Abstract
Antibodies against horseradish peroxidase (anti-HRP) recognize neural specific cell surface antigens in Drosophila and other insects. The nature of these antigens was investigated in Drosophila and found to include a complex set of developmentally regulated proteins. Their common epitope appears to be a carbohydrate that shares features with the sugar moiety of pineapple stem bromelain, a plant glycoprotein whose carbohydrate structure has been determined. A mutation was identified that eliminates staining by the antibody in imaginal and adult neural tissue. Tissue specific glycoconjugates, although widespread in the animal kingdom, are little understood. This mutation provides a unique opportunity to address the consequences of altering a neural specific carbohydrate moiety in an otherwise intact and behaving animal. The mutation maps to 84F. A second mutation, contained on the third chromosome balancer, TM3, eliminates anti-HRP staining in embryos. These mutations appear to be separate genes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayna E. M., Shaper J. H., Shur B. D. Temporally specific involvement of cell surface beta-1,4 galactosyltransferase during mouse embryo morula compaction. Cell. 1988 Apr 8;53(1):145–157. doi: 10.1016/0092-8674(88)90496-5. [DOI] [PubMed] [Google Scholar]
- Bentley D., Caudy M. Navigational substrates for peripheral pioneer growth cones: limb-axis polarity cues, limb-segment boundaries, and guidepost neurons. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):573–585. doi: 10.1101/sqb.1983.048.01.062. [DOI] [PubMed] [Google Scholar]
- Bier E., Ackerman L., Barbel S., Jan L., Jan Y. N. Identification and characterization of a neuron-specific nuclear antigen in Drosophila. Science. 1988 May 13;240(4854):913–916. doi: 10.1126/science.3129785. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burridge K. Direct identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Methods Enzymol. 1978;50:54–64. doi: 10.1016/0076-6879(78)50007-4. [DOI] [PubMed] [Google Scholar]
- Butters T. D., Hughes R. C., Vischer P. Steps in the biosynthesis of mosquito cell membrane glycoproteins and the effects of tunicamycin. Biochim Biophys Acta. 1981 Feb 6;640(3):672–686. doi: 10.1016/0005-2736(81)90097-3. [DOI] [PubMed] [Google Scholar]
- Campbell A. G., Fessler L. I., Salo T., Fessler J. H. Papilin: a Drosophila proteoglycan-like sulfated glycoprotein from basement membranes. J Biol Chem. 1987 Dec 25;262(36):17605–17612. [PubMed] [Google Scholar]
- Caudy M., Bentley D. Pioneer growth cone morphologies reveal proximal increases in substrate affinity within leg segments of grasshopper embryos. J Neurosci. 1986 Feb;6(2):364–379. doi: 10.1523/JNEUROSCI.06-02-00364.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudy M., Bentley D. Pioneer growth cone steering along a series of neuronal and non-neuronal cues of different affinities. J Neurosci. 1986 Jun;6(6):1781–1795. doi: 10.1523/JNEUROSCI.06-06-01781.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J., Shannon L. M. The isolation and characterization of the glycopeptides from horseradish peroxidase isoenzyme C. Biochim Biophys Acta. 1976 Apr 14;427(2):428–442. doi: 10.1016/0005-2795(76)90186-0. [DOI] [PubMed] [Google Scholar]
- Duerr J. S., Quinn W. G. Three Drosophila mutations that block associative learning also affect habituation and sensitization. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3646–3650. doi: 10.1073/pnas.79.11.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan I. W., Kaufman T. C. Cytogenic analysis of chromosome 3 in Drosophila melanogaster: mapping of the proximal portion of the right arm. Genetics. 1975 Aug;80(4):733–752. doi: 10.1093/genetics/80.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985 Mar 7;314(6006):53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- Florman H. M., Wassarman P. M. O-linked oligosaccharides of mouse egg ZP3 account for its sperm receptor activity. Cell. 1985 May;41(1):313–324. doi: 10.1016/0092-8674(85)90084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganetzky B., Wu C. F. Neurogenetics of membrane excitability in Drosophila. Annu Rev Genet. 1986;20:13–44. doi: 10.1146/annurev.ge.20.120186.000305. [DOI] [PubMed] [Google Scholar]
- Glabe C. G., Grabel L. B., Vacquier V. D., Rosen S. D. Carbohydrate specificity of sea urchin sperm bindin: a cell surface lectin mediating sperm-egg adhesion. J Cell Biol. 1982 Jul;94(1):123–128. doi: 10.1083/jcb.94.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelrigg T., Kaufman T. C. Revertants of Dominant Mutations Associated with the Antennapedia Gene Complex of DROSOPHILA MELANOGASTER: Cytology and Genetics. Genetics. 1983 Nov;105(3):581–600. doi: 10.1093/genetics/105.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden J. J., Suzuki D. T. Temperature-sensitive mutations in Drosophila melanogaster. XII. The genetic and developmental effects of dominant lethals on chromosome 3. Genetics. 1973 Mar;73(3):445–458. doi: 10.1093/genetics/73.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh P., Robbins P. W. Regulation of asparagine-linked oligosaccharide processing. Oligosaccharide processing in Aedes albopictus mosquito cells. J Biol Chem. 1984 Feb 25;259(4):2375–2382. [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Antibodies to horseradish peroxidase as specific neuronal markers in Drosophila and in grasshopper embryos. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2700–2704. doi: 10.1073/pnas.79.8.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
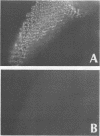
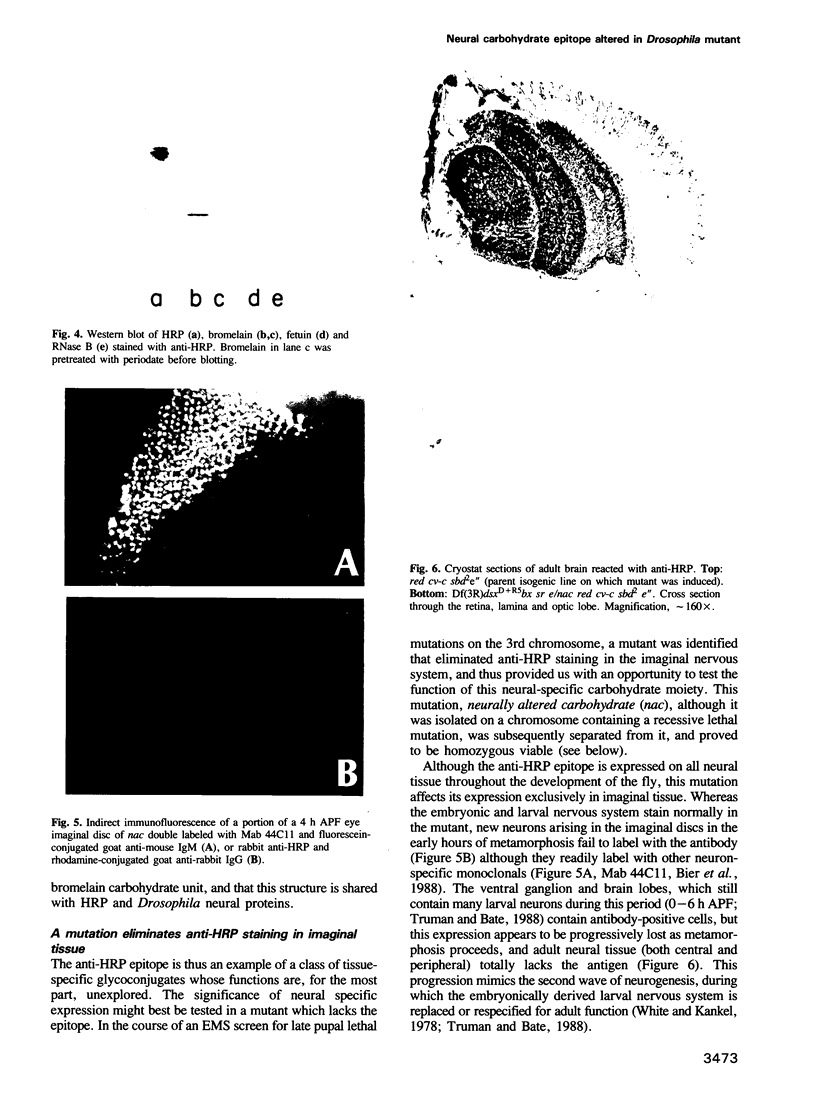
- Jan Y. N., Ghysen A., Christoph I., Barbel S., Jan L. Y. Formation of neuronal pathways in the imaginal discs of Drosophila melanogaster. J Neurosci. 1985 Sep;5(9):2453–2464. doi: 10.1523/JNEUROSCI.05-09-02453.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künemund V., Jungalwala F. B., Fischer G., Chou D. K., Keilhauer G., Schachner M. The L2/HNK-1 carbohydrate of neural cell adhesion molecules is involved in cell interactions. J Cell Biol. 1988 Jan;106(1):213–223. doi: 10.1083/jcb.106.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murray M. A., Schubiger M., Palka J. Neuron differentiation and axon growth in the developing wing of Drosophila melanogaster. Dev Biol. 1984 Aug;104(2):259–273. doi: 10.1016/0012-1606(84)90082-4. [DOI] [PubMed] [Google Scholar]
- Olden K., Bernard B. A., White S. L., Parent J. B. Function of the carbohydrate moieties of glycoproteins. J Cell Biochem. 1982;18(3):313–335. doi: 10.1002/jcb.1982.240180306. [DOI] [PubMed] [Google Scholar]
- Phelps C., Forlani L., Antonini E. The hydrogen ion equilibria of horseradish peroxidase and apoperoxidase. Biochem J. 1971 Sep;124(3):605–614. doi: 10.1042/bj1240605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvaterra P. M., Bournias-Vardiabasis N., Nair T., Hou G., Lieu C. In vitro neuronal differentiation of Drosophila embryo cells. J Neurosci. 1987 Jan;7(1):10–22. doi: 10.1523/JNEUROSCI.07-01-00010.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow P. M., Patel N. H., Harrelson A. L., Goodman C. S. Neural-specific carbohydrate moiety shared by many surface glycoproteins in Drosophila and grasshopper embryos. J Neurosci. 1987 Dec;7(12):4137–4144. doi: 10.1523/JNEUROSCI.07-12-04137.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
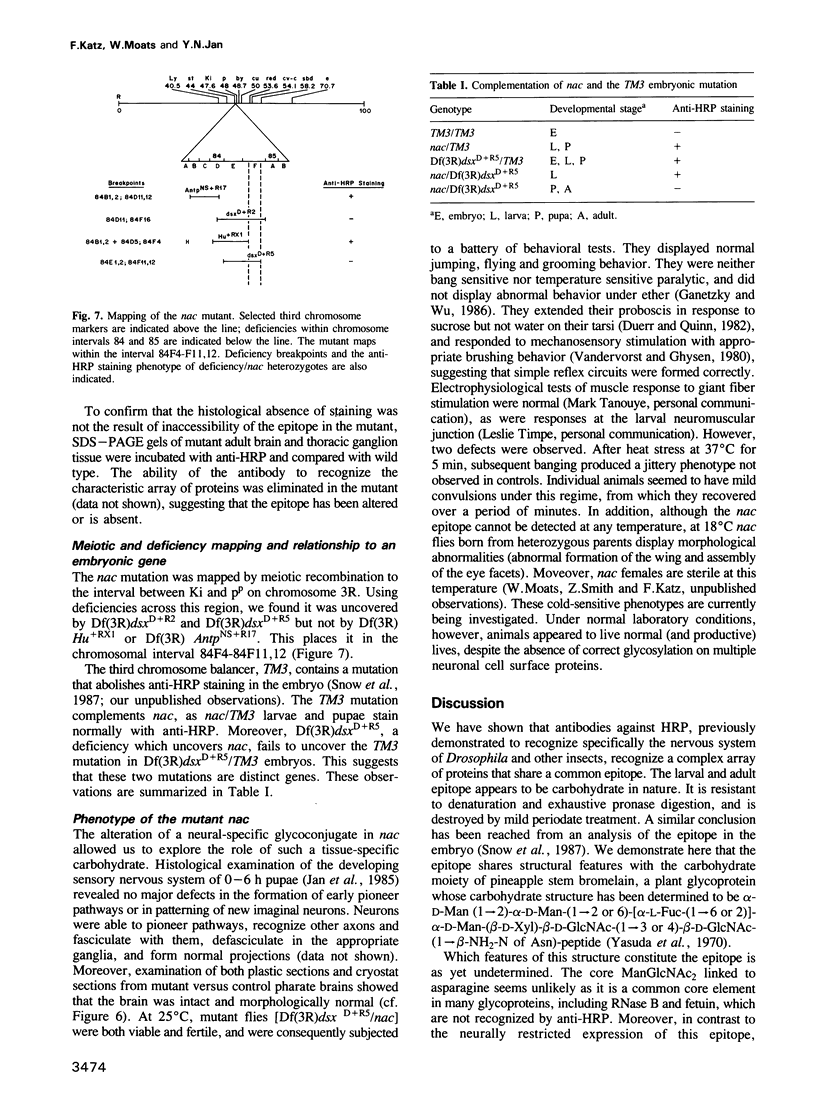
- Truman J. W., Bate M. Spatial and temporal patterns of neurogenesis in the central nervous system of Drosophila melanogaster. Dev Biol. 1988 Jan;125(1):145–157. doi: 10.1016/0012-1606(88)90067-x. [DOI] [PubMed] [Google Scholar]
- Vandervorst P., Ghysen A. Genetic control of sensory connections in Drosophila. Nature. 1980 Jul 3;286(5768):65–67. doi: 10.1038/286065a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Welinder K. G., Smillie L. B. Amino acid sequence studies of horseradish peroxidase. II. Thermolytic peptides. Can J Biochem. 1972 Jan;50(1):63–90. doi: 10.1139/o72-009. [DOI] [PubMed] [Google Scholar]
- White K., Kankel D. R. Patterns of cell division and cell movement in the formation of the imaginal nervous system in Drosophila melanogaster. Dev Biol. 1978 Aug;65(2):296–321. doi: 10.1016/0012-1606(78)90029-5. [DOI] [PubMed] [Google Scholar]
- Yasuda Y., Takahashi N., Murachi T. The composition and structure of carbohydrate moiety of stem bromelain. Biochemistry. 1970 Jan 6;9(1):25–32. doi: 10.1021/bi00803a004. [DOI] [PubMed] [Google Scholar]
- Yednock T. A., Stoolman L. M., Rosen S. D. Phosphomannosyl-derivatized beads detect a receptor involved in lymphocyte homing. J Cell Biol. 1987 Mar;104(3):713–723. doi: 10.1083/jcb.104.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]