Abstract
Both human immunodeficiency virus (HIV) and human herpesvirus (HHV) infections persist lifelong, and almost all individuals infected with HIV are also infected with ≥1 HHV. These coinfections are not independent processes or benign. In this review, we discuss how HHVs, and cytomegalovirus in particular, interact with concurrent HIV infection, and we describe the next steps necessary to understand and address these connections.
Keywords: HIV, Human herpesvirus, cytomegalovirus, epidemiology transmission, pathogenesis, inflammation
The Herpesviridae family comprises more than 120 known viruses adapted to many vertebrate species, including humans [1]. Human herpesviruses (HHVs) have been infecting their vertebrate hosts for hundreds of million years [2]. During this time, they have evolved a number of complex strategies to escape host immune responses allowing lifelong infection. During infection, HHV cycles through latent and active stages, with the active stage being important for the production of viral particles that can infect new hosts. Most viral active stages are largely asymptomatic; however, in the setting of a weakened immune system—especially with decreased cellular immunity, as in organ transplants or coinfection with human immunodeficiency virus (HIV)—episodes of human HHV reactivation are increased and prolonged and can significantly affect morbidity and mortality rates [3, 4]. Here, we review the literature on how HHVs interact with concurrent HIV infection, focusing on cytomegalovirus (CMV), and we discuss the next steps necessary to further elucidate these connections.
RESEARCH STRATEGY AND SELECTION CRITERIA
References for this review were identified through searches of PubMed and Google Scholar with no date restriction using the term HIV in combination with herpesviruses, cytomegalovirus, herpes simplex, immune activation, epidemiology, among others. All included articles were published in English. To obtain the most relevant articles for the focus of our review, we chose articles that were recent and that provided the strongest evidence to support the statements in the review. Specifically, we concentrated on articles published in the past 5 years but also cited older publications when appropriate. For some larger topics that could not be discussed in depth, we cited other reviews that were more specific to that topic. To present the most current level of the field's understanding, we also included high-impact abstracts presented at recent conferences but not yet published.
EPIDEMIOLOGY AND NATURAL HISTORY OF HHV
Herpesviridae are composed of a double-stranded DNA genome contained within a nucleocapsid surrounded by a lipid envelope [5]. There are 8 characterized herpesviruses in human: herpes simplex virus (HHV-1/2; HSV-1/2), varicella zoster virus (HHV-3; VZV), Epstein-Barr virus (HHV-4; EBV), CMV (HHV-5), HHV type 6 (HHV-6), HHV type 7 (HHV-7), and Kaposi sarcoma–associated herpesvirus (HHV-8; KSHV) [5, 6]. These 8 HHVs represent hundreds of millions of years of evolutionary diversification and innovation [2]. It seems that many of these viruses have been infecting the human lineage since before the rise of Homo sapiens [7]. Across all 3 herpesvirus subfamilies (α, β, and γ), the closest relatives of HHV seem to be found in other great ape species (ie, chimpanzees and gorillas) [7, 8]. These viruses seem to have been diverging almost exclusively along the evolutionary lineages of their hosts. A notable exception to this pattern may explain why humans have 2 HSVs but other primate species have only a single simplex virus: HSV-2 seems to be the result of a recent cross-species transmission from the ancestor of modern chimpanzees to a precursor of modern humans (ie, Homo erectus) [7].
Most HHVs are likely to be acquired during childhood or during sexual debut, and they infect most of the human population worldwide, with some regional differences [5, 6]. One exception might be KSHV, which is typically contracted in adulthood in Europe and North America. However, it has a much higher incidence in equatorial Africa (up to 35% by age 21 years), in the so-called KS belt [5]. Most HHVs must come in contact with mucosal surfaces or abraded skin to initiate infection [6]. Clinical manifestations of HHV infections are diverse and range from mild or subclinical disease to encephalitis, pneumonia, and other potentially lethal infections and various types of cancer, including lymphoma, sarcoma, and nasopharyngeal carcinoma [5]. Primary HHV infection in immunocompetent hosts is often asymptomatic or minimally symptomatic, but morbidity and mortality rates can be high when immunocompromised persons are infected, especially with CMV, HSV, or VZV [5, 6].
After primary infection, the virus becomes latent in neural ganglia or blood mononuclear cells in its episomal form [5, 6]. After latency is established, various stimuli can reactivate HHV with clinical manifestations, such as skin vesicles or mucosal ulcers. More commonly, HHV reactivation with shedding is largely asymptomatic [9]. One very common site of HHV shedding is the genital tract. For example, Koelle and Corey [10] found that immunologically normal hosts shed HSV-2 asymptomatically in their anogenital regions a quarter of the days sampled (range, 2% to 75% of days). Another study of immunocompetent adults found that about half of such episodes lasted <12 hours, and about one-third lasted <6 hours [11]. The frequency of shedding of various HHVs in genital secretions varies substantially across studies and is strongly dependent on the geographic location, cohort characteristics, and detection methods. When an infected individual has a compromised immune system, shedding of HHV increases dramatically. For example, almost two-thirds of HIV-infected men asymptomatically shed ≥1 HHV in their genital tract, regardless of CD4+ T-cell count, geographic location, or use of antiretroviral therapy (ART) [12, 13]; CMV and EBV were the viruses most frequently detected during these shedding episodes and were found in about half of all tested semen samples, whereas the other HHVs (HSV, VZV, HHV-6, HHV-7, and HH-8) were found at lower frequencies, between 0% and 17% [12, 13]. Such asymptomatic shedding is probably important for the natural history and transmission dynamics of HHV and interplays with other coinfecting viruses (eg, HIV). Table 1 provides a summary of prevalence and characteristics for the most common HHV infections.
Table 1.
Summary of Prevalence and Characteristics for the Most Common HHV Infections
| HHV Type (Alternate Name) | Subfamily | Estimated Prevalence (%) |
Primary Target Cells | Site of Latency | Route of Transmission | Acute Syndromes | Chronic Syndromes and Other Rare Manifestations | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Children | Adults (US) | Adult (Dev World) | HIV Infection | |||||||
| HHV-1 (HSV-1) |
α | 20–40 | 50–70 | 50–100 | 90–100 | Mucoepithelial | Neuron | Close contact (oral or STD) | Mostly oral (gingivostomatitis) | Episodic reactivation, encephalitis, keratitis, mucocutaneous disease |
| HHV-2 (HSV-2) |
α | 0–5 | 20–50 | 20–60 | 50–90 | Mucoepithelial | Neuron | Close contact (STD) | Mostly genital | Episodic reactivation, encephalitis, keratitis, mucocutaneous disease |
| HHV-3 (VZV) |
α | 50–75 | 85–95 | 50–80 | 90–100 | Mucoepithelial | Neuron | Respiratory and close contact (also STD) | Chickenpox | Episodic reactivation (shingles) |
| HHV-4 (EBV) |
γ | 10–50 | 80–95 | 90–100 | 90–100 | B cells and epithelial cells | B cells | Close contact, saliva, STD, transfusions/transplant, congenital | Infectious mononucleosis | Burkitt lymphoma, CNS lymphoma, posttransplant lymphoproliferative syndrome, nasopharyngeal carcinoma, HIV-associated hairy leukoplakia |
| HHV-5 (CMV) |
β | 10–30 | 40–70 | 40–80 | 90–100 | Monocytes, lymphocytes and epithelial cells | Monocytes, lymphocytes, secretory glands, and possibly others | Close contact, saliva, STD, transfusions/transplant, congenital | Infectious mononucleosis–like syndrome, retinitis and other organ diseases | Reactivation and organ diseases in immunocompromised host |
| HHV-6 | β | 80–100 | 60–100 | 60–100 | 80–100 | T lymphoctes and others | T lymphoctes, monocytes, and others | Close contact (oral) | Roseola infantum | Meningitis, encephalitis and possibly multiple sclerosis |
| HHV-7 | β | 50–80 | 60–100 | 40–100 | 80–100 | T lymphoctes and others | T lymphoctes, monocytes, and others | Close contact (oral) | Roseola infantum | Hepatitis, postinfectious myeloradiculoneuropathy, pitydiasis rosea (?) |
| HHV-8 | γ | <3 | 3–5 | 10–50 | 50–90 | Endothelial cells | Monocytes, dendritic cells, B lymphocytes, and endothelial cells | Close contact (oral or STD) | Lymphadenopathy, diarrhea, rash, fatigue | Kaposi sarcoma, primary effusion lymphoma, multicentric Castleman disease |
Abbreviations: CMV, cytomegalovirus; CNS, central nervous system; Dev World, developing world; EBV, Epstein-Barr virus; HHV, human herpesvirus; HIV, human immunodeficiency virus; HSV, herpes simplex virus; STD, sexually transmitted disease; US, United States; VZV, varicella zoster virus.
EPIDEMIOLOGY OF HHV AND HIV COINFECTIONS
Currently, there are >35 million persons living with HIV-1 worldwide [14]. Unlike HHVs, which have been infecting humans since our species arose [7], HIV is a far younger virus, infecting humans for the past hundred years or so [15]. Whereas new HIV cases are reported in all regions of the world, 95% of new infections occur in individuals who reside in low- and middle-income countries, particularly in the sub-Saharan Africa [16]. In the absence of therapy, infection with HIV-1 results in a progressive loss of immune function marked by depletion of the CD4+ T lymphocytes, leading to opportunistic infections and malignancies characteristic of AIDS. Although both host and viral determinants influence the rate of disease progression, the median time from initial infection to the development of AIDS among untreated individuals ranges from 8 to 10 years [5]. Clinical and immunologic factors associated with disease progression have been extensively investigated, including CD4 T-cell count, HIV-1 subtype, markers of inflammation, HIV RNA levels, opportunistic infections, and coinfection with various HHVs (most frequently CMV, but also HSV, KSHV, and others) [3, 17].
Both HIV and HHV infections persist lifelong, and almost all individuals infected with HIV are also infected with ≥1 HHV. Since the first description of AIDS, coinfections with HHV have been part of the clinical presentation (and responsible for several AIDS-defining conditions) and are among the most common opportunistic infections observed in persons with AIDS, including CMV, HHV-8, HSV, EBV, and VZV [18]. For example, the considerable overlap between the HIV and the HHV epidemics does not seem to be happenstance: the synergy between these 2 largely sexually transmitted viral infections goes beyond similar risk factors for acquisition [9]. Accumulating data show that these 2 types of infections interact both in their epidemiologic niche and at a pathogenesis level by driving viral replication and facilitating transmission.
HHV SHEDDING AND IMPACT ON HIV TRANSMISSION
Several studies have provided convincing evidence that coinfection with some HHVs (most frequently HSV-2 and CMV but also others) play a role in HIV-1 transmission and acquisition, independently from the level of immunosuppression [12, 18, 19]. Three main mechanisms are probably responsible: (1) physical disruption of the mucosal surface creating a portal for HIV entry (especially true for HSV-2), (2) local inflammation with recruitment of activated CD4 T cells targeted by HIV, and (3) enhanced HIV replication with increased level of genital HIV RNA shedding [1, 12, 20]. In fact, HHVs, particularly HSV and CMV, can up-regulate HIV replication directly through a complex interaction with the long terminal repeat region and transactivation of proviral HIV [21] and indirectly through release of inflammatory cytokines and chemokines [18]. Findings of a study published in 2014 suggested that CMV could also be responsible for up-regulation of CCR5 expression in central memory T cells, at least considering cord blood mononuclear cells exposure in vitro [22]. These conditions are often asymptomatic, with only minimal breaks in the mucosa or skin in the genital area. Such theoretical risk is also supported by findings of seroepidemiologic studies that have demonstrated associations between HSV-2 seropositivity and increased risk of HIV acquisition [23].
In the last decade, several clinical trials have investigated the effect of the anti-herpetic drug acyclovir on HIV RNA replication, transmission and disease progression [24, 25]. Interestingly, a mathematical model of HSV-2/HIV-1 coinfection in the high HSV-2 prevalence setting of Kisumu, Kenya, estimated that more than a quarter of HIV-1 infections were attributable to HSV-2 [26]; however, in a randomized trial, 400 mg of acyclovir twice daily did not decrease the risk of HIV-1 transmission despite reducing levels of HIV RNA in blood and genital secretions [25]. This suggests that such treatment may not be enough to completely suppress replication of HSV-2 or that other viruses that are not susceptible to acyclovir, such as CMV, also contribute to HIV transmission. In addition, activated T cells persist at mucosal surfaces for months after cessation of active HSV replication and represent a residual pool of target cells for HIV infection and replication [27].
Findings of some studies suggest that the observed effect of acyclovir on HIV RNA levels might not be mediated by suppression of HSV (or other HHV) but is rather a consequence of direct drug inhibition of acyclovir on HIV replication [28]. This direct drug effect is controversial because acyclovir-associated mutations are typically not observed in HIV isolates from HIV-infected persons treated with acyclovir (or its prodrug valacyclovir) [24]. Interestingly, a very recent randomized trial demonstrated that the effects of valacyclovir on HIV-1 replication are not related to the suppression of HSV-2-mediated inflammation and are consistent with a direct effect of the drug on HIV-1 replication [29]. In the same direction, male circumcision, which has been associated with decreased inflammation in the genital area, was repeatedly associated with reduced risk of sexual HIV and HSV-2 transmission, especially in the heterosexual population [30].
Although the effect of HSV-2 shedding on HIV coinfection is widely documented, it is not the only HHV infection that is common worldwide and might facilitate HIV-1 transmission [13]. All viruses that coinfect and reside in the genital tract may influence each other's virologic dynamics [13]. For example, the genital shedding of HHV (especially HSV, EBV, and CMV) among ART-naive HIV-infected individuals has been associated with increased genital shedding of HIV RNA [12, 13, 19, 20, 24] and with increased HIV transmission [9, 19]. These connections were also observed among coinfected men receiving ART, in whom high-level seminal CMV shedding, but not presence of asymptomatic bacterial coinfections, was associated with HIV RNA shedding, conferring a potential risk for HIV transmission [31]. Along these lines, a study by Gianella et al [32] of HIV-infected and HIV-uninfected men who have sex with men in San Diego, California found that more than one-third of HIV transmissions among these men might be attributable to CMV shedding, compared with 21% for bacterial sexually transmitted infection and 17% for HSV-2. Together, these observations highlight the importance of considering the prevalence of HHV shedding across populations and geographic locations in order to design effective HIV prevention strategies. In any case, a clinical trial is needed to definitively assess the degree to which CMV is causally associated with the shedding and transmission of HIV.
CMV, INFLAMMATION, AND IMMUNOSENESCENCE
Among all HHVs, CMV infection has one of the most dynamic and fierce interactions with the human immune system. In this complex host-virus relationship, the virus elicits and maintains a high frequency of CMV-specific T cells that are engaged in a life-long fight to restrain CMV replication and prevent life-threatening disease [33]. On the other side, CMV developed effective immune evasion strategies to prevent this immune response to clear infection or interfere with viral transmission. In fact, CMV is among the largest of the known viruses to infect humans (with a 230-kb genome), presumably with a large number of T-cell epitopes [33, 34]. Specifically, approximately 10% of both the CD4+ and CD8+ memory T cells circulating in blood are targeted toward CMV, and this percentage can increase to 50% of CD8+ and 30% of CD4+ T cells in older individuals.
As persons age with their CMV infection, the continual immune activation and response to CMV replication basically drive the T-cell repertoire toward a more differentiated T-cell phenotype, with indication of replicative exhaustion and senescence [35]. Therefore, CMV infection may compromise the response to other antigens by both shrinking the remaining T-cell repertoire (in favor of expansion of CMV-specific T cells) and decreasing T-cell diversity. As such, senescent T cells frequently bear antigen specificity toward CMV [36], and the abundance of senescent T cells correlates with a variety of negative outcomes, such as decreased vaccine responsiveness, autoimmunity, frailty, reduction in the T-cell receptor repertoire, cardiovascular disease, and poor responsiveness to new infections [37].
Persistent and intermittent CMV replication is also associated with a large bystander activation of non–CMV-specific T cells [38], probably secondary to the production of several chemokines at the site of CMV replication. Furthermore, CMV encodes both viral homologue chemokines and chemokine-like receptors, which also activate immune cells through a non–antigen-specific mechanism [18, 39, 40]. One recent study demonstrated that some strains of Rhesus CMV have the ability to divert CD8+ T-cell responses away from canonical CMV epitopes that probably constitute the most efficient targets for cytolysis [41]. Therefore, recurrent CMV shedding may further stress the immune resources in HIV infected individuals and accelerate progression to AIDS [3, 4].
In non–HIV-infected populations, there are associations between CMV immunoglobulin (Ig) G levels and increased carotid artery stiffness and ischemic heart disease. One of the largest of these studies was the Sacramento Area Latino Study on Aging (SALSA) [42]. In this population-based study of 1468 adults aged 60–101 years, individuals with the highest anti-CMV IgG titers had the greatest hazard of mortality (all-cause and cardiovascular). In another SALSA analysis, 1204 subjects were screened annually over a 4-year period with a modified Mini-Mental State Examination and a word list/learning test of delayed recall. Again, subjects with the highest anti-CMV IgG concentrations had worse neurocognitive outcomes [42]. In a separate analysis of HIV seronegative individuals older than 65 years, higher CMV IgG levels and higher CMV-specific CD4+ T-cell counts were both independently associated with worse neurocognitive performance and worse functional status [43].
Interestingly, a recent study among HIV/CMV coinfected individuals demonstrated that higher levels of CMV IgG were associated with lower levels of CMV replication, suggesting that increased CMV IgG levels do not primarily reflect frequent CMV reactivations but are more likely the consequence of a stronger immune response to CMV, leading to less reactivation [44]. Therefore, caution should be used when considering CMV IgG a surrogate marker of CMV activity and burden, and it is likely that the immune response to CMV is at least partly to blame for observed disease.
CMV, ACTIVATION/INFLAMMATION, AND END-ORGAN DISEASE IN THE SETTING OF HIV INFECTION
Immune activation is a hallmark of HIV-1 infection and plays an important role in the CD4+ T-cell depletion, immune dysfunction and a variety of clinical conditions [37]. Suppressive ART reduces the levels of T-cell activation and inflammation, but it does not normalize it, and the mechanisms of this persistent immune activation remain unknown. Furthermore, CMV infection induces systemic inflammation not only during primary infection but also during the chronic phase [37]. Indeed both viruses, HIV and CMV, are associated with increased immune activation and inflammation-related morbidities, including neurocognitive impairment, cancer, and cardiovascular disease [37, 45–48]. Because almost all HIV-infected individuals are coinfected with CMV, it is hard to distinguish between HIV, CMV, and combined effects on inflammation and disease progression [18]. Since the beginning of the HIV epidemic, the connections between HIV and CMV have been recognized, and many studies have indicated that CMV accelerates the development of HIV-dependent immunologic abnormalities [36, 45, 46].
Even asymptomatic shedding of CMV in the genital tract has been associated with increased T-cell immune activation and proliferation in peripheral blood [20, 49]. Interestingly, this CMV-associated immune activation occurs even when HIV replication is suppressed with ART [49]. To clarify some of the mechanistic underpinnings of these observations, Dan et al [50] recently investigated associations between asymptomatic CMV replication in the genital tract and programmed death 1 (PD-1) expression on circulating CD4 T cells during suppressive ART and found that PD-1 expression was increased during CMV shedding. Because increased PD-1 expression on T cells has been implicated in the maintenance of the HIV reservoir, HIV disease progression, and the inability of the immune system to adequately control HIV infection [51], the mechanisms connecting CMV, HIV and PD-1 expression deserve further attention.
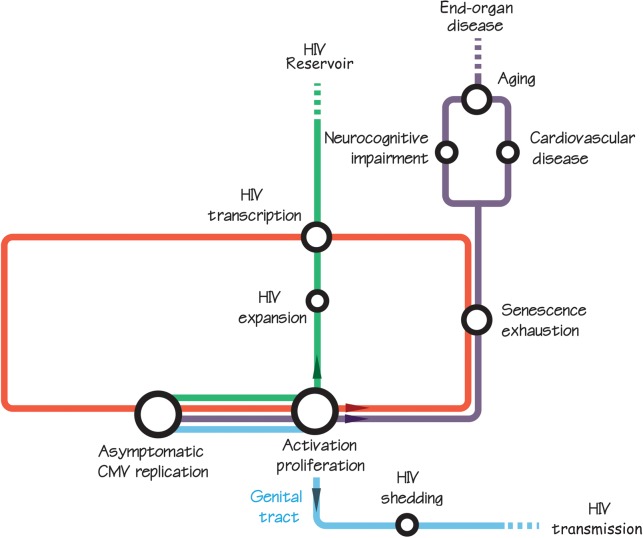
The links between CMV shedding and systemic inflammation during HIV infection was examined in a randomized control study that showed a reduction in T-cell immune activation when HIV-infected individuals were treated with the anti-CMV drug valganciclovir [52]. Although this study was limited, in that only 70% of the subjects had HIV RNA suppression with ART and the sample size was small (n = 30), it did show a sustained reduction in CMV shedding and immune activation 4 weeks after valganciclovir therapy was stopped. This suggests that reducing immune activation might also reduce CMV shedding, and if such a circular feedback is true, then the anti-inflammatory benefit of reducing CMV replication could be greatly compounded with multiple cycles of anti-CMV therapy (see Figure 1). In contrast, a more recent randomized trial of valacyclovir in ART-suppressed HSV-2/HIV–coinfected individuals failed to show decreased systemic immune activation, suggesting that HSV-1/2 is not likely to be responsible for the decrease in immune activation we observed in the valganciclovir trial, further increasing the likelihood that the effect was mediated by CMV and not other HHVs [53].
Figure 1.
Theoretical model connecting asymptomatic cytomegalovirus (CMV) replication with human immunodeficiency virus (HIV) transmission (blue line), larger HIV DNA reservoir (green line), and end-organ disease (purple line). We hypothesize a circular feedback loop between CMV and HIV replication, immune activation and proliferation, and cell dysfunction (senescence and exhaustion) (red loop). In other words, CMV and HIV replication are probably responsible for increasing immune activation and T-cell dysfunction, which in turn further enhance CMV and HIV replication. This persistent immune activation has been repeatedly associated with neurocognitive and cardiovascular disease, aging, and increased viral reservoir, and CMV is probably part of that mechanism.
POSSIBLE BENEFIT OF HHV/HIV COINFECTION
Even if HHV and HIV have many pathogenic codynamics, virus-to-virus interactions are not always negative for the human host. For example, HHV-6 can suppress the replication of CCR5-tropic HIV, the predominant form of transmitted HIV, and HHV-7 replication can down-regulate the CD4 receptor on T cells, consequently reducing the infection of CD4+ T cells by HIV [18]. Further, a recent study observed that ART-naive, recently HIV-1–infected adults coinfected with HSV-2 at the time of HIV-1 acquisition had higher CD4+ T-cell counts over time than individuals infected with HIV alone, and this was independent of HIV RNA levels in blood [54]. Similarly, increased rate of HSV shedding has also been associated with higher CD4+ T-cell count among HIV-infected persons [12]. Similarly, another study showed that mice latently infected with either murine gammaherpesvirus 68 or murine CMV (which are genetically similar to the human pathogens EBV and CMV) are resistant to some bacterial infections [55]. Taken together, interactions between HIV and HHV are complex, and HHV-associated immune stimulation could have both positive and negative effects.
CMV AND HIV DNA RESERVOIR
There is convincing evidence that chronic inflammation and immune activation helps maintain the HIV reservoir during ART [37]. To draw the link between CMV shedding, systemic inflammation, and maintenance of the HIV reservoir, recent studies have demonstrated that asymptomatic CMV seminal shedding is associated with increased levels of total HIV DNA in both ART-naive individuals [56] and individuals suppressed during long-term ART [49]. Furthermore, in a large longitudinal study of 108 individuals followed up since the earliest phase of HIV infection, there was a significant positive association between longitudinal levels of CD4+ T-cell–associated HIV DNA in blood and the frequency of detectable CMV DNA in blood cells [57]. In addition, in vitro studies demonstrated that CMV infection may facilitate HIV DNA entry in cells that are ordinarily nonpermissive, such as fibroblasts [58]. Although the observational design of these studies does not allow causality to be inferred, and the investigators did not specifically evaluate the replication competent HIV DNA subset, this finding does support the theory that asymptomatic CMV replication could drive local and systemic immune activation with a subsequent increase in the HIV reservoir. It may follow that targeting drivers of chronic inflammation, such as CMV, might be important to consider in curative strategies for HIV. Confirming this hypothesis will require a large randomized, placebo-controlled clinical trial of antiviral therapy aimed at stopping CMV shedding in the male genital tract as a way to reduce the HIV reservoir. Such a trial will be difficult with currently approved anti-CMV therapies, given their inherent toxic effects [47], but newer anti-CMV therapies may hold such promise.
CONCLUSIONS
The virologic and immunologic connections between HHV and HIV are close and complex. A detailed knowledge of host-mediated interactions between HHV and HIV is necessary, not only to understand the complex mechanisms of HIV infection, but also for the development of new anti-HIV therapies. Interactions between HHV (especially CMV) and HIV will be difficult to address with available technologies. Current anti-CMV therapies are toxic and not applicable on a large scale or for prolonged periods of time. Newer and less toxic anti-CMV drugs (eg, brincidofovir [59] or letermovir [60]) are in development and should be evaluated as part of future clinical trials. Of course, none of these drugs will eradicate HHV, and prolonged course of therapy will be necessary and might still not be enough to reverse the inflammatory process initiated with cellular infection. Therapeutic or prophylactic vaccines against CMV (and other HHVs) are also in development, but it is unclear whether an additional (vaccine-induced) stimulation of the immune system might further enhance the inflammatory process. Therefore, more interventional studies are needed to determine whether suppression of HHV replication (not just HSV) will have a positive effect on HIV disease progression and transmission. In particular, considering the strong association between CMV and HIV-1, such trials should test the effect of anti-CMV drugs to help determine whether anti-CMV therapies can help in the fight against HIV-related disease and perhaps in the effort to find a cure for HIV infection.
Notes
Acknowledgments. We are very thankful to Peter Hunt, Leonid Margolis, and Christophe Vanpouille for their useful comments and feedback.
Financial support. This work was supported by the Department of Veterans Affairs, the National Institutes of Health (grants AI43638, AI100665, MH097520, DA034978, AI036214, AI007384, AI027763, AI106039, AI074621, AI110181, 7-UM1 AI068636-07, P30-AI027763, UL1TR000100, and amfAR grant 108537 [with support from Foundation for AIDS and Immune Research]), and the James B. Pendleton Charitable Trust.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Van de Perre P, Segondy M, Foulongne V, et al. Herpes simplex virus and HIV-1: deciphering viral synergy. Lancet Infect Dis 2008; 8:490–7. [DOI] [PubMed] [Google Scholar]
- 2.McGeoch DJ, Cook S, Dolan A, Jamieson FE, Telford EA. Molecular phylogeny and evolutionary timescale for the family of mammalian herpesviruses. J Mol Biol 1995; 247:443–58. [DOI] [PubMed] [Google Scholar]
- 3.Griffiths PD. CMV as a cofactor enhancing progression of AIDS. J Clin Virol 2006; 35:489–92. [DOI] [PubMed] [Google Scholar]
- 4.Webster A, Lee CA, Cook DG, et al. Cytomegalovirus infection and progression towards AIDS in haemophiliacs with human immunodeficiency virus infection. Lancet 1989; 2:63–6. [DOI] [PubMed] [Google Scholar]
- 5.Dollard SC, Butler LM, Jones AM, et al. Substantial regional differences in human herpesvirus 8 seroprevalence in sub-Saharan Africa: insights on the origin of the “Kaposi's sarcoma belt”. Int J Cancer 2010; 127:2395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fishman JA. Overview: cytomegalovirus and the herpesviruses in transplantation. Am J Transplant 2013; 13:1–8. [DOI] [PubMed] [Google Scholar]
- 7.Wertheim JO, Smith MD, Smith DM, Scheffler K, Kosakovsky Pond SL. Evolutionary origins of human herpes simplex viruses 1 and 2. Mol Biol Evol 2014; 31:2356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavergne A, Donato D, Gessain A, et al. African great apes are naturally infected with roseoloviruses closely related to human herpesvirus 7. J Virol 2014; 88:1321–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnabas RV, Celum C. Infectious co-factors in HIV-1 transmission herpes simplex virus type-2 and HIV-1: new insights and interventions. Curr HIV Res 2012; 10:228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koelle DM, Corey L. Herpes simplex: insights on pathogenesis and possible vaccines. Annu Rev Med 2008; 59:381–95. [DOI] [PubMed] [Google Scholar]
- 11.Mark KE, Wald A, Magaret AS, et al. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis 2008; 198:1141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gianella S, Morris SR, Anderson C, et al. Herpesviruses and HIV-1 drug resistance mutations influence the virologic and immunologic milieu of the male genital tract. AIDS 2013; 27:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lisco A, Munawwar A, Introini A, et al. Semen of HIV-1-infected individuals: local shedding of herpesviruses and reprogrammed cytokine network. J Infect Dis 2012; 205:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.UNAIDS. Gap report 2014. Available at: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf. Accessed 15 February 2015. [Google Scholar]
- 15.Worobey M, Gemmel M, Teuwen DE, et al. Direct evidence of extensive diversity of HIV-1 in Kinshasa by 1960. Nature 2008; 455:661–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet 2014; 384:258–71. [DOI] [PubMed] [Google Scholar]
- 17.Tan DH, Murphy K, Shah P, Walmsley SL. Herpes simplex virus type 2 and HIV disease progression: a systematic review of observational studies. BMC Infect Dis 2013; 13:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lisco A, Vanpouille C, Margolis L. Coinfecting viruses as determinants of HIV disease. Curr HIV/AIDS Rep 2009; 6:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gianella S, Morris SR, Vargas MV, et al. The role of seminal shedding of herpesviruses in HIV-1 transmission. J Infect Dis 2012; 207:257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gianella S, Strain MC, Rought SE, et al. Associations between virologic and immunologic dynamics in blood and in the male genital tract. J Virol 2012; 86:1307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Margolis DM, Rabson AB, Straus SE, Ostrove JM. Transactivation of the HIV-1 LTR by HSV-1 immediate-early genes. Virology 1992; 186:788–91. [DOI] [PubMed] [Google Scholar]
- 22.Johnson EL, Howard CL, Thurman J, Pontiff K, Johnson ES, Chakraborty R. CMV upregulates expression of CCR5 in central memory TCM cord blood mononuclear cells which may facilitate in utero HIV-1 transmission. J Infect Dis 2015; 211:187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baggaley RF, Griffin JT, Chapman R, et al. Estimating the public health impact of the effect of herpes simplex virus suppressive therapy on plasma HIV-1 viral load. AIDS 2009; 23:1005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HN, Wang J, Hughes J, et al. Effect of acyclovir on HIV-1 set point among herpes simplex virus type 2-seropositive persons during early HIV-1 infection. J Infect Dis 2010; 202:734–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med 2010; 362:427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abu-Raddad LJ, Magaret AS, Celum C, et al. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS One 2008; 3:e2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Posavad CM, Zhao L, Mueller DE, et al. Persistence of mucosal T-cell responses to herpes simplex virus type 2 in the female genital tract. Mucosal Immunol 2015; 8:115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanpouille C, Lisco A, Introini A, et al. Exploiting the anti-HIV-1 activity of acyclovir: suppression of primary and drug-resistant HIV isolates and potentiation of the activity by ribavirin. Antimicrob Agents Chemother 2012; 56:2604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanpouille C, Lisco A, Grivel JC, et al. Valacyclovir decreases plasma HIV-1 RNA in HSV-2 seronegative individuals: a randomized placebo-controlled crossover trial. Clin Infect Dis 2015; doi:10.1093/cid/civ172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Templeton DJ. Male circumcision to reduce sexual transmission of HIV. Curr Opin HIV AIDS 2010; 5:344–9. [DOI] [PubMed] [Google Scholar]
- 31.Gianella S, Smith DM, Vargas MV, et al. Shedding of HIV and human herpesviruses in the semen of effectively treated HIV-1-infected men who have sex with men. Clin Infect Dis 2013; 57:441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gianella S, Scheffler K, Mehta SR, et al. Seminal shedding of CMV and HIV transmission among men who have sex with men [abstract 1020]. In: 22nd Conference on Retroviruses and Opportunistic Infections (CROI) Seattle, WA, 23–26 February 2015. [Google Scholar]
- 33.Sylwester AW, Mitchell BL, Edgar JB, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 2005; 202:673–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Margolick JB, Bream JH, et al. Heterogeneity of CD4+ and CD8+ T-cell responses to cytomegalovirus in HIV-infected and HIV-uninfected men who have sex with men. J Infect Dis 2014; 210:400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fletcher JM, Vukmanovic-Stejic M, Dunne PJ, et al. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J Immunol 2005; 175:8218–25. [DOI] [PubMed] [Google Scholar]
- 36.Appay V, Fastenackels S, Katlama C, et al. Old age and anti-cytomegalovirus immunity are associated with altered T-cell reconstitution in HIV-1-infected patients. AIDS 2011; 25:1813–22. [DOI] [PubMed] [Google Scholar]
- 37.Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep 2012; 9:139–47. [DOI] [PubMed] [Google Scholar]
- 38.McNally JM, Zarozinski CC, Lin MY, Brehm MA, Chen HD, Welsh RM. Attrition of bystander CD8 T cells during virus-induced T-cell and interferon responses. J Virol 2001; 75:5965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dammermann W, Bochmann D, Bentzien F, et al. CMV specific cytokine release assay in whole blood is optimized by combining synthetic CMV peptides and toll like receptor agonists. J Immunol Methods 2014; 414:82–90. [DOI] [PubMed] [Google Scholar]
- 40.Suni MA, Ghanekar SA, Houck DW, et al. CD4+CD8dim T lymphocytes exhibit enhanced cytokine expression, proliferation and cytotoxic activity in response to HCMV and HIV-1 antigens. Eur J Immunol 2001; 31:2512–20. [DOI] [PubMed] [Google Scholar]
- 41.Hansen SG, Sacha JB, Hughes CM, et al. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science 2013; 340:1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol 2010; 172:363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vescovini R, Biasini C, Telera AR, et al. Intense antiextracellular adaptive immune response to human cytomegalovirus in very old subjects with impaired health and cognitive and functional status. J Immunol 2010; 184:3242–9. [DOI] [PubMed] [Google Scholar]
- 44.Gianella S, Morris SR, Tatro E, et al. Virologic correlates of anti-CMV IgG levels in HIV-1 infected men. J Infect Dis 2014; 209:452–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sacre K, Hunt PW, Hsue PY, et al. A role for cytomegalovirus-specific CD4+CX3CR1+ T cells and cytomegalovirus-induced T-cell immunopathology in HIV-associated atherosclerosis. AIDS 2012; 26:805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Detels R, Leach CT, Hennessey K, et al. Persistent cytomegalovirus infection of semen increases risk of AIDS. J Infect Dis 1994; 169:766–8. [DOI] [PubMed] [Google Scholar]
- 47.Soderberg-Naucler C. Treatment of cytomegalovirus infections beyond acute disease to improve human health. Expert RevAnti Infect Ther 2014; 12:211–22. [DOI] [PubMed] [Google Scholar]
- 48.Lichtner M, Cicconi P, Vita S, et al. Cytomegalovirus coinfection is associated with an increased risk of severe non-AIDS-defining events in a large cohort of HIV-infected patients. J Infect Dis 2015; 211:178–86. [DOI] [PubMed] [Google Scholar]
- 49.Gianella S, Massanella M, Richman DD, et al. Cytomegalovirus replication in semen is associated with higher levels of proviral HIV DNA and CD4+ T cell activation during antiretroviral treatment. J Virol 2014; 88:7818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dan J, Massanella M, Spina C, et al. Effect of CMV and HIV replication on T cell exhaustion and senescence during ART [abstract 300]. In: 22nd Conference on Retroviruses and Opportunistic Infections (CROI). Seattle, WA, 23–26 February 2015. [Google Scholar]
- 51.Cockerham LR, Jain V, Sinclair E, et al. Programmed death-1 expression on CD4+ and CD8+ T cells in treated and untreated HIV disease. AIDS 2014; 28:1749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunt PW, Martin JN, Sinclair E, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis 2011; 203:1474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yi TJ, Walmsley S, Szadkowski L, et al. A randomized controlled pilot trial of valacyclovir for attenuating inflammation and immune activation in HIV/herpes simplex virus 2-coinfected adults on suppressive antiretroviral therapy. Clin Infect Dis 2013; 57:1331–8. [DOI] [PubMed] [Google Scholar]
- 54.Barbour JD, Sauer MM, Sharp ER, et al. HIV-1/HSV-2 co-infected adults in early HIV-1 infection have elevated CD4+ T cell counts. PLoS One 2007; 2:e1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barton ES, White DW, Cathelyn JS, et al. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 2007; 447:326–9. [DOI] [PubMed] [Google Scholar]
- 56.Gianella S, Anderson CM, Vargas MV, et al. CMV DNA in semen and blood is associated with higher levels of proviral HIV DNA. J Infect Dis 2012; 207:898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gianella S, Anderson C, Var SR, et al. Detectable CMV in PBMC is associated with slower HIV DNA decay during suppressive ART [abstract 375]. In: 22nd Conference on Retroviruses and Opportunistic Infections (CROI) Seattle, WA, 23–26 February 2015. [Google Scholar]
- 58.Margalith M, D'Aquila RT, Manion DJ, et al. HIV-1 DNA in fibroblast cultures infected with urine from HIV-seropositive cytomegalovirus (CMV) excretors. Arch Virol 1995; 140:927–35. [DOI] [PubMed] [Google Scholar]
- 59.Marty FM, Winston DJ, Rowley SD, et al. CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med 2013; 369:1227–36. [DOI] [PubMed] [Google Scholar]
- 60.Verghese PS, Schleiss MR. Letermovir treatment of human cytomegalovirus infection antiinfective agent. Drugs Future 2013; 38:291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]