Abstract
Background. Inactivated influenza vaccine (IIV) is recommended during pregnancy to prevent influenza infection and its complications in pregnant women and their infants. However, the extent to which pregnancy modifies the antibody response to vaccination remains unclear, and prior studies have focused primarily on hemagglutinin inhibition (HI) titers. A more comprehensive understanding of how pregnancy modifies the humoral immune response to influenza vaccination will aid in maximizing vaccine efficacy.
Methods. Healthy pregnant women and control women were studied prior to, 7 days after, and 28 days after vaccination with IIV. HI titers, microneutralization (MN) titers, and the frequency of circulating plasmablasts were evaluated in pregnant versus control women.
Results. Pregnant women and control women mount similarly robust serologic immune responses to IIV, with no significant differences for any influenza strain in postvaccination geometric mean HI or MN titers. HI and MN titers correlate, though MN titers demonstrate more robust changes pre- versus postvaccination. The induction of circulating plasmablasts is increased in pregnant women versus controls (median fold-change 2.60 vs 1.49 [interquartile range, 0.94–7.53 vs 0.63–2.67]; P = .03).
Conclusions. Pregnant women do not have impaired humoral immune responses to IIV and may have increased circulating plasmablast production compared to control women.
Keywords: hemagglutinin inhibition, influenza, plasmablast, pregnancy, vaccination, viral neutralization
Influenza infection presents serious risks to the health of pregnant women who suffer disproportionately high levels of morbidity and mortality when compared to control women [1, 2]. This increased burden of disease has been particularly prominent during pandemic years: pregnant women infected with influenza experienced mortality rates 27- to 45-fold higher than controls in 1918 [3] and approximately 6-fold higher in 2009 [4–6]. Seasonal influenza increases the risk of hospitalization approximately 3-fold over baseline during pregnancy [7, 8]. Inactivated influenza vaccine (IIV) has demonstrated safety during pregnancy [9, 10], is currently recommended for all pregnant women [10], and reduces influenza-like illness both in pregnant women and their newborns [11–14]. However, there is somewhat limited, and occasionally conflicting, data describing the serologic response to influenza vaccination during pregnancy, which has important implications for efforts to protect this vulnerable population.
Two studies performed in 1964 and 1979 demonstrated comparable serologic responses to influenza vaccination in pregnant women compared to control women [15, 16]. More recent analyses of a 2009 monovalent pandemic influenza A (pH1N1) vaccine and the trivalent IIV in pregnant women revealed that seroconversion rates were equivalent during pregnancy and the postpartum period, and that seroprotection was attained in >90% of pregnant women [17, 18]. These data suggest that humoral responses to influenza vaccination are maintained during pregnancy. However, Schlaudecker et al [19] recently performed a direct comparison of hemagglutination inhibition (HI) responses to seasonal IIV in pregnant and control women. Though pregnant and control women achieved seroconversion and seroprotection at comparable rates, consistent with earlier studies, pregnant women had significantly decreased geometric mean titers (GMTs) to the influenza A strains in the vaccine (influenza A/California [H1N1] and influenza A/Perth [H3N2]), but not to the influenza B/Brisbane virus [19]. This raises the possibility that pregnant women may have attenuated responses to influenza vaccination that could place them at risk.
One potential limitation of the existing studies of influenza vaccine responses during pregnancy is that they largely relied on HI titers as the sole measure of humoral immunity, though additional measures of humoral immune function may provide a more complete picture. While HI titers measure the ability of serum/plasma to inhibit red blood cell agglutination by influenza, influenza microneutralization (MN) assays directly assess antibody function by determining the ability of serum/plasma antibodies to prevent viral entry. Though MN titers generally correlate with HI titers, the MN assay is more sensitive and specific [20–22], and is accurate in evaluating individuals with impaired HI responses to influenza vaccination, such as those with HIV [23] and for strains such as avian influenza [24]. Another consideration is that serum volume expansion during pregnancy may alter immunoglobulin levels, potentially affecting the concentration of influenza-specific antibodies. One longitudinal study found that total serum immunoglobin G (IgG) increased during the first trimester, but decreased throughout the second and third trimesters [25]. A distinct measure of humoral responses is the induction of plasmablasts, recently activated B cells that peak in systemic circulation approximately 7 days following vaccination and secrete antibodies specific to vaccine epitopes [26]. Elderly populations that have poor HI responses to IIV also have diminished plasmablast production [27].
This study was therefore designed to comprehensively explore humoral immune responses to IIV during pregnancy. In addition to HI responses, we compared total serum IgG levels, functional neutralizing antibodies using MN titers, and the generation of circulating plasmablasts following vaccination with IIV between pregnant and control women.
METHODS
Participants and Study Design
We performed an observational study during the 2012–2013 influenza season, recruiting 25 healthy pregnant women aged 18–42 in their second and third trimesters of pregnancy who were receiving influenza vaccine as part of their standard prenatal care at the Obstetrics Clinic at Lucile Packard Children's Hospital at Stanford University [28]. Exclusion criteria included morbid obesity (prepregnancy BMI > 40), concomitant illnesses, immunosuppressive medications, receipt of blood products within the previous year, and known fetal abnormalities. Venous blood was collected prior to vaccination, 7 days following vaccination, and 28 days following vaccination, and separated into serum/plasma and peripheral blood mononuclear cell (PBMC) components. Twenty-one pregnant women completed all time points. Serum samples from control (nonpregnant) women aged 18–42 (n = 19) immunized during the 2012–2013 influenza season were obtained from the annual National Institutes of Health–sponsored studies of influenza vaccination, conducted at Stanford's Clinical and Translational Research Unit. All control women also had venous blood collections prior to vaccination, 7 days, and 28 days following vaccination. Because only 11 of the control women immunized in 2012–2013 had PBMCs available, PBMCs from additional control women were obtained from the 2010–2011 (n = 11) and 2011–2012 (n = 7) influenza seasons. All participants received the seasonal IIV vaccine recommended for the given year and underwent venous blood draws at the same time points. This study was performed in accordance with the Declaration of Helsinki and approved by the Stanford University Institutional Review Board; written informed consent was obtained from all participants.
Serum and PBMC Isolation
PBMCs were isolated from whole blood by Ficoll-Paque (GE Healthcare, Pittsburg, Pennsylvania) and cryopreserved in 90% fetal bovine serum (Thermo Scientific, Waltham, Massachusetts) + 10% dimethyl sulfoxide (Sigma-Aldrich, St. Louis, Missouri). Plasma was collected in pregnant women in heparinized tubes and serum was collected in controls. HI and MN titers in laboratory controls were comparable in serum and plasma, consistent with published literature [29, 30].
Hemagglutinin Inhibition
HI assays were performed at the Stanford Human Immune Monitoring Center using a standard technique [31]. The HI titer was defined as the reciprocal of the dilution of the last concentration of serum inhibiting hemagglutination. Serum samples that tested negative at a dilution of 1:10 were assigned a titer of 5. Samples were run in duplicate and results were averaged if values differed by 1 dilution factor. If values differed by 2 or more dilutions, the sample was re-run.
Viral Microneutralization
Assays were performed using a standard technique [32]: briefly, serial dilutions of heat-inactivated serum samples were mixed with vaccine-matched influenza virus at 100× the tissue culture infectious dose 50 (TCID50) and incubated for 1 hour at 37°C, followed by addition of 1.5 × 104 Madin-Darby canine kidney epithelial cells (ATCC, Manassas, Virginia) and incubation for 18–20 hours. The monolayers were washed with phosphate-buffered saline (PBS) (Thermo Scientific, Waltham, Massachusetts) and fixed with 80% cold acetone for 10 minutes. Influenza nucleoprotein (NP) was detected by enzyme-linked immunosorbent assay (ELISA) using anti-influenza A or B NP monoclonal antibody (Abcam, Cambridge, Massachusetts, clones: C43 [influenza A], B017 [influenza B]), followed by goat anti-mouse IgG–horseradish peroxidase, and development with 1-Step Ultra TMB for 10 minutes, stopping with 1 M sulfuric acid (Fisher). Absorbance was read at 450 nm. Wells were normalized to internal positive and negative controls on each plate, and analyzed by fitting a 4-parameter logistic curve and determining the dilution at which 50% of virus was neutralized. MN titers were reported as the reciprocal of this dilution.
Serum IgG Quantification
Total levels of circulating serum IgG were quantified by ELISA. Serum samples were diluted 1:10 000 and assayed using an IgG Human ELISA Kit (Abcam, Cambridge, Massachusetts). A standard curve was used to fit a 4-parameter logistic regression to determine concentrations, which were then adjusted for dilution and reported as mg/mL.
Plasmablast Quantification
Antibodies to the surface markers CD19, CD20, CD3, CD38, CD27, CD14, CD7, and CD56 were obtained and conjugated for mass cytometry as noted in Supplementary Table 1 using MaxPar X8 labeling kits (DVS Sciences, Sunnyvale, California). Detailed staining protocols for mass cytometry have been described [33–35]. Briefly, cryopreserved PBMCs were thawed and washed with Roswell Park Memorial Institute medium supplemented with 10% fetal bovine serum (Thermo Scientific, Waltham, Massachusetts) and 50 U/mL benzonase (EMD Millipore, Billerica, Massachusetts). Two million PBMCs were transferred to 96-well deep well plates (Sigma) and resuspended in 25 µM cisplatin for 1 minute as a live-dead stain [36] followed by quenching with 100% serum. Cells were then surface stained for 30 minutes on ice using the antibodies listed in Supplementary Table 1. Cells were suspended overnight with iridium interchelator (DVS Sciences) and 2% paraformaldehyde in PBS, then washed once in PBS and twice in water before acquisition on a CyTOF mass cytometer instrument (DVS Sciences) as described [35]. Four Element Calibration Beads (DVS Sciences) were used to normalize across days [37]. Flow cytometry standard files were analyzed with FlowJo software v9.7.5 (Tree Star, Ashland, Oregon).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism v 6.0d (GraphPad Software, San Diego, California) and R [38]. Pregnant and control participant characteristics were compared using Mann–Whitney U tests for continuous variables and Fisher exact test for discrete variables. Wilcoxon signed-rank tests were used to compare paired pre- and postvaccination values within groups. Fisher exact test was used to compare percentages of women in each group that met HI criteria for seroconversion (≥4-fold increase in titer) and seroprotection (postvaccination titer ≥40). Modeling of the effect of pregnancy while controlling for baseline HI titers was performed by analysis of covariance (ANCOVA) with confidence intervals obtained using bootstrapping.
RESULTS
Cohort Demographics
HI and MN titers to the vaccine strains were compared between 21 pregnant and 19 control women, all vaccinated during the 2012–2013 influenza season. Demographics of the cohort are summarized in Table 1. Pregnant women were older, less likely to have been vaccinated in the prior year, and equally divided between the second and third trimester at the time of enrollment. Because only 11 control women vaccinated in 2012–2013 had PBMC samples available to assess plasmablast frequencies, and the induction of plasmablasts is not expected to be type-specific for a particular vaccine strain, PBMCs from additional control women, vaccinated during the 2010–2011 (n = 11) and 2011–2012 (n = 7), were also evaluated for plasmablast induction to compare pregnant (n = 21) with control (n = 29) women.
Table 1.
Characteristics of Pregnant and Non-pregnant (Control) Women
| Characteristic | Pregnant (n = 20) | Control (N = 18) | Statistic |
|---|---|---|---|
| Age Median, (Range) | 31 (19–40) | 26 (19–33) | P = .004 |
| Race/ethnicity | P = .14 | ||
| White | 4 (19.0) | 8 (44.4) | |
| Asian | 4 (19.0) | 6 (33.3) | |
| Hispanic | 9 (42.8) | 4 (22.2) | |
| Other | 3 (19.0) | 0 (0) | |
| IIV in Prior Year | 9 (42.9) | 19 (100) | P = .001 |
| 2nd Trimester | 11 (55.0) | ||
| 3rd Trimester | 9 (45.0) |
Data are number (%) of participants unless specified. Age: Mann–Whitney U test. Race/prior IIV: Fisher's exact T test.
Abbreviation: IIV, inactivated influenza vaccination.
HI Titers in Pregnant and Control Women
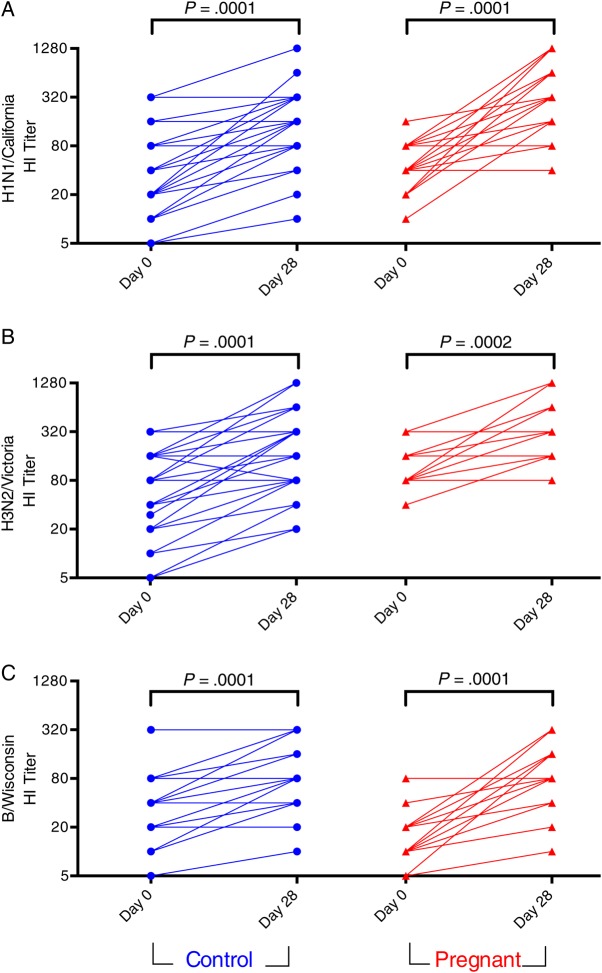
We compared pre- and postimmunization HI titers between pregnant and control women (Table 2). Preimmunization, pregnant women had a trend for lower baseline HI GMTs to pH1N1 (P = .09), equivalent titers to H3N2/Victoria, but significantly lower HI titers (GMT) to the B/Wisconsin influenza strain (P = .02), possibly reflecting the lower frequency of self-reported vaccination in the pregnant group. Following vaccination, there were no significant differences in GMTs to any of the influenza strains between pregnant and control women, and rates of seroprotection (postimmunization GMT > 40) were also equivalent (Table 2). The fold-increase in antibody production following immunization, measured as the geometric mean ratio (GMR) between post- and prevaccination titers revealed greater induction of antibodies pH1N1 (P = .013) and B/Wisconsin (P = .001), but not H3N2 (P = .83) in pregnant women. Pregnant women were also more likely to seroconvert to pH1N1 (P = .05) and B/Wisconsin (P = .03), but not H3N2/Victoria (P = 1.0). The increased GMR and seroconversion rates in pregnant women are likely related to the lower prevaccine titers, as previously reported [18]. Postimmunization titers were significantly higher than prevaccine titers for all 3 strains in both pregnant women and controls (Figure 1).
Table 2.
Strain Specific HI and MN Responses Pre- and Post-influenza Vaccination
| Influenza Subtype, Analyte | HI |
MN |
||||
|---|---|---|---|---|---|---|
| Pregnant Women (n = 20) | Nonpregnant Women (n = 18) | P Value | Pregnant Women (n = 20) | Nonpregnant Women (n = 18) | P Value | |
| A/California/7/2009 | ||||||
| Prevaccine (GMT) | 44.4 (31.2–63.2) | 74.1 (44.3–123.8) | .09 | 43.9 (20.0–96.4) | 227.9 (87.1–596.6) | .008 |
| Postvaccine (GMT) | 343.0 (209.6–561.3) | 209.5 (136.5–321.6) | .12 | 474.5 (223.3–1008.0) | 411.5 (219.2–772.5) | .76 |
| Fold Increase (GMR) | 7.7 (4.0–15.0) | 2.8 (1.8–4.5) | .013 | 10.8 (3.1–38.0) | 1.8 (0.5–6.9) | .048 |
| Seroconversion (%) | 70.0 (45.7–88.1) | 33.3 (13.3–59.0) | .05 | NA | NA | |
| Seroprotection (%) | 100 (NA) | 100 (NA) | 1.00 | NA | NA | |
| A/Victoria/361/2011 | ||||||
| Prevaccine (GMT) | 113.1 (86.5–148.0) | 99.2 (64.1–153.5) | .70 | 54.9 (34.1–88.5) | 131.4 (74.1–232.9) | .019 |
| Postvaccine (GMT) | 234.3 (154.8–354.4) | 230.9 (133.1–400.6) | .94 | 266.8 (166.1–428.6) | 535.4 (346.1–828.1) | .03 |
| Fold Increase (GMR) | 2.1 (1.4–3.0) | 2.3 (1.4–3.8) | .83 | 4.9 (2.3–10.2) | 4.1 (2.1–7.9) | .71 |
| Seroconversion (%) | 30.0 (11.9–54.3) | 27.8 (9.7–53.5) | 1.00 | NA | NA | |
| Seroprotection (%) | 100 (83.2–100.0) | 94.4 (72.7–99.9) | .47 | NA | NA | |
| B/Wisconsin/1/2010 | ||||||
| Prevaccine (GMT) | 14.6 (10.3–20.9) | 29.4 (17.8–48.7) | .02 | 18.8 (6.4–54.7) | 70.1 (37.4–131.3) | .04 |
| Postvaccine (GMT) | 74.6 (50.4–110.5) | 52.4 (31.7–86.7) | .25 | 165.8 (81.1–339.3) | 176.5 (115.9–268.9) | .87 |
| Fold Increase (GMR) | 5.1 (3.1–8.5) | 1.8 (1.3–2.5) | .001 | 8.8 (2.0–38.3) | 2.5 (1.8–3.6) | .097 |
| Seroconversion (%) | 60.0 (36.1–80.9) | 22.2 (6.4–47.6) | .03 | NA | NA | |
| Seroprotection (%) | 90.0 (68.3–98.8) | 77.8 (52.4–93.6) | .39 | NA | NA | |
Data are geometric means (95% confidence interval), unless defined otherwise.
Abbreviations: GMR, geometric mean ratio; GMT, geometric mean titer; HI, hemagglutination inhibition; MN, microneutralization; NA, not applicable.
Figure 1.
HI titers to A/H1N1/California/2009 (pH1N1) (A), A/H3N2/Victoria/2011 (B), and B/Wisconsin/2010 (C) before (Day 0) and after (Day 28) IIV administration in pregnant and control women. Titers are the reciprocal of the highest serum dilution capable of preventing hemagglutination of red blood cells. Lines connect data points from individuals. Data points represent average of technical replicates. Abbreviations: HI, hemagglutination inhibition; IIV, inactivated influenza vaccine.
In light of the differences in vaccination history between pregnant and control women (Table 1), we stratified prevaccine HI titers based on history of vaccination in the prior year (Supplementary Figure 1). There was a significant elevation in prevaccine titers for pH1N1, but not for H3N2/Victoria or B/Wisconsin. To account for baseline differences between groups, we used ANCOVA to assess the effect of pregnancy on GMR while controlling for prevaccine titers. For H3N2/Victoria, pregnancy was not significantly associated with GMR when controlling for baseline HI titer (P = .46, Supplementary Figure 2); however, for pH1N1 and B/Wisconsin, the GMR remained significantly greater in pregnant women after controlling for baseline HI titer (P = .016 and .014, respectively, Supplementary Figure 2). These results suggest that pregnancy status had a greater influence on the induction of antibodies than did prior vaccination history for pH1N1 and B/Wisconsin, but not for H3N2/Victoria.
Assessment of Pre- and Post-IIV MN Titers
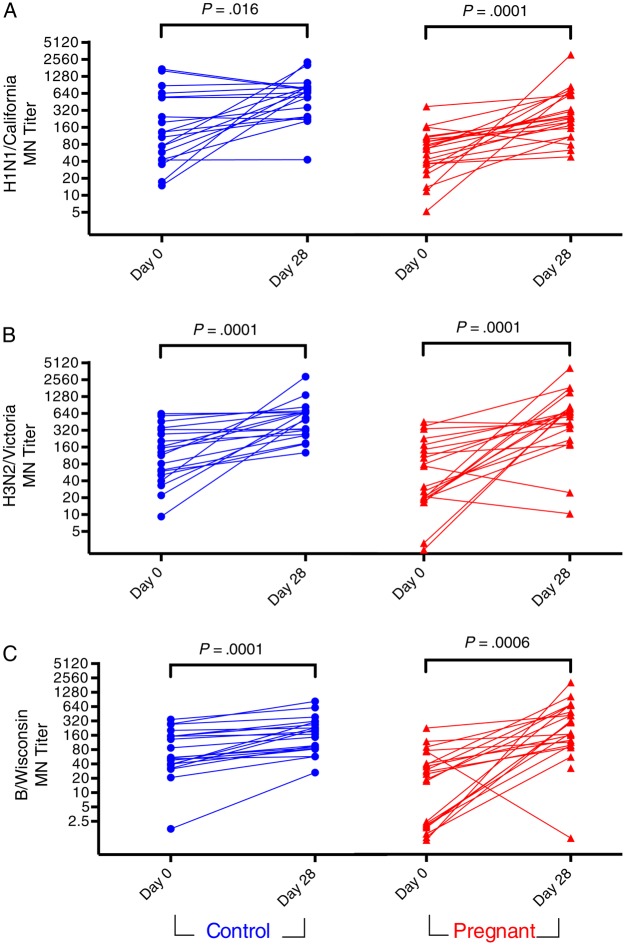
To assess whether there were more subtle differences between pregnant and control women in influenza-specific antibody induction, we evaluated MN titers (Table 2 and Figure 2). Baseline MN titers to pH1N1 (P = .008), A/H3N2/Victoria (P = .019), and B/Wisconsin (P = .033) were significantly lower in pregnant women (Table 2). As reported previously for nonpregnant women [23], HI and MN GMRs were significantly correlated in both pregnant and control women (Supplementary Figure 3). Postvaccination MN GMTs were not significantly different between pregnant women and controls for pH1N1 and B/Wisconsin, but titers were significantly lower in pregnant women for H3N2/Victoria (P = .029) (Table 2). Pregnant women had significantly greater MN GMR to pH1N1 (P = .048) but not to H3N2/Victoria (P = .71) or B/Wisconsin (P = .097). Both pregnant and control women displayed significantly increased MN titers against all 3 strains following vaccination (Figure 2). After controlling for baseline titer using an ANCOVA model, pregnancy was not associated with deficits in the induction of neutralizing antibodies to any of the 3 influenza strains tested (Supplementary Figure 2).
Figure 2.
MN titers to A/H1N1/California/2009 (pH1N1) (A), A/H3N2/Victoria/2011 (B), and B/Wisconsin/2010 (C) before (Day 0) and after (Day 28) IIV administration in pregnant and control women. Titers are the reciprocal of the highest serum dilution factor with less than 50% infection, normalized to positive controls. Lines connect data points from individuals. Data points represent average of technical replicates. Abbreviations: IIV, inactivated influenza vaccine; MN, microneutralization.
Levels of Total Serum Immunoglobulin in Pregnant and Control Women
Given the increased blood volume during pregnancy, we quantified total serum IgG in pregnant and control women (Figure 3). We found that pregnant women have significantly higher IgG concentrations before immunization (P = .042), but that difference is not seen postvaccination (P = .788), suggesting that differences in total IgG concentration did not account for the observed differences in the HI or MN GMRs. Prior to immunization, pregnant women had more variability in IgG concentration with a trend for a lower prevaccination IgG concentration as pregnancy progressed (Supplementary Figure 4). Normalization of HI and MN to total IgG levels did not influence the observed differences in titers based on pregnancy status (not shown).
Figure 3.
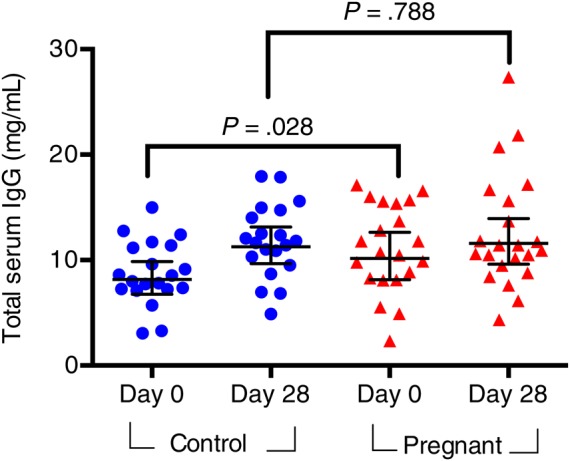
Total serum IgG concentrations in pregnant and control women, before and after IIV. Pre- and postvaccination levels were compared using a Mann–Whitney U test. Error bars represent geometric mean with 95% confidence intervals. Abbreviations: IgG, immunoglobin G; IIV, inactivated influenza vaccine.
B-cell and Plasmablast Frequencies in Pregnant and Control Women
To determine whether pregnancy or IIV was associated with alterations in antibody-producing cells, we quantified B cells and plasmablasts by mass cytometry (Supplementary Figure 5). In pregnant but not control women, B-cell frequencies significantly declined between the prevaccination and 28-day postvaccination and postpartum time points (Figure 4). However, the frequency of B cells did not significantly differ between pregnant and control women at any time point (Figure 4).
Figure 4.
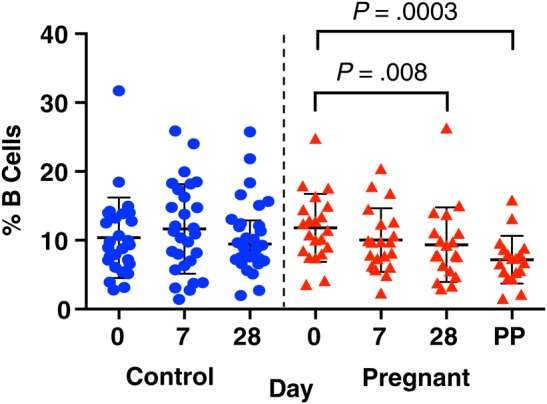
B-cell frequencies as a percentage of live PBMCs in control (circles) and pregnant (triangles) women prevaccination, 7 days postvaccination, 28 days postvaccination, and 6 weeks postpartum in the pregnant group. Abbreviations: PBMCs, peripheral blood mononuclear cells; PP, postpartum.
Both pregnant and control women had significantly increased plasmablast frequencies 7 days postvaccination (median, 0.38 vs 1.32%, P = .003 for pregnant; median, 0.37 vs 0.46%, P = .03 for control) (Figure 5A). There was no difference in the baseline plasmablast frequency in pregnant versus control women; however, pregnant women had a significantly higher frequency of plasmablasts at day 7 (P = .03) and a larger fold-change in plasmablast frequency (mean, 2.60 vs 1.49 [interquartile range 0.95–7.53 vs 0.63–2.67]; P = .03) (Figure 5B). There were no significant differences between pregnant women and controls when the analysis was limited to the small number of controls enrolled in 2012, but this may reflect a lack of statistical power. Though some, but not all, studies have found that plasmablast frequency correlates with antibody production [26, 39], the plasmablast frequency at day 7 was not correlated with HI or MN fold-change (Supplementary Figure 6). In an ANCOVA model, pregnancy remained predictive of increased plasmablast induction following IIV while controlling for baseline HI averaged between all 3 strains (P = .012). Thus, pregnant women appeared to have increased production of plasmablasts following IIV compared to control women, further suggesting that the humoral immune response to IIV is maintained during pregnancy.
Figure 5.
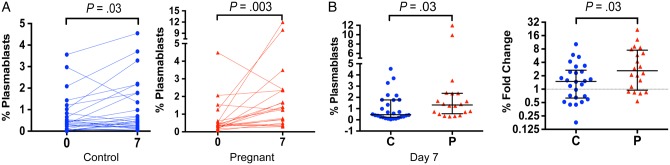
Plasmablast frequencies in pregnant women and controls. A, Plasmablast frequencies prevaccination and 7 days postvaccination in control (circles) and pregnant (triangles) were compared longitudinally using a Wilcoxon rank-sum test. B, Plasmablast frequencies at 7 days postvaccination and fold-changes (day 7 postvaccination/prevaccination) were compared by Mann–Whitney U test in control and pregnant women. All error bars indicate the median and interquartile range. Abbreviations: C, control; P, pregnant.
DISCUSSION
Here we performed a comprehensive analysis of humoral response to IIV, including HI and MN titers, serum IgG quantification, and plasmablast induction in pregnant women compared to control women. We found that pregnant women mounted robust antibody responses to IIV, reaching equivalent rates of seroprotection and postvaccine HI titers. Furthermore, pregnant women mounted equivalent neutralizing antibody titers to pH1N1 and to B/Wisconsin, though the titers pre- and postvaccination were lower against H3/Victoria. In addition, pregnant women appeared to induce plasmablasts as, or perhaps even more, robustly than did control women. Collectively, these data suggest that pregnancy does not inhibit the antibody responses to IIV, consistent with the clinical data that IIV is highly efficacious in pregnant women and reduces morbidity for both pregnant women and their children [11–14].
The fact that pregnant women in our study had lower prevaccination antibody titers than control women is not surprising, given that a smaller proportion of pregnant women reported being vaccinated in the previous season. Vaccination in the prior year has previously been linked to a reduced odds of seroconversion [18, 40], but not lower postvaccination GMTs, consistent with our findings. We also observed no evidence of a deficit in functional antibodies during pregnancy, as HI and MN titers were significantly correlated. Our data agree with several other studies that indicated that pregnant women mount adequate immune responses to influenza vaccine [18, 41, 42]. However, the equivalent postvaccination HI titers differ from the recently published data by Schlaudecker et al [19], who demonstrated a 40%–50% decrease in the postvaccination GMTs for pH1N1 and H3N2/Perth in pregnant women compared to controls. The discrepancy could relate to several factors, including year-to-year differences in vaccine formulation and study population. The fact that the HI titers observed in the control women were very similar between the Schlaudecker et al study in 2011–2012 and our study in 2012–2013 implies that differences in the vaccine strain may not have been the primary factor. One significant difference between the studies is that our cohort had lower rates of prior vaccination. In the Schlaudecker et al study, >95% of both pregnant and control women had been vaccinated in the prior season. Their result could imply that pregnant women are less adept at mounting a response to a secondary challenge, though this will require further study. Notably, currently only 50% of pregnant women receive IIV before or during pregnancy [43].
Given the differences in vaccination history between pregnant women and controls in our study, we sought to evaluate the extent to which the baseline antibody titers influenced the GMRs. A multivariate linear regression (ANCOVA) analysis suggested that the significantly increased induction of neutralizing antibodies to pH1N1 was driven primarily by pregnancy status, not by prevaccine titers. This implies that pregnant women may have more robust responses, at least to some vaccine strains to which prior exposure was limited, though this result will require validation with larger cohorts of women of known vaccination history.
In addition to serologic responses, we also sought to evaluate B-cell and plasmablast frequencies in pregnant women compared to control women. Longitudinal data in pregnant women suggest that B-cell frequencies are significantly depressed during the third trimester of pregnancy [42], consistent with our results. However, several lines of evidence suggest that pregnant women may produce more antibodies, potentially compensating for a decrease in frequency. First, estrogen, which peaks in the third trimester of pregnancy, increases immunoglobulin production from B cells in vitro [44]. Second, women generally respond more robustly than men to IIV vaccine, suggesting that estrogen may enhance antibody production in vivo [45]. Third, B-cell-mediated autoimmune diseases such as lupus are generally exacerbated by pregnancy [46]. Plasmablasts, which are important mediators of the humoral immune response [47, 48], were robustly induced in pregnant women, suggesting that humoral immune responses are maintained during pregnancy.
Limitations of this study include the relatively small sample size and the significant differences in the vaccination history between pregnant women and controls. While we did our best to account for these differences, further study with larger cohorts will be necessary to determine whether there are consistent differences in the ability of pregnant women to respond to influenza vaccine across multiple years and vaccine strains. An additional limitation is that we detected plasmablasts by mass cytometry, a technology that does not allow the cells to be sorted. As a result, we were unable to quantify with certainty the percentage of influenza-specific plasmablasts, though prior studies have indicated that plasmablast frequency is significantly correlated with number of influenza-specific plasmablasts 7 days following vaccination (P = .02) [49].
Our findings have important implications in the context of pregnancy because IIV prevents influenza in infants through passive transfer of antibodies via the placenta and breastfeeding [19, 50]. Maternal vaccination significantly reduces the risk of infant influenza-related hospitalization and intensive-care unit admission during the first 6 months of life, a time when infants benefit from passively transferred antibodies but are ineligible to receive IIV [11–14]. If postvaccination GMT titers were significantly reduced in pregnant women relative to controls, strategies could be considered to further boost titers and the duration of protection for infants. However, the HI and MN data from this cohort of pregnant women suggest that such interventions may not be necessary. Overall, our comprehensive evaluation of the humoral immune response to IIV in pregnant women versus control women revealed no consistent functional deficits in production of antibodies to influenza. Further, plasmablast production may be increased during pregnancy, providing further evidence of a robust humoral immune response. While further studies will need to evaluate whether pregnant women mount less robust responses to secondary challenges, these findings provide an immunologic correlate for the clinical observations that influenza vaccination is efficacious at preventing disease in pregnant women and their infants.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the pregnant and control study volunteers for their participation. We thank Kanta Subbarao, MBBS, MPH, at the National Institutes of Health for providing influenza virus; V. Camille Roque for her outstanding assistance with pregnant patient enrollment; Holden Maecker and Yael Rosenburg-Hasson for performing HI titers. We also thank Stanford–Lucile Packard Children's Hospital staff who enrolled the control volunteers: Clinic Manager Sue Swope, RN; Research Nurses Tony Trela and Nancy Mastman; Clinical Research Assistants Ashima Goel, Raquel Fleischmann, Kyrsten Spann, and Sushil Batra, who provided regulatory support, scheduling, and data entry; and phlebotomist Michele Ugur.
Financial support. This work was supported by a 2013 Clinical Scientist Development Award #2013099 from the Doris Duke Charitable Foundation (C. A. B.), the McCormick Faculty Award (C. A. B.), an Infrastructure and Opportunity Fund (C. A. B.) as part of the Stanford Human Immunology Project Consortium (HIPC) Grant U19AI090019 (M. M. D.) and U19A1057229 (M. M. D.), the Stanford Child Health Research Institute–Stanford Clinical and Translational Science Award grant number UL1 TR000093 (A. W. K.), a National Institutes of Health (NIH) Training Grant: Viral Infections in Children T32 AI78896-05 (A. W. K.), a Smith Family Stanford Graduate Fellowship (N. L. B.), and an NIH/National Center for Research Resources Clinical and Translational Science Award UL1 RR025744.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. N Engl J Med 2014; 370:2211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabriel G, Arck PC. Sex, immunity and influenza. J Infect Dis 2014; 209(Suppl 3):S93–9. [DOI] [PubMed] [Google Scholar]
- 3.Harris JW. Influenza occurring in pregnant women: a statistical study of thirteen hundred and fifty cases. JAMA 1919; 72:978–80. [Google Scholar]
- 4.Jamieson DJ, Honein MA, Rasmussen SA, et al. Articles. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009; 374:451–8. [DOI] [PubMed] [Google Scholar]
- 5.Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA 2010; 303:1517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louie JK, Jean C, Acosta M, Samuel MC, Matyas BT, Schechter R. A review of adult mortality due to 2009 pandemic (H1N1) influenza A in California. PLOS One 2011; 6:e18221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol 1998; 148:1094–102. [DOI] [PubMed] [Google Scholar]
- 8.Dodds L, McNeil SA, Fell DB, et al. Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant women. Can Med Assoc J 2007; 176:463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munoz FM. Safety of influenza vaccines in pregnant women. Am J Obstet Gynecol 2012; 207:S33–7. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices—United States, 2013–2014. MMWR Recomm Rep 2013; 62:1. [PubMed] [Google Scholar]
- 11.Eick AA, Uyeki TM, Klimov A, et al. Maternal influenza vaccination and effect on influenza virus infection in young infants. Arch Pediatr Adolesc Med 2011; 165:104–11. [DOI] [PubMed] [Google Scholar]
- 12.Poehling KA, Szilagyi PG, Staat MA, et al. Impact of maternal immunization on influenza hospitalizations in infants. Am J Obstet Gynecol 2011; 204:S141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359:1555–64. [DOI] [PubMed] [Google Scholar]
- 14.Benowitz I, Esposito DB, Gracey KD, Shapiro ED, Vázquez M. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis 2010; 51:1355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray DL, Imagawa DT, Okada DM, St Geme JW. Antibody response to monovalent A/New Jersey/8/76 influenza vaccine in pregnant women. J Clin Microbiol 1979; 10:184–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hulka JF. Effectiveness of polyvalent influenza vaccine in pregnancy. Report of a controlled study during an outbreak of Asian influenza. Obstet Gynecol 1964; 23:830–7. [PubMed] [Google Scholar]
- 17.Jackson LA, Patel SM, Swamy GK, et al. Immunogenicity of an inactivated monovalent 2009 H1N1 influenza vaccine in pregnant women. J Infect Dis 2011; 204:854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sperling RS, Engel SM, Wallenstein S, et al. Immunogenicity of trivalent inactivated influenza vaccination received during pregnancy or postpartum. Obstet Gynecol 2012; 119:631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlaudecker EP, McNeal MM, Dodd CN, Ranz JB, Steinhoff MC. Pregnancy modifies the antibody response to trivalent influenza immunization. J Infect Dis 2012; 206:1670–3. [DOI] [PubMed] [Google Scholar]
- 20.Gross PA, Davis AE. Neutralization test in influenza: use in individuals without hemagglutination inhibition antibody. J Clin Microbiol 1979; 10:382–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grund S, Adams O, Wählisch S, Schweiger B. Comparison of hemagglutination inhibition assay, an ELISA-based micro-neutralization assay and colorimetric microneutralization assay to detect antibody responses to vaccination against influenza A H1N1 2009 virus. J Virol Methods 2011; 171:369–73. [DOI] [PubMed] [Google Scholar]
- 22.Kilbourne ED. Comparative efficacy of neuraminidase-specific and conventional influenza virus vaccines in induction of antibody to neuraminidase in humans. J Infect Dis 1976; 134:384–94. [DOI] [PubMed] [Google Scholar]
- 23.Benne CA, Kroon FP, Harmsen M, Tavares L, Kraaijeveld CA, De Jong JC. Comparison of neutralizing and hemagglutination-inhibiting antibody responses to influenza A virus vaccination of human immunodeficiency virus-infected individuals. Clin Diagn Lab Immunol 1998; 5:114–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowe T, Abernathy RA, Hu-Primmer J, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol 1999; 37:937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amino N, Tanizawa O, Miyai K. Changes of serum immunoglobulins IgG, IgA, IgM, and IgE during pregnancy. Obstet Gynecol 1978; 52:415–20. [PubMed] [Google Scholar]
- 26.Wrammert J, Smith K, Miller J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 2008; 453:667–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasaki S, Sullivan M, Narvaez CF, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest 2011; 121:3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kay AW, Fukuyama J, Aziz N, et al. Enhanced natural killer-cell and T-cell responses to influenza A virus during pregnancy. Proc Natl Acad Sci USA 2014; 111:14506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Defang GN, Martin NJ, Burgess TH, et al. Comparative analysis of hemagglutination inhibition titers generated using temporally matched serum and plasma samples. PLOS One 2012; 7:e48229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jegaskanda S, Weinfurter JT, Friedrich TC, Kent SJ. Antibody-dependent cellular cytotoxicity is associated with control of pandemic H1N1 influenza virus infection of macaques. J Virol 2013; 87:5512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Black S, Nicolay U, Vesikari T, et al. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J 2011; 30:1081–5. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. Serological diagnosis of influenza by microneutralization assay. Geneva, Switzerland: World Health Organization, 2010. http://www.who.int/influenza/gisrs_laboratory/2010_12_06_serological_diagnosis_of_influenza_by_microneutralization_assay.pdf. Accessed 25 September 2013. [Google Scholar]
- 33.Newell EW, Sigal N, Bendall SC, Nolan GP, Davis MM. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity 2012; 36:142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bendall SC, Simonds EF, Qiu P, et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 2011; 332:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horowitz A, Strauss-Albee DM, Leipold M, et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med 2013; 5:208ra145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fienberg HG, Simonds EF, Fantl WJ, Nolan GP, Bodenmiller B. A platinum-based covalent viability reagent for single-cell mass cytometry. Cytometry A 2012; 81:467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finck R, Simonds EF, Jager A, et al. Normalization of mass cytometry data with bead standards. Cytometry A 2013; 83:483–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria, 2013. [Google Scholar]
- 39.Bentebibel SE, Lopez S, Obermoser G, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Trans Med 2013; 5:176ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972; 70:767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsatsaris V. Maternal immune response and neonatal seroprotection from a single dose of a monovalent nonadjuvanted 2009 influenza A(H1N1) vaccine: a single-group trial. Ann Intern Med 2011; 155:733. [DOI] [PubMed] [Google Scholar]
- 42.Kraus TA, Engel SM, Sperling RS, et al. Characterizing the pregnancy immune phenotype: results of the viral immunity and pregnancy (VIP) study. J Clin Immunol 2011; 32:300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention. Influenza vaccination coverage among pregnant women—United States, 2012–13 influenza season. MMWR Recomm Rep 2013; 62:777–80. [PMC free article] [PubMed] [Google Scholar]
- 44.Kanda N, Tamaki K. Estrogen enhances immunoglobulin production by human PBMCs. J Allergy Clin Immunol 1999; 103:282–8. [DOI] [PubMed] [Google Scholar]
- 45.Klein F, Halper-Stromberg A, Horwitz JA, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature 2013; 492:118–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsokos GC. Systemic lupus erythematosus. N Engl J Med 2011; 365:2110–21. [DOI] [PubMed] [Google Scholar]
- 47.He X-S, Sasaki S, Narvaez CF, et al. Plasmablast-derived polyclonal antibody response after influenza vaccination. J Immunol Methods 2011; 365:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol 2003; 3:822–9. [DOI] [PubMed] [Google Scholar]
- 49.Nakaya HI, Wrammert J, Lee EK, et al. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol 2011; 12:786–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steinhoff MC, Omer SB, Roy E, et al. Influenza immunization in pregnancy—antibody responses in mothers and infants. N Engl J Med 2010; 362:1644–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.