 months of ART initiation, coincides with the typical timing of clinical IRIS [14], and suggests that the altered immune response and the inflammatory process seen after initiation of ART may temporarily enhance shedding of different viruses. It should be noted that no clinically manifest IRIS symptoms were reported in the trial, so the increased CMV and HSV-2 shedding seen in these women would be indicative of a subclinical IRIS effect. This enhanced viral shedding early during immune reconstitution may skew the immune system toward an anti-CMV directed response, which has been associated with increased risk of end-organ disease [15].
Further studies are needed to determine the possible clinical significance and long-term effects of asymptomatic CMV reactivation in ART treated individuals.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We are grateful to the study participants and the Rakai Community Advisory Board whose commitment and cooperation made this study possible. We would also like to thank Dr Davey Smith for helpful discussions in planning the study and Dr Elizabeth Colantuoni for her useful suggestions on the statistical analyses.
Financial support. This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, and in part through program project grant PO1 AI-30731-19, P30-AI027763, P30 AI036214, AI100665, 7-UM1 AI068636-07, R24AI106039, UL1TR000100. A. A. R. T. was supported by the National Institutes of Health (NIH) 1K23AI093152-01A1 and the Doris Duke Charitable Foundation Clinician Scientist Development Award (#22006.02). This project has been funded in part with federal funds from the National Cancer Institute, NIH, under Contract No. HHSN261200800001E. M. K. G. was supported by NIH T32AI102623 and the Doris Duke Charitable Foundation.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gianella S, Morris SR, Vargas MV, et al. The role of seminal shedding of herpesviruses in HIV-1 transmission. J Infect Dis 2012; 207:257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson EL, Howard CL, Thurman J, Pontiff K, Johnson ES, Chakraborty R. CMV upregulates expression of CCR5 in central memory TCM cord blood mononuclear cells which may facilitate in utero HIV-1 transmission. J Infect Dis 2015; 211:187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gianella S, Massanella M, Richman DD, et al. Cytomegalovirus replication in semen is associated with higher levels of proviral HIV DNA and CD4+ T cell activation during antiretroviral treatment. J Virol 2014; 88:7818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell C, Hitti J, Paul K, et al. Cervicovaginal shedding of HIV type 1 is related to genital tract inflammation independent of changes in vaginal microbiota. AIDS Res Hum Retroviruses 2011; 27:35–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoenfisch AL, Dollard SC, Amin M, et al. Cytomegalovirus (CMV) shedding is highly correlated with markers of immunosuppression in CMV-seropositive women. J Med Microbiol 2011; 60:768–74. [DOI] [PubMed] [Google Scholar]
- 6.Mostad SB, Kreiss JK, Ryncarz AJ, et al. Cervical shedding of cytomegalovirus in human immunodeficiency virus type 1-infected women. J Med Virol 1999; 59:469–73. [PubMed] [Google Scholar]
- 7.Silver MI, Paul P, Sowjanya P, et al. Shedding of Epstein-Barr virus and cytomegalovirus from the genital tract of women in a periurban community in Andhra Pradesh, India. J Clin Microbiol 2011; 49:2435–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broccolo F, Cassina G, Chiari S, et al. Frequency and clinical significance of human beta-herpesviruses in cervical samples from Italian women. J Med Virol 2008; 80:147–53. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds SJ, Makumbi F, Newell K, et al. Effect of daily aciclovir on HIV disease progression in individuals in Rakai, Uganda, co-infected with HIV-1 and herpes simplex virus type 2: a randomised, double-blind placebo-controlled trial. Lancet Infect Dis 2012; 12:441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med 2010; 362:427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tobian AA, Grabowski MK, Serwadda D, et al. Reactivation of herpes simplex virus type 2 after initiation of antiretroviral therapy. J Infect Dis 2013; 208:839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heagerty PJ, Zeger SL. Lorelogram: a regression approach to exploring dependence in longitudinal categorical responses. J Am Stat Assoc 1998; 93:150–62. [Google Scholar]
- 13.Muller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10:251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis 2009; 48:101–7. [DOI] [PubMed] [Google Scholar]
- 15.Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep 2012; 9:139–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.