Abstract
Background. Human immunodeficiency virus (HIV) infection leads to lower rates of hepatitis C virus (HCV) clearance after acute infection, higher HCV viremia, and accelerated progression of HCV-related fibrosis. The mechanisms underlying this acceleration of HCV progression by HIV are poorly understood, but HIV-induced dysfunction in the anti-HCV humoral immune response may play a role.
Methods. To define the effect of HIV coinfection on the anti-HCV antibody response, we measured anti-HCV envelope binding antibody titers, neutralizing antibody (nAb) titers, and nAb breadth of serum from HCV-infected subjects isolated longitudinally before and after incident HIV infection.
Results. A significant reduction in HCV envelope-specific binding antibody and nAb titers was detected in subjects with CD4+ T-cell counts <350/mm3 after HIV infection, and subjects with CD4+ T-cell counts <200/mm3 also showed a reduction in nAb breadth. Subjects who maintained CD4+ T-cell counts ≥350/mm3 displayed little to no decline in antibody levels.
Conclusions. Depletion of CD4+ T cells by HIV infection results in a global decline in the anti-HCV envelope antibody response, including binding antibody titers, nAb titers, and nAb breadth.
Keywords: HCV, HIV, coinfection, neutralizing antibody
Approximately 170 million persons are infected with hepatitis C virus (HCV) worldwide [1]. In the United States and other developed countries, transmission of HCV occurs primarily through injection drug use. Because this is also a common mode of human immunodeficiency virus (HIV) acquisition, approximately 16% of HIV-infected persons in the United States and Europe also have HCV infection [2], and HCV-related liver disease is a major cause of death in persons with HIV infection [3, 4].
HIV infection leads to lower rates of HCV clearance after acute infection, higher HCV viremia, accelerated progression of HCV-related fibrosis, and decreased rates of sustained virologic response after HCV treatment with pegylated interferon and ribavirin [5–10]. The mechanisms underlying this acceleration of HCV progression by HIV are poorly understood, but HIV infection leads to destruction and functional impairment of helper CD4+ T cells, which may lead to dysfunction in the HCV-specific humoral immune response [11–14].
In a longitudinal study by Netski et al [15] of HCV-infected subjects with incident HIV infection, antibody titers against nonstructural HCV proteins fell after HIV infection, and this decrease was greatest in individuals with more CD4+ T-cell loss. This confirms an association between HIV-induced CD4+ T-cell loss and a reduction in some antibody responses against HCV, but it remains unclear whether antibodies against nonstructural proteins influence HCV disease progression, because these antibodies do not block HCV infection [16].
Antibodies against HCV envelope (E1E2) may have a more direct effect on viral replication, because E1E2 is exposed on the surface of HCV virions, and some antibodies against HCV E1E2, known as neutralizing antibodies (nAbs), can block HCV infection [17–19]. A subset of these nAbs, known as broadly neutralizing antibodies, can block infection by multiple diverse HCV variants [20–22]. Numerous studies have shown that nAbs against HCV exert immune pressure driving evolution in HCV E1E2, and more recent studies have demonstrated that early high-titer and broad nAb responses against HCV are associated with HCV clearance [23–28]. Therefore, measurement of antibody responses against HCV E1E2, particularly nAb responses, in HIV/HCV-coinfected individuals may be particularly relevant for understanding the impact of HIV on HCV disease progression.
We used multiple assays to measure anti-E1E2 binding antibody titers, nAb titers, and nAb breadth in serum from chronically HCV-infected subjects, isolated before and after incident HIV coinfection, to determine the effect of HIV infection on levels of these HCV E1E2-specific antibodies. Previously, testing of anti-HCV nAb breadth was limited by the lack of a diverse, representative panel of HCV isolates for use in neutralization assays. However, a diverse panel of genotype 1 HCV pseudoparticles (HCVpp) was recently developed [26], allowing us to measure the effect of incident HIV coinfection on the breadth as well as the titer of the anti-HCV nAb response. Changes in antibody levels were correlated with CD4+ T-cell counts after HIV infection to define the relationship between CD4+ T-cell depletion and impairment of the anti-HCV humoral immune response.
SUBJECTS, MATERIALS, AND METHODS
Subjects
The AIDS Linked to the Intravenous Experience (ALIVE) Cohort
Between 1988 and 1989, 2921 persons who inject drugs from Baltimore, Maryland, were recruited into the AIDS Linked to the Intravenous Experience (ALIVE) cohort to study the natural history of HIV infection [29]. At enrollment, HCV antibody was detected in 89% of subjects. By March 2002, a total of 309 previously HIV-seronegative persons had acquired HIV-1 infection. For a previous study, 29 of these 309 subjects were selected who had detectable HCV RNA before and after HIV infection, ≥1 visit before HIV infection and 2 visits after HIV infection, and adequate serum volumes for multiple antibody assays [15]. For the current study, 28 of the original 29 subjects were used, based on serum sample availability. If multiple pre-HIV samples were available, the sample collected closest to HIV infection was used.
All subjects were antiretroviral naive at enrollment. Only 1 received highly active antiretroviral therapy (HAART) during the course of the study, because collection of most samples predated HAART availability. Post-HIV samples were chosen based on sample availability and longest duration from initial HIV infection. Ten additional chronically HCV-infected control subjects from the ALIVE cohort, who did not acquire HIV infection, were selected based on availability of serum with a time between samples that would result in a median for the control group that was at least the median time between samples for the test subjects. Based on self-report, none of the test or control subjects received HCV treatment during the study. Informed consent was obtained from all subjects and this research was approved by the Johns Hopkins Institutional Review Board.
HCV E1E2 Enzyme-Linked Immunosorbent Assay
HCV E1E2–specific antibodies were detected with an enzyme-linked immunosorbent assay (ELISA), as described elsewhere [30]. Only 27 of the 28 study subjects were assessed owing to lack of adequate serum samples from 1 subject. Briefly, cells were transfected with an H77 envelope expression construct expressing the E1 and E2 proteins, and cell lysates were harvested at 48–72 hours. Plates were coated with 500 ng of Galanthus nivalis lectin (Sigma) and blocked with phosphate-buffered saline containing 0.5% Tween 20 and 5% nonfat dry milk, and H77 E1E2–containing cell lysates were added. Serum samples were assayed at 2-fold serial dilutions, starting at 1:50 and binding detected with horseradish peroxidase–conjugated anti-human immunoglobulin G secondary antibody (BD-Pharmingen). Twice the mean optical density of normal human serum wells on the same plate was used as a cutoff for positivity. Reported titers are the highest dilution still positive by ELISA. Samples from both time points for each subject were tested in the same batch.
HCV Neutralization Assays
HCVpp were generated by cotransfection of pNL4-3.Luc.R−E− plasmid and an expression plasmid containing the H77 HCV E1E2, as described elsewhere [16, 31]. Virus-containing medium was collected at 48 and 72 hours, pooled, and stored in aliquots at −80°C. For nAb titer experiments, 2- or 3-fold dilutions of heat inactivated serum, starting at 1:50, were incubated with HCVpp for 1 hour at 37°C and added to Hep3B hepatoma cells (American Type Culture Collection) for 5 hours, after which the virus-containing medium was removed. After 72 hours, cells were lysed, and luciferase activity, measured in relative light units (RLUs), was detected in a luminometer (Berthold Technologies). Pseudoparticle infection was measured in the presence of test serum (HCVppRLUtest) or HCV-negative normal human serum (HCVppRLUcontrol) at the same dilution. The percentage of neutralization was calculated as . End point neutralization titers are reported as the dilution of plasma that resulted in 50% inhibition of HCVpp infectivity (50% inhibitory dose [ID50]), as calculated by nonlinear regression (Graphpad Prism 6, version 6.05). Negative control pseudoparticles expressing no envelope protein produced RLU values ≥5-fold lower than HCVpp. Samples from both time points for each subject were tested in the same batch.
Assessment of nAb Breadth Against Library HCVpp
Development of a library of genotype 1 E1E2-expressing lentiviral pseudoparticles for measurement of nAb breadth was described elsewhere [26]. Of the 19 HCVpp described in the initial panel, 11 (1b34, 1a31, 1a53, 1b09, 1b38, 1a154, 1a157, 1b20, 1a80, 1a129, and 1b58) were selected for this study, based on reproducible infectivity and maximization of E1E2 sequence diversity among clones and to represent a range of neutralization sensitivity based on prior testing with HCV-positive plasma samples [26]. Owing to limitations in available serum from some subjects, neutralizing breadth was measured at 2 time points in 15 of the 28 study subjects, chosen to represent a range of CD4+ T-cell counts. Infection with HCVpp was measured in the presence of test serum (HCVppRLUtest) or HCV-negative normal human serum (HCVppRLUcontrol) at a 1:100 dilution. Nonspecific neutralization or enhancement of pseudoparticle infection by each serum sample was also measured by quantitating infection of pseudoparticles with MLV envelope in the presence of test serum (MLVppRLUtest) or HCV-negative normal human serum (MLVppRLUcontrol) at a 1:100 dilution. The percentage neutralization for each HCVpp was calculated and adjusted for nonspecific neutralization or enhancement, using the following formula:
Positive neutralization of each of the HCVpp was noted when neutralization was >25%, which was >2 standard deviations above the mean neutralization of negative control MLV pseudoparticles by all 25 serum samples tested.
Statistical Analysis
ELISA titers below the level of detection were assigned a titer of 1:25 and serum samples still ELISA positive at a 1:51 200 dilution were assigned that value for analysis. The nAb titers below the level of detection were assigned an ID50 value of 1:25 for analysis. Wilcoxon signed rank test was used to calculate significance of changes in binding antibody titer, nAb titer, and number of HCVpp neutralized; when normality was satisfied, paired t tests were used. Rank sum tests were used to compare change in binding titer, nAb titer, and nAb breadth between study groups; when normality was satisfied, t tests were used.
RESULTS
Subjects
Longitudinal analyses of antibody responses against HCV E1E2 proteins were performed for 10 HCV-monoinfected controls and 28 HCV-infected subjects before and after they acquired HIV. Longitudinal serum samples were tested in an HCV E1E2 ELISA to assess the total anti-HCV E1E2 antibody response, as well as in HCVpp neutralization assays to measure nAb titers and nAb breadth. Characteristics of the 28 coinfected and 10 monoinfected subjects are shown in Table 1. All subjects were HCV seropositive at the time of entry into the ALIVE study. The median time between the pre- and post-HIV visits was 80.5 months (range, 22.6–153.5 months). For monoinfected controls, the median time between serum samples was 124.3 months (range, 72.4–128.2 months). The median CD4+ T-cell count at the time of the second serum sample was 284/mm3 (range, 7–725/mm3) for the coinfected subjects and 1105/mm3 (663–1137/mm3) for the monoinfected controls.
Table 1.
Demographic and Viral Characteristics of Study Subjectsa
| Characteristic | Coinfected Subjects (n = 28) | Monoinfected Subjects (n = 10) |
|---|---|---|
| Race | ||
| African American | 27 (96) | 8 (80) |
| Other | 1 (4) | 2 (20) |
| Sex | ||
| Male | 18 (64) | 6 (60) |
| Female | 10 (36) | 4 (40) |
| Age at HIV seroconversion or 1st sample,b median (range), y | 36 (26–51) | 40 (35–51) |
| Time between ALIVE study entry and pre-HIV sample, median (range), mo | 41.9 (0.6–137.7) | … |
| Time between pre-HIV sample and HIV seroconversion, median (range), mo | 8.9 (2.9–14.1) | … |
| Time between HIV seroconversion and post-HIV sample, median (range), mo | 72.0 (16.6–141.1) | … |
| Time between 1st and 2nd samples, median (range), mo | 80.5 (22.6–153.5) | 124.3 (72.4–128.2) |
| CD4+ T-cell count at 2nd sample, median (range), cells/mm3 | 284 (7–725) | 1105 (663–1137)c |
| CD4+ T-cell countc at 2nd sample, cells/mm3 | ||
| 0–199 | 10 (36) | … |
| 200–349 | 8 (29) | … |
| ≥350 | 10 (36) | … |
| HIV RNA at 2nd sample, median (range), copies/mL | 54796 (542–759 003) | … |
| Any antiretroviral therapy | 7 (25) | … |
| HAART | 1 (4) | … |
| HCV genotyped | ||
| 1 | 25 (96) | 7 (100) |
| 2 | 1 (4) | 0 (0) |
| Injection drug usee | ||
| 1st samplef | ||
| None | 6 (22) | 4 (40) |
| <1/d | 8 (30) | 4 (40) |
| ≥1/d | 13 (48) | 2 (20) |
| 2nd sample | ||
| None | 14 (50) | 8 (80) |
| <1/d | 3 (11) | 1 (10) |
| ≥1/d | 11 (39) | 1 (10) |
Abbreviations: ALIVE, AIDS Linked to the Intravenous Experience; HAART, highly active antiretroviral therapy; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
a Unless otherwise indicated, data represent No. (%) of subjects.
b Age at HIV seroconversion for HIV/HCV coinfected subjects and age at first sample for HCV monoinfected subjects.
c The CD4+ T-cell count was not measured for all monoinfected subjects (n = 3).
d The HCV genotype was unavailable for 2 coinfected subjects (n = 26) and 3 monoinfected subjects (n = 7).
e Injection drug use in the 6 months before the clinic visit.
f Information unavailable for 1 coinfected subject (n = 27).
Anti-HCV E1E2 Binding Antibody Titer Stability During Chronic HCV Monoinfection Versus Decline After Incident HIV Coinfection
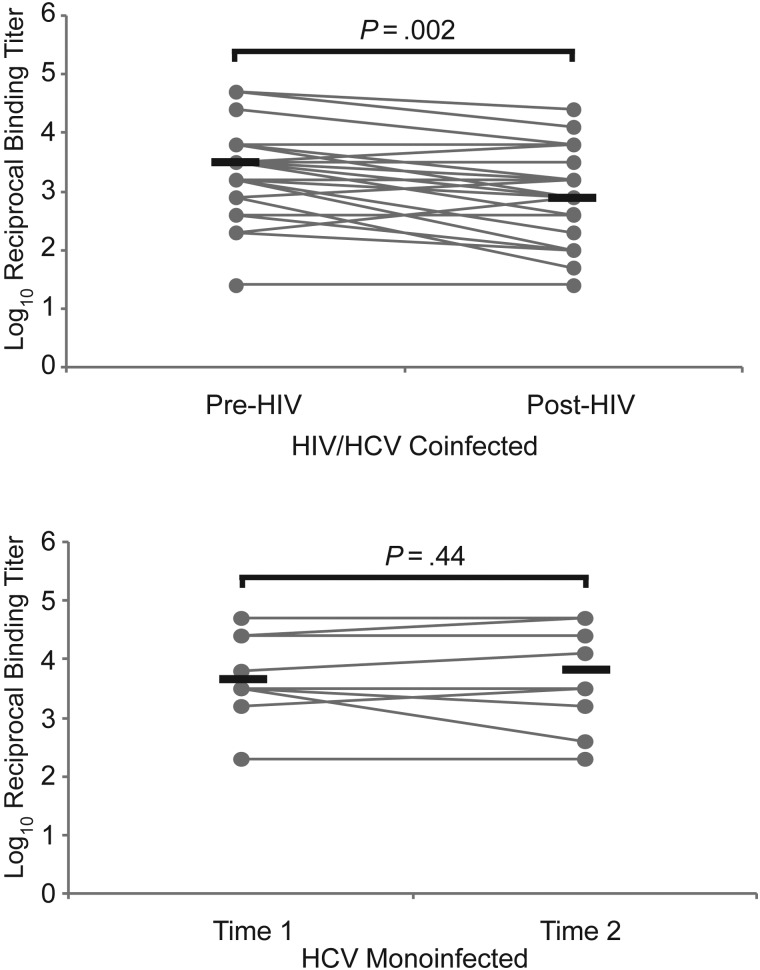
The stability of HCV E1E2 binding antibody titers was measured in 10 subjects who were chronically infected with HCV and never acquired HIV and in 27 HCV-infected subjects with incident HIV coinfection. For each HCV-monoinfected subject, binding antibody titers were assessed in 2 serum samples collected approximately 124 months apart (median, 124.3 months; range, 72.4–128.2 months). As shown in Figure 1, binding antibody titers remained stable over this time period (median log10 reciprocal titer, 3.7 vs 3.8; P = .44). In contrast, in 27 subjects who acquired HIV, anti-E1E2 binding titers declined significantly (median log10 reciprocal titer, 3.5 pre-HIV vs 2.9 post-HIV; P = .002)
Figure 1.
Anti–hepatitis C virus (HCV) envelope binding antibody titers are stable during chronic HCV monoinfection but decline after incident human immunodeficiency virus (HIV) infection. Titers of anti-HCV envelope (E1E2) antibody were measured in serum samples isolated from 27 HCV-infected subjects before and after incident HIV infection. Titers were also measured in 10 HCV-monoinfected control subjects at 2 longitudinal time points. Gray line represents titers for individual subjects measured at 2 time points; black lines, medians. Enzyme-linked immunosorbent assay (ELISA) titers below the level of detection were assigned a titer of 1:25, and serum samples still ELISA positive at a 1:51 200 dilution were assigned that value for comparison analysis. Wilcoxon signed rank test was used to calculate significance of changes; when normality was satisfied, paired t tests were used.
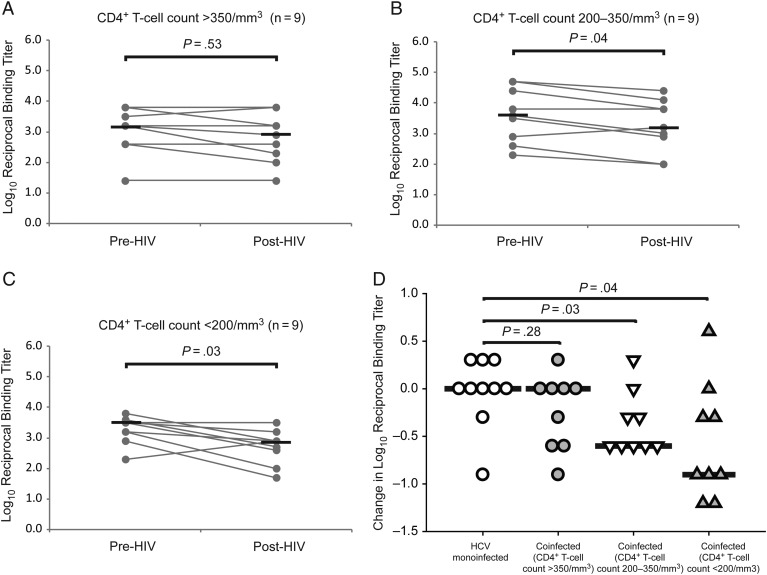
Decline in Anti-HCV Envelope Binding Antibody Titers After Incident HIV Infection in Subjects With CD4+ T-Cell Loss
To define the role of CD4+ T-cell depletion in the decline in binding antibody titers, we divided subjects with incident HIV infection into 3 groups of 9 subjects: those with post-HIV CD4+ T-cell counts >350/mm3, 200–350/mm3, or <200 cells/mm3. As shown in Figure 2A, binding antibody titers were relatively stable in those who maintained CD4+ T-cell counts >350/mm3 (median log10 reciprocal binding titer, 3.2 pre-HIV vs 2.9 post-HIV; P = .53). Subjects with CD4+ T-cell counts of 200–350/mm3 or <200/mm3 both experienced significant declines in binding antibody titers (median log10 reciprocal binding titer, 3.5 pre-HIV vs 3.2 post-HIV [P = .04] and 3.5 pre-HIV vs 2.9 post-HIV [P = .03], respectively; Figure 2B and 2C). As shown in Figure 2D, the longitudinal change in anti-E1E2 binding antibody titer was similar between HCV-monoinfected and HIV/HCV-coinfected subjects who maintained CD4+ T-cell counts >350/mm3 (median change in log10 reciprocal binding titer, 0 vs 0; P = .28), whereas those with counts of 200–350/mm3 or <200 /mm3 showed a greater decline in binding antibody titers than monoinfected controls (median change in log10 reciprocal binding titer, 0 vs −0.6 [P = .03] and 0 vs −0.9 [P = .04], respectively). Together, these data suggest that anti-E1E2 binding antibody titers decline more in subjects who acquire HIV and that the decline covaries with CD4+ T-cell loss.
Figure 2.
Decline in anti–hepatitis C virus (HCV) envelope binding antibody titers after incident human immunodeficiency virus (HIV) infection occurs in subjects with CD4+ T-cell loss. A–C, Anti-HCV envelope titers were stable after incident HIV infection in subjects with CD4+ T-cell counts >350/mm3 but declined significantly in subjects with CD4+ T-cell counts of 200–350/mm3 or <200/mm3. Gray line represent titers for individual subjects measured at 2 time points; black lines, medians. Enzyme-linked immunosorbent assay (ELISA) titers below the level of detection were assigned a titer of 1:25, and serum samples still ELISA positive at a 1:51 200 dilution were assigned that value for comparison analysis. Wilcoxon signed rank test was used to calculate significance of changes; when normality was satisfied, paired t tests were used. D, Comparison of the change in binding antibody titers over time in HCV-monoinfected controls and HIV/HCV-coinfected subjects stratified by post-HIV CD4+ T-cell count. Symbols represent changes for individual subjects; black lines, medians. Rank sum tests were used to calculate significance; when normality was satisfied, t tests were used.
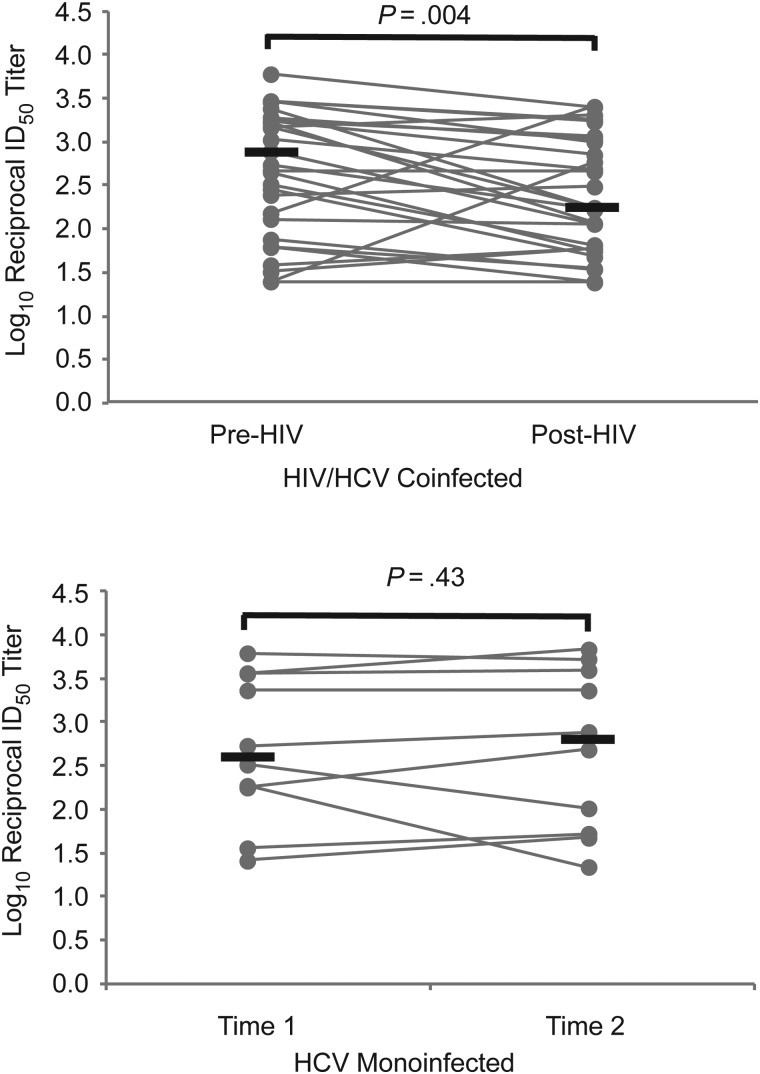
Anti-HCV nAb Titer Stability During Chronic HCV Monoinfection Versus Decline After Incident HIV Infection
The nAb titers were measured in the same longitudinal serum samples from 10 HCV-monoinfected subjects and 28 HCV-infected subjects with incident HIV infection. As shown in Figure 3, nAb titers in HCV-monoinfected subjects remained generally stable over this time period (median log10 reciprocal ID50 titer, 2.6 vs 2.8; P = .43). However, in 28 subjects who acquired HIV, nAb titers declined significantly (median log10 reciprocal ID50 titer, 2.7 pre-HIV vs 2.2 post-HIV; P = .004).
Figure 3.
Anti–hepatitis C virus (HCV) neutralizing antibody (nAb) titers are stable during chronic HCV monoinfection but decline after incident human immunodeficiency virus (HIV) infection. The 50% inhibitory dose (ID50) titers of nAb against a heterologous HCV isolate (H77) were measured in serum samples isolated from 28 HCV-infected subjects before and after incident HIV infection. Titers were also measured in 10 HCV-monoinfected control subjects at 2 longitudinal time points. Gray lines represent titers for individual subjects measured at 2 time points; black lines, medians. Any nAb titers below the level of detection were assigned an ID50 value of 1:25 for comparison analysis. Wilcoxon signed rank test was used to calculate significance of changes; when normality was satisfied, paired t tests were used.
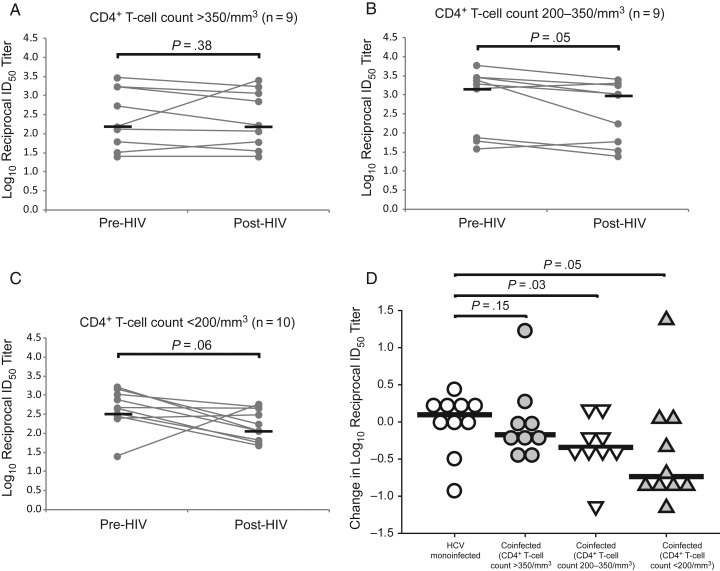
Decline in nAb Titers After Incident HIV Infection in Subjects With CD4+ T-Cell Loss
To determine whether decline in nAb titers covaries with CD4+ T-cell counts, subjects with incident HIV infection were again divided into 3 groups by post-HIV CD4+ T-cell count: >350/mm3 (n = 9), 200–350/mm3 (n = 9), and <200/mm3 (n = 10). As shown in Figure 4A, nAb titers were stable in those who maintained CD4+ T-cell counts >350/mm3 (median log10 reciprocal nAb titer, 2.2 pre-HIV vs 2.2 post-HIV; P = .38). Subjects with post-HIV CD4+ T-cell counts of 200–350/mm3 experienced significant declines in nAb titer (median log10 reciprocal ID50 titer, 3.3 pre-HIV vs 3.0 post-HIV; P = .05; Figure 4B). Subjects with CD4+ T-cell counts <200/mm3 also showed a trend toward declining nAb titer (median log10 reciprocal ID50 titer, 2.7 pre-HIV vs 2.2 post-HIV; P = .06; Figure 4C), although this did not achieve statistical significance, most likely owing to small sample size and 1 subject with a rise in ID50 titer from 1:25 to 1:587. As shown in Figure 4D, the change in anti-E1E2 nAb titers did not differ significantly between HCV-monoinfected and HIV/HCV-coinfected subjects who maintained CD4+ T-cell counts >350/mm3 (median change in log10 reciprocal ID50 titer, 0.10 vs −0.17; P = .15), whereas subjects with CD4+ T-cell counts of 200–350/mm3 or <200/mm3 showed a greater decline in nAb titers than monoinfected controls (median change in log10 reciprocal ID50 titer, 0.10 vs −0.34 [P = .03] and 0.10 vs −0.74 [P = .05], respectively). Overall, these data suggest that E1E2 nAb titers decline more in subjects who acquire HIV and that the decline covaries with CD4+ T-cell loss.
Figure 4.
Decline in anti–hepatitis C virus (HCV) neutralizing antibody (nAb) titers after incident human immunodeficiency virus (HIV) infection occurs in subjects with CD4+ T-cell loss. A–C, Anti-HCV nAb titers were stable after incident HIV infection in subjects with CD4+ T-cell counts >350/mm3, declined significantly in those with counts of 200–350/mm3, and trended downward in those with counts <200/mm3. Gray lines represents titers for individual subjects measured at 2 time points; black lines, medians. Any nAb titers below the level of detection were assigned a 50% inhibitory dose (ID50) value of 1:25 for comparison analysis. Wilcoxon signed rank test was used to calculate significance of changes; when normality was satisfied, paired t tests were used. D, Comparison of the change in nAb titers over time in HCV-monoinfected controls and HIV/HCV-coinfected subjects stratified by post-HIV CD4+ T-cell counts. Symbols represent changes for individual subjects; black lines indicate medians. Rank sum tests were used to calculate significance; when normality was satisfied, t tests were used.
Decline in Breadth of the nAb Response After Incident HIV Infection
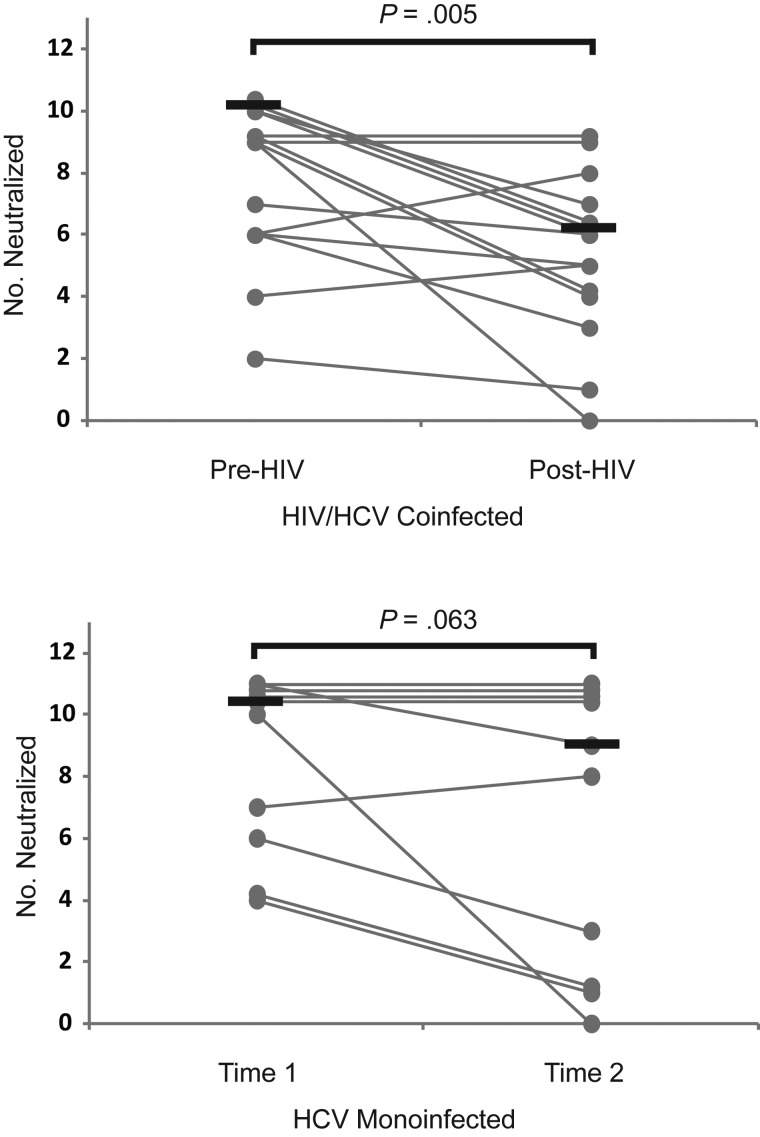
Because HCV varies within an individual at any instant (quasispecies diversity) and over time within an infected individual (divergence), an effective nAb response should neutralize multiple antigenically diverse HCV variants [32, 33]. This ability can be estimated by nAb breadth, the ability of serum to neutralize multiple diverse heterologous HCV isolates [19]. To measure nAb breadth, 2 longitudinal serum samples from the 10 HCV-monoinfected controls and 15 of the 28 HIV/HCV-coinfected subjects were tested for their ability to neutralized 11 clonal heterologous genotype 1 HCVpp. Interestingly, despite stable E1E2 binding titers, HCV-monoinfected controls showed a trend toward decreasing nAb breadth over time, although 4 of 5 subjects with the greatest nAb breadth maintained those responses (Figure 5). Subjects with incident HIV infection showed a significant decline in nAb breadth after HIV infection (median number of HCVpp neutralized, 9 pre-HIV vs 6 post-HIV; P = .005).
Figure 5.
Breadth of the neutralizing antibody response declines after incident human immunodeficiency virus (HIV) infection. Serum samples isolated from 15 hepatitis C virus (HCV) –infected subjects before and after incident HIV infection were tested for their ability to neutralize 11 clonal heterologous genotype 1 HCV pseudoparticles (HCVpp). Serum samples from 10 HCV-monoinfected control subjects at 2 longitudinal time points were used as controls. Gray lines represents number of HCVpp neutralized (neutralizing breadth) for individual subjects measured at 2 time points; black lines, medians (overlapping lines are dithered for clarity). Positive neutralization of each of the HCVpp was noted when neutralization was >25%, which was >2 standard deviations above the mean neutralization of negative control murine leukemia virus pseudoparticles by all 25 serum samples tested. Wilcoxon signed rank test was used to calculate significance of the change in number of HCVpp neutralized; when normality was satisfied, paired t tests were used.
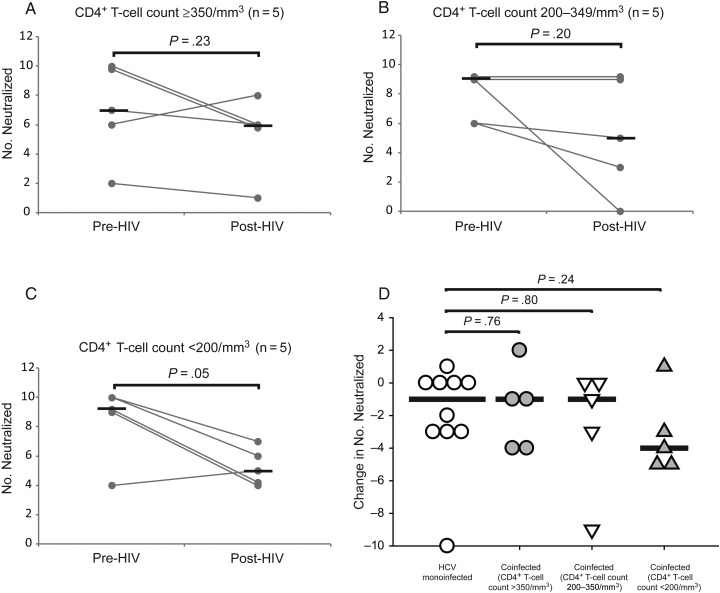
Decrease in nAb Breadth After Incident HIV Infection in Subjects With CD4+ T-Cell Loss
To define the role of CD4+ T-cell depletion in decreasing nAb breadth, subjects with incident HIV infection were divided into 3 groups of 5 subjects according to post-HIV CD4+ T-cell counts: ≥350/mm3, 200–349/mm3, and <200/mm3. As shown in Figure 6A, nAb breadth was relatively stable in those who maintained CD4+ T-cell counts >350/mm3 (median number of HCVpp neutralized, 7 pre-HIV vs 6 post-HIV; P = .23). Subjects with CD4+ T-cell counts of 200–350/mm3 also did not show a statistically significant decline in nAb breadth. Interestingly, despite the small sample size, subjects with CD4+ T-cell counts <200/mm3 showed a significant decline in nAb breadth after HIV infection (median number of HCVpp neutralized, 9 pre-HIV vs 5 post-HIV; P = .05). As shown in Figure 6D, the change in anti-E1E2 nAb breadth did not differ significantly between HCV-monoinfected and HIV/HCV-coinfected subjects, although there was a trend toward greater loss of breadth in coinfected subjects with post-HIV CD4+ T-cell counts <200/mm3 (median change in number neutralized, −1 for HCV-monoinfected vs −4 for coinfected subjects with CD4+ T-cell counts <200/mm3; P = .24). Overall, these results suggest that that decline in nAb breadth, like declines in anti-E1E2 binding antibody titer and nAb titer, covaries with CD4+ T-cell loss.
Figure 6.
Decrease in anti hepatitis C virus (HCV) neutralizing antibody breadth after incident human immunodeficiency virus (HIV) infection occurs in subjects with CD4+ T-cell loss. A–C, The decrease in neutralizing breadth after incident HIV infection was not significant in subjects with CD4+ T-cell counts ≥350/mm3, but breadth declined significantly in subjects with counts <200/mm3. Grey lines represent number of HCVpp neutralized (neutralizing breadth) for individual subjects measured at 2 time points; black lines, medians (overlapping lines are dithered for clarity). Positive neutralization of each of the HCVpp was noted when neutralization was >25%, which was >2 standard deviations above the mean neutralization of negative control murine leukemia virus pseudoparticles by all 25 serum samples tested. Wilcoxon signed rank test was used to calculate significance of the change in number of HCVpp neutralized; when normality was satisfied, paired t tests were used. D, Comparison of the change in the number of HCVpp neutralized over time in HCV-monoinfected controls and HIV/HCV-coinfected subjects stratified by post-HIV CD4+ T-cell count. Symbols represent changes for individual subjects; black lines, medians. Rank sum tests were used to calculate significance; normality was satisfied, t tests were used.
DISCUSSION
In this investigation, we demonstrated a reduction in anti-HCV E1E2-specific binding antibody titers, nAb titers, and nAb breadth after HIV infection and showed that this decrease is associated with a loss of CD4+ T cells. By using a longitudinal study design, we showed that reductions in antibody levels occurred in individual subjects over time, reducing any bias associated with interpersonal differences in baseline antibody levels. The loss of HCV-specific nAbs has important implications for understanding the disease interactions that occur during coinfection with HIV and HCV.
Our results extend earlier work demonstrating a reduction in HCV-specific antibody responses after HIV infection. Using the same subjects presented in the current study, we previously showed that HIV infection was associated with a reduction in antibodies specific for HCV nonstructural proteins [15]. By measuring levels of HCV E1E2-specific antibodies, particularly nAbs, as well as nAb breadth, the present study reinforces our previous findings and also provides results of more functional relevance, because nAbs can block HCV infection in vitro and in vivo, are associated with clearance of acute HCV infection, and potentially modulate chronic HCV infection [13, 14, 20, 23, 24, 26–28, 34–36]. Therefore, the decline in the anti-HCV nAb response in the setting of HIV/HCV coinfection may provide a mechanistic explanation for the acceleration of HCV progression by HIV.
We saw little effect of HIV infection on HCV antibody levels in subjects who maintained CD4+ T-cell counts >350/mm3. This is not surprising, given the established role of CD4+ T cells in B-cell function. However, a previous cross-sectional study that similarly showed lower HCV nAb titers in HIV/HCV-coinfected subjects did not find an association with CD4+ T-cell counts [37]. One explanation may be that the longitudinal design of the current study better controls for variables such as baseline nAb titers, which vary between subjects. In addition, all the subjects in the cross-sectional study were receiving HAART with well-controlled HIV viremia, whereas few subjects in the current study received HIV treatment. The ability of HAART to increase CD4+ T-cell numbers is well established, but the effects of this reconstitution on antibody production are less well known.
It is noteworthy that binding titers, nAb titers, and nAb breadth rose in a several subjects despite HIV infection, suggesting that other factors in addition to absolute CD4+ T-cell count may influence the development and maintenance of nAb responses. Of note, antibody titers and breadth rose in only 1 subject with a post-HIV CD4+ T-cell count <200/mm3. This subject was unusual in that only 18 days separated his or her ALIVE enrollment and first documented HCV seropositivity from the first time point used in this study, so it is possible that this subject was still in the acute/early period of HCV infection at the pre-HIV time point. Numerous studies have shown that nAb titers against HCV tend to rise over time early in infection, so this may explain the rise in anti-HCV antibody titers in this subject despite a decline in CD4+ T-cell count [23, 24].
This work also raises additional questions that warrant further investigation. To our knowledge, before this study, anti-HCV nAb breadth had never been measured longitudinally over >5 years of chronic HCV infection. Although we observed a statistically significant reduction in nAb breadth in HIV/HCV-coinfected subjects, we also observed an unexpected trend toward declining breadth in HCV-monoinfected controls, and the magnitude of the reduction did not differ significantly between HIV/HCV-coinfected and HCV-monoinfected subjects. Although the lack of statistical difference between groups may be due to small sample sizes, the trend toward declining nAb breadth in monoinfected subjects is interesting, given the relative stability of binding and nAb titers in the same subjects. The decline in breadth may reflect the CD4+ T-cell exhaustion late in chronic HCV infection that has been well documented in numerous studies [38–42].
In summary, this study demonstrates a CD4+ T-cell–dependent decrease in anti-E1E2 binding antibody titers, nAb titers, and nAb breadth after infection with HIV. Early high titer nAb responses have been associated with clearance of acute HCV infection and probably also play a role in the control of chronic HCV infection. Decline in the anti-HCV nAb response in the setting of HIV/HCV coinfection may provide a mechanistic explanation for the acceleration of HCV progression by HIV.
Notes
Acknowledgments. We appreciate the input and assistance of the AIDS Linked to the Intravenous Experience (ALIVE) study staff, particularly Jacquie Astemborski for assistance with sample acquisition and demographic data, and the ALIVE participants.
Financial support. This work was supported by the National Institutes of Health (grants K08 AI102761, U19 AI088791, R37 DA013806, R01 DA012568, and R56 DA004334) and the Johns Hopkins University Center for AIDS Research (grant P30 AI094189).
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013; 57:1333–42. [DOI] [PubMed] [Google Scholar]
- 2.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis 2002; 34:831–7. [DOI] [PubMed] [Google Scholar]
- 3.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006; 166:1632–41. [DOI] [PubMed] [Google Scholar]
- 4.van der Helm J, Geskus R, Sabin C, et al. Effect of HCV infection on cause-specific mortality after HIV seroconversion, before and after 1997. Gastroenterology 2013; 144:751–60. [DOI] [PubMed] [Google Scholar]
- 5.Sh M, Cox A, Hoover DR, et al. Protection against persistence of hepatitis C. Lancet 2002; 359:1478–83. [DOI] [PubMed] [Google Scholar]
- 6.Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis 2001; 33:562–9. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Samaniego J, Rodriguez M, Berenguer J, et al. Hepatocellular carcinoma in HIV-infected patients with chronic hepatitis C. Am J Gastroenterol 2001; 96:179–83. [DOI] [PubMed] [Google Scholar]
- 8.Thomas DL, Astemborski J, Vlahov D, et al. Determinants of the quantity of hepatitis C virus RNA. J Infect Dis 2000; 181:844–51. [DOI] [PubMed] [Google Scholar]
- 9.Eyster ME, Fried MW, Di Bisceglie AM, Goedert JJ. Increasing hepatitis C virus RNA levels in hemophiliacs: relationship to human immunodeficiency virus infection and liver disease. Blood 1994; 84:1020–3. [PubMed] [Google Scholar]
- 10.Zinkernagel AS, von Wyl V, Ledergerber B, et al. Eligibility for and outcome of hepatitis C treatment of HIV-coinfected individuals in clinical practice: the Swiss HIV cohort study. Antivir Ther 2006; 11:131–42. [PubMed] [Google Scholar]
- 11.Vergis EN, Mellors JW. Natural history of HIV-1 infection. Infect Dis Clin North Am 2000; 14:809–25, v-vi. [DOI] [PubMed] [Google Scholar]
- 12.Alimonti JB, Ball TB, Fowke KR. Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J Gen Virol 2003; 84:1649–61. [DOI] [PubMed] [Google Scholar]
- 13.Lake-Bakaar G, Dustin L, McKeating J, Newton K, Freeman V, Frost SD. Hepatitis C virus and alanine aminotransferase kinetics following B-lymphocyte depletion with rituximab: evidence for a significant role of humoral immunity in the control of viremia in chronic HCV liver disease. Blood 2007; 109:845–6. [DOI] [PubMed] [Google Scholar]
- 14.Bjoro K, Froland SS, Yun Z, Samdal HH, Haaland T. Hepatitis C infection in patients with primary hypogammaglobulinemia after treatment with contaminated immune globulin. N Engl J Med 1994; 331:1607–11. [DOI] [PubMed] [Google Scholar]
- 15.Netski DM, Mosbruger T, Astemborski J, Mehta SH, Thomas DL, Cox AL. CD4+ T cell-dependent reduction in hepatitis C virus-specific humoral immune responses after HIV infection. J Infect Dis 2007; 195:857–63. [DOI] [PubMed] [Google Scholar]
- 16.Netski DM, Mosbruger T, Depla E, et al. Humoral immune response in acute hepatitis C virus infection. Clin Infect Dis 2005; 41:667–75. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Keck ZY, Foung SK. Neutralizing antibody response to hepatitis C virus. Viruses 2011; 3:2127–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards V, Tarr AW, Urbanowicz R, Ball J. The role of neutralizing antibodies in hepatitis C virus infection. J Gen Virol 2012; 93(pt 1):1–19. [DOI] [PubMed] [Google Scholar]
- 19.Ball JK, Tarr AW, McKeating JA. The past, present and future of neutralizing antibodies for hepatitis C virus. Antiviral Res 2014; 105:100–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Law M, Maruyama T, Lewis J, et al. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med 2008; 14:25–7. [DOI] [PubMed] [Google Scholar]
- 21.Keck ZY, Xia J, Wang Y, et al. Human monoclonal antibodies to a novel cluster of conformational epitopes on HCV e2 with resistance to neutralization escape in a genotype 2a isolate. PLoS Pathog 2012; 8:e1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlsen TH, Pedersen J, Prentoe JC, et al. Breadth of neutralization and synergy of clinically relevant human monoclonal antibodies against HCV genotypes 1a, 1b, 2a, 2b, 2c, and 3a. Hepatology 2014; 60:1551–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dowd KA, Netski DM, Wang XH, Cox AL, Ray SC. Selection pressure from neutralizing antibodies drives sequence evolution during acute infection with hepatitis C virus. Gastroenterology 2009; 136:2377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pestka JM, Zeisel MB, Blaser E, et al. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci U S A 2007; 104:6025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L, Fisher BE, Thomas DL, Cox AL, Ray SC. Spontaneous clearance of primary acute hepatitis C virus infection correlated with high initial viral RNA level and rapid HVR1 evolution. Hepatology 2012; 55:1684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osburn WO, Snider AE, Wells BL, et al. Clearance of hepatitis C infection is associated with the early appearance of broad neutralizing antibody responses. Hepatology 2014; 59:2140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Hahn T, Yoon JC, Alter H, et al. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology 2007; 132:667–78. [DOI] [PubMed] [Google Scholar]
- 28.Logvinoff C, Major ME, Oldach D, et al. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc Natl Acad Sci U S A 2004; 101:10149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirk GD, Astemborski J, Mehta SH, et al. Assessment of liver fibrosis by transient elastography in persons with hepatitis C virus infection or HIV-hepatitis C virus coinfection. Clin Infect Dis 2009; 48:963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keck ZY, Li SH, Xia J, et al. Mutations in hepatitis C virus E2 located outside the CD81 binding sites lead to escape from broadly neutralizing antibodies but compromise virus infectivity. J Virol 2009; 83:6149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu M, Zhang J, Flint M, et al. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc Natl Acad Sci U S A 2003; 100:7271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dienstag JL. The natural history of chronic hepatitis C and what we should do about it. Gastroenterology 1997; 112:651–5. [DOI] [PubMed] [Google Scholar]
- 33.Ray SC, Fanning L, Wang XH, Netski DM, Kenny-Walsh E, Thomas DL. Divergent and convergent evolution after a common-source outbreak of hepatitis C virus. J Exp Med 2005; 201:1753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrarca A, Rigacci L, Caini P, et al. Safety and efficacy of rituximab in patients with hepatitis C virus-related mixed cryoglobulinemia and severe liver disease. Blood 2010; 116:335–42. [DOI] [PubMed] [Google Scholar]
- 35.Farci P, Shimoda A, Wong D, et al. Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proc Natl Acad Sci U S A 1996; 93:15394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Jong YP, Dorner M, Mommersteeg MC, et al. Broadly neutralizing antibodies abrogate established hepatitis C virus infection. Sci Trans Med 2014; 6:254ra129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castelain S, Schnuriger A, Francois C, et al. Low levels of hepatitis C virus (HCV) neutralizing antibodies in patients coinfected with HCV and human immunodeficiency virus. J Infect Dis 2008; 198:332–5. [DOI] [PubMed] [Google Scholar]
- 38.Raghuraman S, Park H, Osburn WO, Winkelstein E, Edlin BR, Rehermann B. Spontaneous clearance of chronic hepatitis C virus infection is associated with appearance of neutralizing antibodies and reversal of T-cell exhaustion. J Infect Dis 2012; 205:763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Penna A, Pilli M, Zerbini A, et al. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology 2007; 45:588–601. [DOI] [PubMed] [Google Scholar]
- 40.Kasprowicz V, Schulze zur Wiesch J, Kuntzen T, et al. High PD-1 expression on HCV-specific CD8+ and CD4+ T cells during acute hepatitis C irrespective of clinical outcome. J Virol 2008; 82:3154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006; 443:350–4. [DOI] [PubMed] [Google Scholar]
- 42.Cox AL, Mosbruger T, Mao Q, et al. Cellular immune selection with hepatitis C virus persistence in humans. J Exp Med 2005; 201:1741–52. [DOI] [PMC free article] [PubMed] [Google Scholar]