Abstract
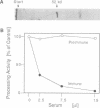
Two proteins co-operate in the proteolytic cleavage of mitochondrial precursor proteins: the mitochondrial processing peptidase (MPP) and the processing enhancing protein (PEP). In order to understand the structure and function of this novel peptidase, we have isolated mutants of Saccharomyces cerevisiae which were temperature sensitive in the processing of mitochondrial precursor proteins. Here we report on the mif2 mutation which is deficient in MPP. Mitochondria from the mif2 mutant were able to import precursor proteins, but not to cleave the presequences. The MPP gene was isolated. MPP is a hydrophilic protein consisting of 482 amino acids. Notably, MPP exhibits remarkable sequence similarity to PEP. We speculate that PEP and MPP have a common origin and have evolved into two components with different but mutually complementing functions in processing of precursor proteins.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cheng M. Y., Pollock R. A., Hendrick J. P., Horwich A. L. Import and processing of human ornithine transcarbamoylase precursor by mitochondria from Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4063–4067. doi: 10.1073/pnas.84.12.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy J. G., Fenton W. A., Rosenberg L. E. Processing of pre-ornithine transcarbamylase requires a zinc-dependent protease localized to the mitochondrial matrix. Biochem Biophys Res Commun. 1982 Mar 15;105(1):1–7. doi: 10.1016/s0006-291x(82)80002-8. [DOI] [PubMed] [Google Scholar]
- Daum G., Gasser S. M., Schatz G. Import of proteins into mitochondria. Energy-dependent, two-step processing of the intermembrane space enzyme cytochrome b2 by isolated yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13075–13080. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Vassarotti A., Garrett J., Geller B. L., Takeda M., Douglas M. G. The amino terminus of the yeast F1-ATPase beta-subunit precursor functions as a mitochondrial import signal. J Cell Biol. 1986 Feb;102(2):523–533. doi: 10.1083/jcb.102.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiard B. Structure, expression and regulation of a nuclear gene encoding a mitochondrial protein: the yeast L(+)-lactate cytochrome c oxidoreductase (cytochrome b2). EMBO J. 1985 Dec 1;4(12):3265–3272. doi: 10.1002/j.1460-2075.1985.tb04076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl F. U., Ostermann J., Guiard B., Neupert W. Successive translocation into and out of the mitochondrial matrix: targeting of proteins to the intermembrane space by a bipartite signal peptide. Cell. 1987 Dec 24;51(6):1027–1037. doi: 10.1016/0092-8674(87)90589-7. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Schmidt B., Wachter E., Weiss H., Neupert W. Transport into mitochondria and intramitochondrial sorting of the Fe/S protein of ubiquinol-cytochrome c reductase. Cell. 1986 Dec 26;47(6):939–951. doi: 10.1016/0092-8674(86)90809-3. [DOI] [PubMed] [Google Scholar]
- Hawlitschek G., Schneider H., Schmidt B., Tropschug M., Hartl F. U., Neupert W. Mitochondrial protein import: identification of processing peptidase and of PEP, a processing enhancing protein. Cell. 1988 Jun 3;53(5):795–806. doi: 10.1016/0092-8674(88)90096-7. [DOI] [PubMed] [Google Scholar]
- Hay R., Böhni P., Gasser S. How mitochondria import proteins. Biochim Biophys Acta. 1984 Jan 27;779(1):65–87. doi: 10.1016/0304-4157(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich A. L., Kalousek F., Fenton W. A., Pollock R. A., Rosenberg L. E. Targeting of pre-ornithine transcarbamylase to mitochondria: definition of critical regions and residues in the leader peptide. Cell. 1986 Feb 14;44(3):451–459. doi: 10.1016/0092-8674(86)90466-6. [DOI] [PubMed] [Google Scholar]
- Horwich A. L., Kalousek F., Mellman I., Rosenberg L. E. A leader peptide is sufficient to direct mitochondrial import of a chimeric protein. EMBO J. 1985 May;4(5):1129–1135. doi: 10.1002/j.1460-2075.1985.tb03750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Schatz G. The cleavable prepiece of an imported mitochondrial protein is sufficient to direct cytosolic dihydrofolate reductase into the mitochondrial matrix. FEBS Lett. 1984 Dec 10;178(2):306–310. doi: 10.1016/0014-5793(84)80622-5. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalousek F., François B., Rosenberg L. E. Isolation and characterization of ornithine transcarbamylase from normal human liver. J Biol Chem. 1978 Jun 10;253(11):3939–3944. [PubMed] [Google Scholar]
- Kalousek F., Orsulak M. D., Rosenberg L. E. Newly processed ornithine transcarbamylase subunits are assembled to trimers in rat liver mitochondria. J Biol Chem. 1984 May 10;259(9):5392–5395. [PubMed] [Google Scholar]
- Keng T., Alani E., Guarente L. The nine amino-terminal residues of delta-aminolevulinate synthase direct beta-galactosidase into the mitochondrial matrix. Mol Cell Biol. 1986 Feb;6(2):355–364. doi: 10.1128/mcb.6.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McAda P. C., Douglas M. G. A neutral metallo endoprotease involved in the processing of an F1-ATPase subunit precursor in mitochondria. J Biol Chem. 1982 Mar 25;257(6):3177–3182. [PubMed] [Google Scholar]
- Miura S., Mori M., Amaya Y., Tatibana M. A mitochondrial protease that cleaves the precursor of ornithine carbamoyltransferase. Purification and properties. Eur J Biochem. 1982 Mar 1;122(3):641–647. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Hartl F. U., Guiard B., Neupert W. Mitochondrial precursor proteins are imported through a hydrophilic membrane environment. Eur J Biochem. 1987 Dec 1;169(2):289–293. doi: 10.1111/j.1432-1033.1987.tb13610.x. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Hartl F. U., Neupert W. Import of proteins into mitochondria: a multi-step process. Eur J Biochem. 1988 Aug 1;175(2):205–212. doi: 10.1111/j.1432-1033.1988.tb14185.x. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Tropschug M., Neupert W. Mitochondrial protein import: nucleoside triphosphates are involved in conferring import-competence to precursors. Cell. 1987 Jun 19;49(6):815–823. doi: 10.1016/0092-8674(87)90619-2. [DOI] [PubMed] [Google Scholar]
- Schleyer M., Neupert W. Transport of proteins into mitochondria: translocational intermediates spanning contact sites between outer and inner membranes. Cell. 1985 Nov;43(1):339–350. doi: 10.1016/0092-8674(85)90039-x. [DOI] [PubMed] [Google Scholar]
- Schmidt B., Wachter E., Sebald W., Neupert W. Processing peptidase of Neurospora mitochondria. Two-step cleavage of imported ATPase subunit 9. Eur J Biochem. 1984 Nov 2;144(3):581–588. doi: 10.1111/j.1432-1033.1984.tb08505.x. [DOI] [PubMed] [Google Scholar]
- Schwaiger M., Herzog V., Neupert W. Characterization of translocation contact sites involved in the import of mitochondrial proteins. J Cell Biol. 1987 Jul;105(1):235–246. doi: 10.1083/jcb.105.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stueber D., Ibrahimi I., Cutler D., Dobberstein B., Bujard H. A novel in vitro transcription-translation system: accurate and efficient synthesis of single proteins from cloned DNA sequences. EMBO J. 1984 Dec 20;3(13):3143–3148. doi: 10.1002/j.1460-2075.1984.tb02271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassarotti A., Chen W. J., Smagula C., Douglas M. G. Sequences distal to the mitochondrial targeting sequences are necessary for the maturation of the F1-ATPase beta-subunit precursor in mitochondria. J Biol Chem. 1987 Jan 5;262(1):411–418. [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]
- Witte C., Jensen R. E., Yaffe M. P., Schatz G. MAS1, a gene essential for yeast mitochondrial assembly, encodes a subunit of the mitochondrial processing protease. EMBO J. 1988 May;7(5):1439–1447. doi: 10.1002/j.1460-2075.1988.tb02961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe M. P., Ohta S., Schatz G. A yeast mutant temperature-sensitive for mitochondrial assembly is deficient in a mitochondrial protease activity that cleaves imported precursor polypeptides. EMBO J. 1985 Aug;4(8):2069–2074. doi: 10.1002/j.1460-2075.1985.tb03893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe M. P., Schatz G. Two nuclear mutations that block mitochondrial protein import in yeast. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4819–4823. doi: 10.1073/pnas.81.15.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwizinski C., Neupert W. Precursor proteins are transported into mitochondria in the absence of proteolytic cleavage of the additional sequences. J Biol Chem. 1983 Nov 10;258(21):13340–13346. [PubMed] [Google Scholar]
- Zwizinski C., Schleyer M., Neupert W. Proteinaceous receptors for the import of mitochondrial precursor proteins. J Biol Chem. 1984 Jun 25;259(12):7850–7856. [PubMed] [Google Scholar]