Abstract
Study Objectives:
Older adults and patients with dementia often have disrupted circadian activity rhythms (CARs). Disrupted CARs are associated with health declines and could affect cognitive aging. We hypothesized that among older women, weaker CARs would be associated with poorer cognitive function 5 y later.
Design:
Prospective observational study.
Setting:
Three US clinical sites.
Participants:
There were 1,287 community-dwelling older women (82.8 ± 3.1 y) participating in an ongoing prospective study who were free of dementia at the baseline visit.
Measurements and Results:
Baseline actigraphy was used to determine CAR measures (amplitude, mesor, and rhythm robustness, analyzed as quartiles; acrophase analyzed by peak activity time < 13:34 and > 15:51). Five years later, cognitive performance was assessed with the Modified Mini-Mental Status Examination (3MS), California Verbal Learning Task (CVLT), digit span, Trail Making Test B (Trails B), categorical fluency, and letter fluency. We compared cognitive performance with CARs using analyses of covariance adjusted for a number of health factors and comorbidities. Women in the lowest quartile for CAR amplitude performed worse on Trails B and categorical fluency compared to women in the highest quartile (group difference (d) = 30.42 sec, d = -1.01 words respectively, P < 0.05). Women in the lowest quartile for mesor performed worse on categorical fluency (d = -0.86 words, P < 0.05). Women with a later acrophase performed worse on categorical fluency (d = -0.69 words, P < 0.05). Controlling for baseline Mini-Mental State Examination and sleep factors had little effect on our results.
Conclusions:
Weaker circadian activity rhythm patterns are associated with worse cognitive function, especially executive function, in older women without dementia. Further investigation is required to determine the etiology of these relationships.
Citation:
Walsh CM, Blackwell T, Tranah GJ, Stone KL, Ancoli-Israel S, Redline S, Paudel M, Kramer JH, Yaffe K. Weaker circadian activity rhythms are associated with poorer executive function in older women. SLEEP 2014;37(12):2009-2016.
Keywords: actigraphy, cognition, cognitive impairment, executive function, verbal memory
INTRODUCTION
With the rapidly expanding older adult population, it is essential to identify risk factors and possibly modifiable constructs associated with cognitive impairment. One area that has shown substantial promise is sleep, given that sleep disturbance increases with age1–3 and is associated with greater impairment in cognitive performance.4–8 Both physiologic and behavioral circadian patterns9–15 have been shown to be more disrupted with increased age, with an advanced phase and flattening of the amplitude of the circadian cycle as measured by body temperature, melatonin, and behavioral activity.12–15 Little is known, however, about the relationship between circadian activity rhythms (CARs) and cognition in older adults. The onset of mild cognitive impairment (MCI) and dementia is associated with weaker circadian activity rhythmicity, smaller amplitude of activity, lower mesor of activity, and a delayed activity phase.16–18 Disrupted behavioral CARs negatively affect longevity in older adults19,20 and individuals with dementia.21 Overall, this suggests that weaker circadian patterns are associated with both aging and cognitive decline in older adults, but that healthy aging is associated with both a behavioral and physiological phase advancement, whereas disease-related aging (such as cognitive impairment) is associated with an activity phase delay.
Previous research has shown that disrupted CARs are associated with increased risk of MCI and dementia 5 years later22 and in a cross-sectional study older adults with weaker CAR amplitude had poorer executive function.8 However, no study has investigated the relationship between CARs and prospective cognitive performance in different types of cognitive processes. Therefore, it remains unclear whether disrupted CARs are associated with generalized or specific cognitive impairment. Making the distinction between generalized and focused cognitive impairment associated with previous CARs could suggest potential underlying mechanisms. Further, understanding if all cognition or a specific domain is more likely to be impaired in those with weaker CAR will allow for more directed care-planning.
The objective of this study was to determine if CARs were associated with prospective cognitive function in global cognition, verbal memory, working memory, or executive function. In this study, we used the same cohort of subjects previously reported on,22 using slightly more stringent inclusion criteria. Therefore, based on the previous study using this same cohort,22 as well as other cohorts,8 we hypothesized that individuals with weaker CAR amplitude, lower mesor, or delayed acrophase would have poorer performance on cognitive tests, especially those related to executive function 5 y later.
METHODS
Participants
Between 1986 and 1988, 9,704 community-dwelling Caucasian women age 65 y and older were recruited into a longitudinal Study of Osteoporotic Fractures (SOF) at one of four centers: Baltimore, MD; Minneapolis, MN; Portland, OR; and Monongahela Valley, near Pittsburgh, PA.23 From 1997 to 1998, an additional 662 African-American women were recruited to the study.24 Women were originally excluded from the study if they had a history of bilateral hip replacement or were unable to walk unassisted.
For this analysis we included women who had actigraphy measured at the Year 15 visit (2002-2004, our study baseline) and an expanded cognitive assessment at the Year 20 visit (2006-2008), which included participants from three of the four clinic sites. Though the African-American cohort was recruited several years into the original study, we will refer to all subjects' visits as the year from the start of the original study, e.g., all testing done in 2002-2004 for both the original and the African-American cohort will be referred to as the Year 15 visit. Of the 2,549 women with a minimum of 24 h of CAR data at Year 15, 1,777 also participated in the Year 20 visit. Of these, 1,323 underwent cognitive testing at the year 20 (2006-2008) visit. We excluded 25 women who had either a Mini-Mental State Examination (MMSE) score below 24, a medical history of dementia, or were taking medication for dementia at year 15. Because of the relationship between sleep disrupted breathing and cognitive impairment, we excluded 11 women with self-reported sleep apnea at Year 15. The final analytic cohort was 1,287. The final cohort used in this study mostly overlaps with the same subjects used in the previously published study reporting that weaker CAR was associated with increased risk of MCI and dementia 5 y later.22 The institutional review boards at each site approved the study, and written informed consent was obtained from all subjects.
Measures of CARs
Activity data was computed from wrist actigraphy measured using the Sleep-watch-O (Ambulatory Monitoring, Inc., Ardsley, NY) actigraphy wristband. Actigraphs were worn on the nondominant wrist for three 24-h periods. Activity was measured by a piezoelectric biomorph-ceramic cantilevered beam, which generates a voltage each time the actigraph is moved. Data were continuously collected and averaged into 1-min epochs, with activity measured in arbitrary units of counts/min. Activity data were collected using the proportional integration mode (PIM) of data collection, a high-resolution measurement of the area under the rectified conditioned transducer signal (area under the curve). Circadian activity rhythm variables were calculated from the activity data using a five-parameter extension of the 24-h cosine curve, or in other words, a nonlinear transformation to the cosine curve to describe the shape of the rest/activity rhythm across the 24-h period.25 There were four calculated variables of interest: amplitude, mesor, rhythm robustness, and acrophase. Amplitude (counts/min) is the difference between the peak and nadir of the activity fitted curve. Mesor (counts/min) is the mean of the activity fitted curve. Robustness (pseudo F-statistic) represents the rhythmicity of the circadian activity pattern in which the pseudo-F statistic calculates the overall fit of the raw activity data to the fitted curve. Acrophase (hours) is the time of day of the peak of the fitted curve.
CAR measures of amplitude, mesor, and robustness were subdivided into quartiles, with individuals in the lowest amplitude, mesor, or robustness quartile having the weakest CAR. Alternatively, acrophase quartiles were assessed based on previously published activity periods in older women.20 For this measure, individuals with early, mean, and late acrophase activity showed peak activity prior to 13:34, between 13:34 and 15:51, and after 15:51, respectively.
We also used other measures of sleep assessed at Year 15 (baseline) to control for potential sleep confounds in our analyses. Subjective sleep duration was assessed from the self-reported Pittsburgh Sleep Quality Index26 (PSQI) using a 0-3 scale (0 represents over 7 h of sleep per night, whereas 3 indicates less than 5 h of sleep per night). Objective total sleep time was assessed using actigraphy measures. Subjective sleepiness was assessed using the Epworth Sleepiness Scale27 (ESS). Finally, in addition to providing self-reported information based on physician diagnoses of sleep apnea (used as an exclusion criteria), participants indicated through self-report if they ever stop breathing while sleeping as an additional subjective marker for potential sleep apnea.
Measures of Cognitive Performance
Cognitive tasks were measured at the SOF Year 20 visit. Cognitive variables included measures of global cognition, verbal memory, and executive functioning. Global cognition was assessed using the 3MS, which is an expanded and more sensitive version of the MMSE.28 Verbal memory was assessed using the California Verbal Learning Test-Second Edition, Short Form (CVLT-II SF) 10-min delayed free recall.29 Working memory was assessed using the Digits Span Backwards test (Wechsler Adult Intelligence Scale). Executive functioning was assessed using Trail Making Test B (Trails B), categorical fluency and letter fluency. Trails B measures executive task-switching, attention, visual scanning, and motoric speeds.30 Both the categorical fluency and the letter fluency tasks measure executive function, verbal production, and language. In all cognitive testing higher scores indicated better performance except for Trails B, where lower scores indicated better performance.
At the Year 15 (baseline) visit, the 30-item MMSE was used as a measure of global cognition. Performance on MMSE was used as a covariate in our analyses to control for initial differences in overall cognition.
Baseline Cohort Characteristics
All subjects completed self-reported questionnaires at this study baseline (Year 15), which included education level, medical history, alcohol use, caffeine intake, and physical activity (number of blocks walked per day). Self-reported physician diagnoses were assessed for conditions including stroke, diabetes, hypertension, chronic obstructive pulmonary disease (COPD), and coronary heart disease. Medication use was based on medications used in the past 30 days and coded with a medication coding dictionary for categorization.31 Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared (kg/m2). Depressive symptoms were measured using the 15-item Geriatric Depression Scale32 (GDS-SF).
Statistical Analysis
Characteristic variables known to affect either cognition or CAR patterns were analyzed to determine their relationship with each of the four CAR measures (at Year 15) and global cognition (3MS, at Year 20) using analyses of variance (ANOVAs) or correlations for continuous normally distributed variables, and Kruskal-Wallis tests for skewed and categorical variables across all subjects. These analyses identified characteristics of interest to use as covariates in our analyses. Follow-up analyses were used to confirm group differences between women in the highest and lowest amplitude quartiles.
Our research question focused on cognitive differences between those individuals with higher versus lower CARs. The cognitive variables were Blom transformed33 (a rank-based inverse normal transformation) using all 1,287 subjects. We used ANOVAs and ANCOVAs to determine if CARs were associated with cognitive performance 5 y later. Cognitive performance was compared between individuals with the strongest (highest quartile, Q4) and weakest (lowest quartile, Q1) CAR amplitude, mesor, or robustness. Cognitive performance was also compared between individuals with the earlier and later CAR acrophase.
Covariate models were constructed to better understand the relationship between CAR predictors and cognitive performance. In Models 1-3, ANOVAs were adjusted for baseline (Year 15) characteristic variables, which were related (P < 0.10) to either the global cognitive score (3MS) at Year 20, or at least one of the baseline CAR measures (amplitude, mesor, robustness, or acrophase). We first adjusted for demographics and medical covariates (Model 1), which were: age, race, education, BMI, depressive symptoms, use of antidepressants or benzodiazepines, caffeine use, number of blocks walked per day, history of coronary heart disease, COPD, hypertension, diabetes, and stroke. To determine the CAR/cognitive relationship independent of baseline global cognition, we adjusted for MMSE in addition to demographics and medical covariates (Model 2). To better isolate CAR from potential sleep related effects on cognition, we adjusted for subjective total sleep time, objective total sleep time, and sleepiness in addition to demographics, medical covariates, and baseline cognition (Model 3). All statistical analyses were performed using SPSS 20 (IBM Corp.).
Analyses were repeated restricting the cohort to individuals with a minimum of 72 h of good actigraphy recordings (n = 1,149).
RESULTS
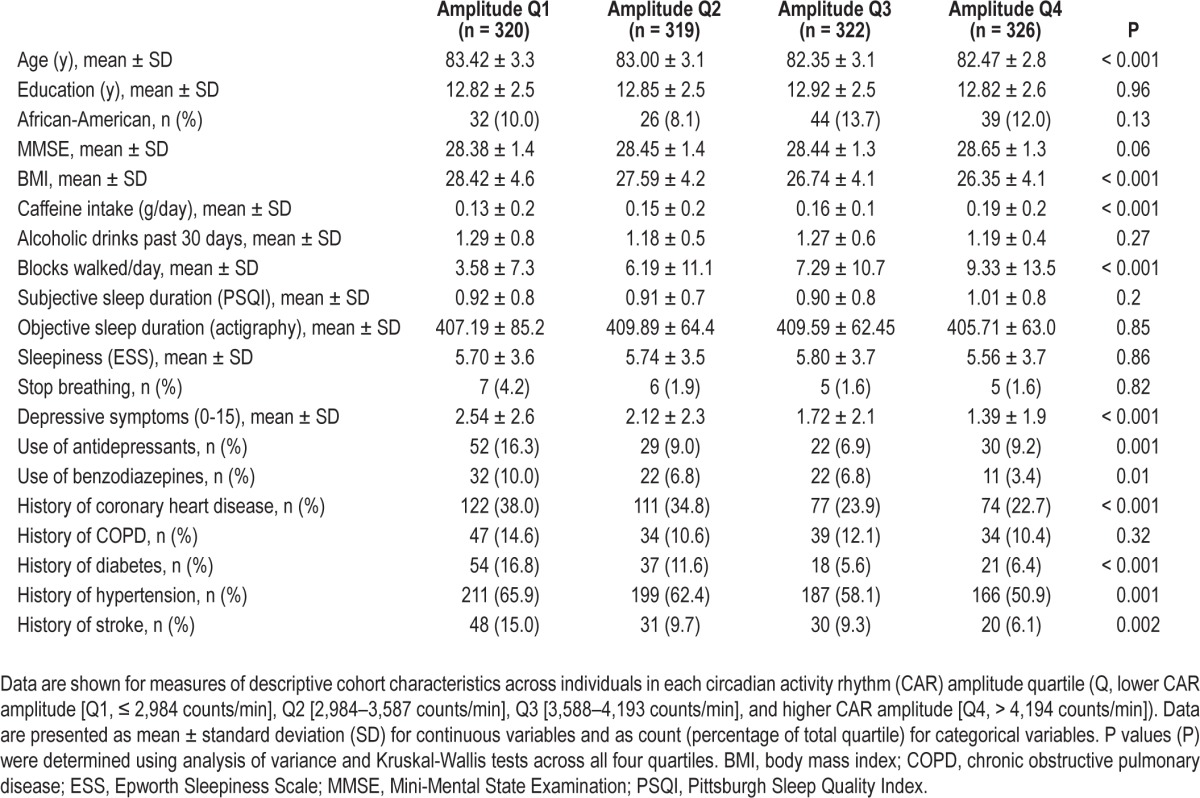
CAR amplitude was significantly associated with several cohort characteristics (Table 1). Specifically analyzing differences between women in the highest and lowest CAR amplitude quartiles, women with smaller amplitude: were older (P < 0.001), had higher BMI (P < 0.001), lower MMSE (P < 0.05), used less caffeine (P < 0.001), walked fewer blocks per day (P < 0.001), and had lower sleep efficiency (P < 0.001). Further, women with smaller CAR amplitude had higher levels of depression (P < 0.001) and medical comorbidities, and used more medications.
Table 1.
Year 15 baseline characteristics for women in each circadian activity rhythm amplitude quartile.
Unadjusted Model
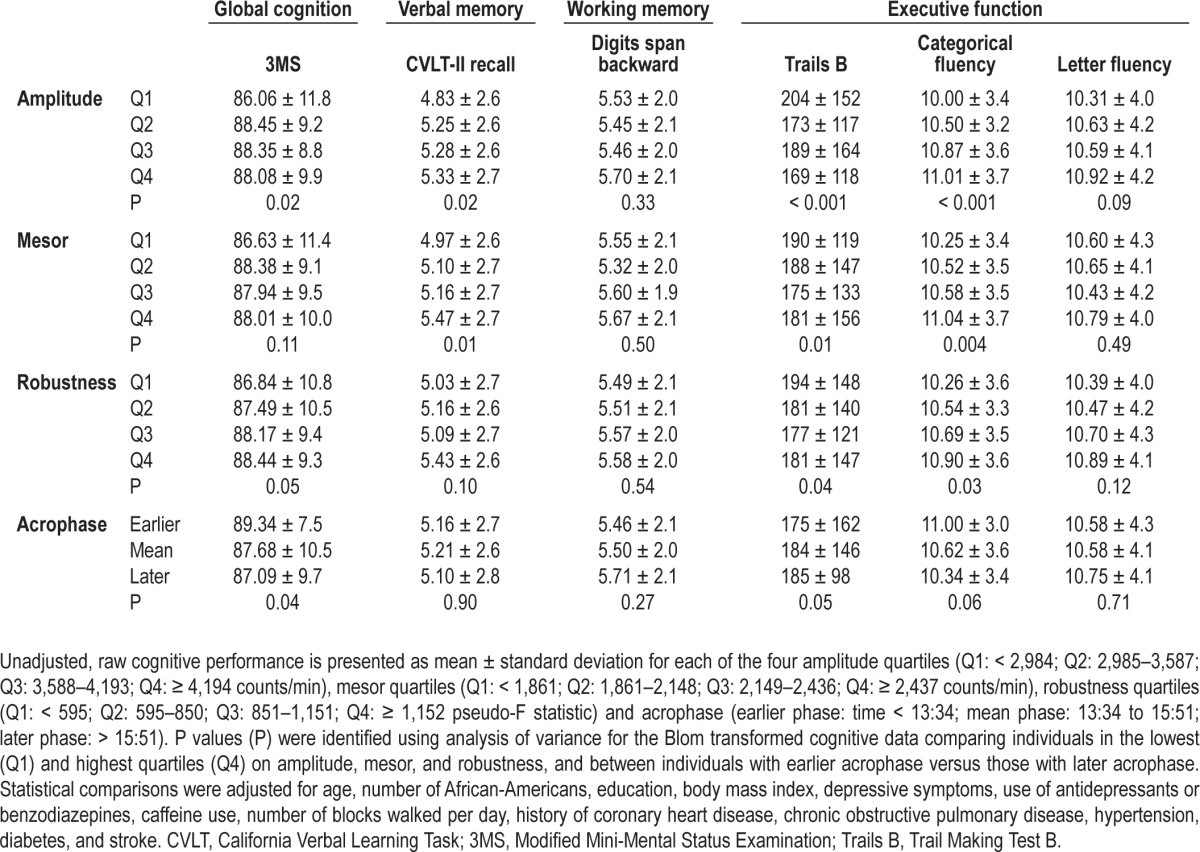
Blom transformed cognitive performance was compared between women in the highest (Q4) and lowest quartiles (Q1) of CAR amplitude, mesor, and robustness, and between the earlier and later acrophase periods (Table 2). Women in the lowest CAR amplitude quartile performed significantly worse than women in the highest quartile on global cognition (P < 0.05), verbal memory recall (P < 0.05), and two of the executive function tasks, Trails B (P < 0.001) and categorical fluency (P < 0.001). Women in the lowest CAR mesor quartile performed significantly worse than women in the highest quartile on verbal memory recall (P = 0.01) and on two of the executive function tasks, Trails B (P = 0.01) and categorical fluency (P < 0.01). Women in the lowest CAR robustness quartile performed significantly worse than women in the highest quartile on global cognition (P = 0.05), and two of the executive function tasks, Trails B (P < 0.05) and categorical fluency (P < 0.05). In addition, women with a later peak activity phase performed significantly worse than women with an earlier acrophase on global cognition (P < 0.05) and Trails B (P = 0.05).
Table 2.
Cognitive performance based on circadian activity rhythm predictors 5 years earlier.
Adjusting for Demographics and Medical Covariates (Model 1)
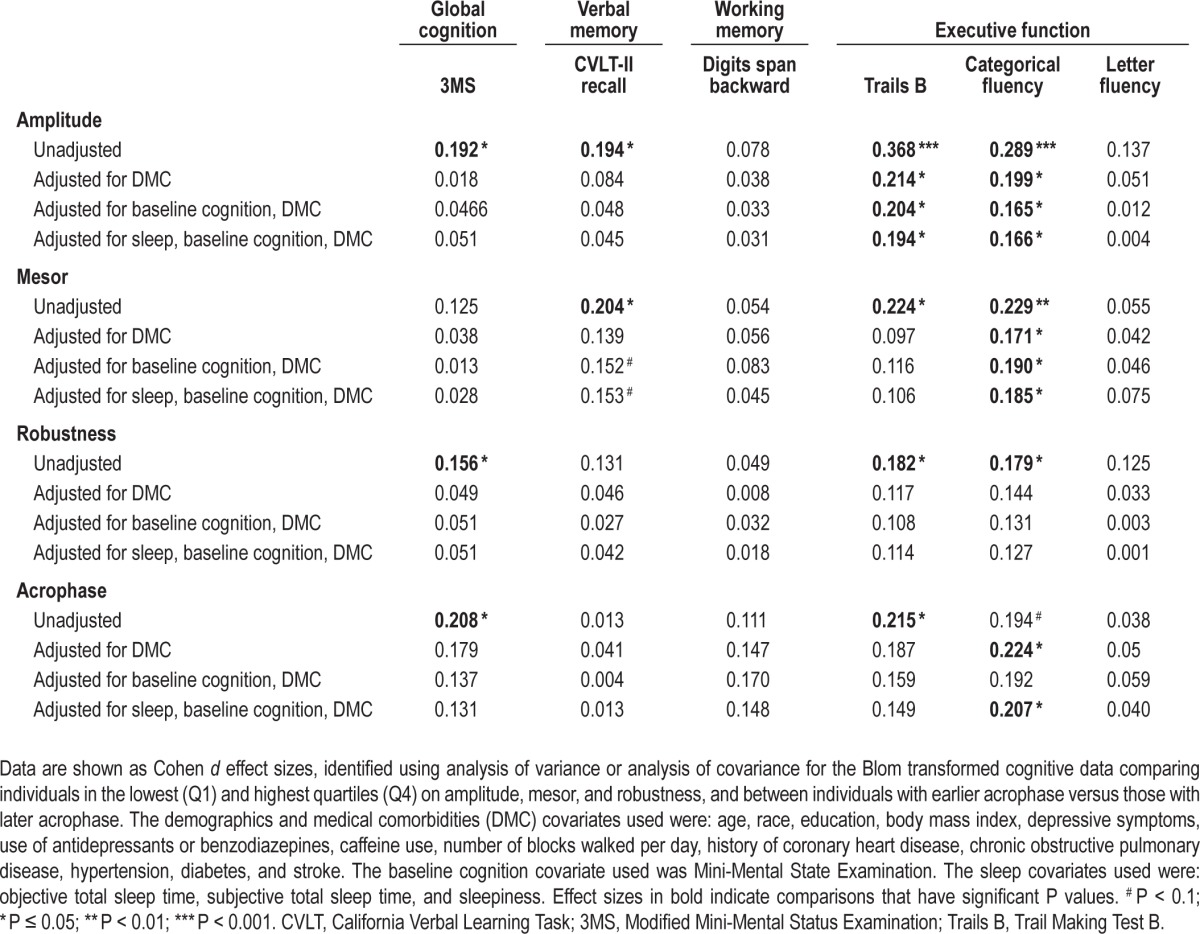
After adjusting for a number of comorbidities and health-related factors, only executive function continued to be significantly associated with CAR amplitude, mesor, and acrophase (Table 3). Specifically, as compared to women with higher amplitude (Q4), those with smaller amplitude (Q1) performed significantly worse on Trails B (P < 0.05) and categorical fluency (P < 0.05). Women with smaller mesor (Q1) were significantly worse on categorical fluency (P < 0.05) as compared to women with higher mesor (Q4). Women with an earlier acrophase performed significantly better on categorical fluency (P < 0.05) than those with a later phase.
Table 3.
Cohen d effect sizes across models of analyses comparing cognitive performance based on circadian activity rhythm predictors 5 years earlier.
Adjusting for Demographics, Medical Covariates, and Baseline Cognition (Model 2)
To control for the contribution of baseline differences in global cognition contributing to our results, we adjusted for MMSE in addition to demographics and medical covariates. Women with smaller CAR amplitude and CAR mesor performed significantly worse on executive function measures (amplitude: Trails B [P < 0.05] and categorical fluency [P < 0.05]; mesor: categorical fluency [P < 0.05]) independent of baseline global cognition.
Adjusting for Demographics, Medical Covariates, Baseline Cognition, and Sleep (Model 3)
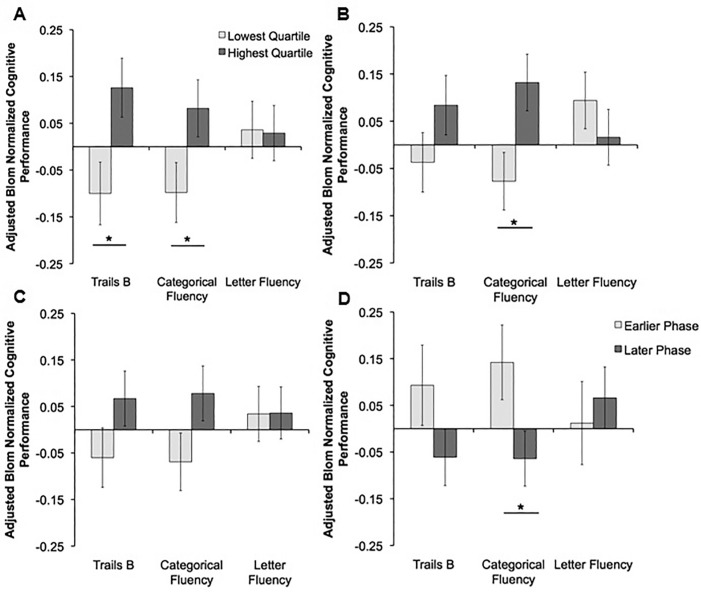
To reduce the contribution of sleep in our investigation of the relationship between CAR and cognitive function, we controlled for sleep, in addition to global cognition, demographics, and medical covariates. Women with smaller CAR amplitude and mesor performed significantly worse on executive function measures (amplitude: Trails B [P < 0.05] and categorical fluency [P < 0.05]; mesor: categorical fluency [P < 0.05]). In addition, women with a later acrophase performed significantly worse on categorical fluency (P < 0.05) than those with an earlier phase. The group comparisons for the executive function tasks analyzed using this model, adjusting for demographics, medical covariates, baseline cognition, and sleep are shown in Figure 1. No other group differences were identified.
Figure 1.
The relationship between circadian activity rhythms and measures of executive function adjusting for sleep, baseline global cognition, and health- related factors (Model 3). Blom transformed executive performance adjusted for demographics, medical covariates, baseline cognition, and sleep is presented as estimated marginal means ± standard error for (A) amplitude, (B) mesor, (C) robustness, and (D) acrophase. Analyses of covariance (ANCOVAs) were used to statistically compare cognitive performance between individuals in the lowest quartile (light gray) and the highest quartile (dark gray) for amplitude, mesor, and robustness (pseudo-F statistic). Similarly, ANCOVAs were used to statistically compare cognitive performance for individuals with an earlier phase of peak activity (light gray) and later phase of peak activity (dark gray). Data for Trail Making Test B (Trails B) are inverted; therefore for all three cognitive variables the higher on the y-axes the better the performance. Statistical comparisons were adjusted for age, number of African-Americans, education, body mass index, Min-Mental State Examination, depressive symptoms, use of antidepressants or benzodiazepines, caffeine use, number of blocks walked per day, objective total sleep time, subjective total sleep time, sleepiness, history of coronary heart disease, chronic obstructive pulmonary disease, hypertension, diabetes and stroke. * P ≤ 0.05.
When the data were restricted to only individuals with a minimum of 72 h of good actigraphy recordings, the results were nearly identical (data not shown).
DISCUSSION
Our study investigated the relationship between CAR and prospective performance on individual cognitive tasks. We found that some measures of CAR are positively associated with performance on cognitive testing, in particular, executive function, 5 y later. Furthermore, we found that of the cognitive tasks assessed, executive function alone was positively associated with CAR measures, independent of baseline MMSE, sleep factors, and a number of comorbidities and health factors. Here we discuss these findings with respect to cognitive function, dementia and cardiovascular function.
Sleep and Executive Function
Our study supports and extends the previous cross-sectional findings that CAR amplitude was positively associated with executive function in older adults.8 It is possible that sleep and CARs may differentially affect cognition. In older adults, increased sleep disturbance is associated with deficits in executive function, working memory, and global cognition.4,5,7 By covarying for sleep measures in addition to baseline cognition, demographics, and medical covariates (Model 3), we isolated the CAR/cognitive relationship from the contribution of sleep. In light of previous findings, our study suggests that both CARs and sleep may have distinct relationships with cognitive aging.
Understanding the relationship-flow between dementia and CARs has been challenging. Previous studies have shown that CARs are disrupted in individuals with dementia16,18,34,35 and that CAR disruption increases as dementia worsens.17,36 However, it has been unclear whether disrupted CARs lead to dementia, dementia leads to CAR disruption, or if these processes occur in parallel. It could be argued that the disrupted CARs seen with worsening dementia may result from an individual no longer being independent and as active on a daily basis. Our results suggest, however, that independent of cognitive impairment (Model 2 adjusting for demographics, medical covariates, and baseline cognition), CARs may lead to worse cognitive function. This suggests that CAR disruption is prior to the cognitive decline and therefore is not caused by the dementia itself and does not occur in parallel.
Our group previously reported that disrupted CARs (low amplitude, low mesor, and weak robustness) were associated with increased all-cause mortality20 and an increased likelihood for developing MCI or dementia 5 y later.22 Based on the previous studies, poorer performance on global cognition and memory tasks in those with previously weaker CARs may have been predicted. However, our results suggest that the weaker CAR-associated cognitive impairment is found predominantly in the executive function domain. Individuals with vascular disease have a much higher specificity toward declines in executive function,37 even as compared with individuals with predominant hippocampal atrophy who show more widespread cognitive declines.38
Further, individuals with vascular dementia have weaker CARs than those with Alzheimer disease.39 The pattern of cognitive impairment associated with previously disrupted CARs in our cohort is therefore similar to the pattern seen with vascular disease moreso than with other cognitive decline-associated diseases such as Alzheimer disease. In other words, disrupted CARs may be associated with the later development of vascular dementia. To date, there have been no studies investigating CAR patterns prior to the onset of vascular dementia. It is possible that CAR modulation of cardiovascular regulation could alter executive functioning.
Cardiovascular Measures and Sleep Quality
In our analyses, we adjusted our models for a number of cardiovascular factors and continued to find a cognitive deficit pattern suggestive of cardiovascular disease, indicating that CARs may mediate cardiovascular health beyond typically measured factors. To date, there has been little research on the relationship between CARs and risk for vascular dementia. However, the cardiovascular system has a circadian rhythm vulnerable to changes in sleep/waking activity. For example, there is a circadian pattern for the timing and severity of a cardiovascular event.40–42 Weaker or disrupted CARs are associated with increased risk of cardiovascular disease, in particular stroke and coronary heart disease.43–45 These data suggest that disrupted CARs predate cardiovascular disease. The mechanism for this is likely caused by a desynchronized circadian pattern between the central clock and peripheral clocks that regulate factors such as heart rate and blood pressure, leading to the disrupted regulation of these factors.46,47 Therefore, disrupted CARs increase risk of cardiovascular disease and based on our study CARs may also increase the risk for declines in executive function. Further research, with the addition of neuroimaging, is required to determine whether altered CARs can predict the later onset of specific neurodegenerative diseases such as vascular dementia. Studies are also needed to examine whether synchronizing or strengthening both the physiological and behavioral CARs would prevent cognitive decline. Thus, CARs could be a modifiable construct to manage cognitive loss.
Caveats and Conclusions
The strengths of this work include reporting on a large cohort of older adult women followed prospectively. Controlling for multiple variables and in particular for baseline MMSE and sleep factors, we were able to describe a unique relationship between CARs and cognitive performance 5 y later. Another strength of this study is that we assessed a battery of six cognitive tests, measuring different domains of cognitive function. However, our study is limited to a single sex and primarily Caucasian population; thus, generalizability is a concern. A further limitation of this study is that we did not have detailed cognitive function tests at baseline to control for initial differences in executive function. As previously described, we removed individuals from our analyses with likely dementia (MMSE below 24 or reported dementia) and controlled for baseline MMSE. However, we are unable to establish longitudinal changes in specific cognitive function in this study. An additional limitation to our study was that although we controlled for a number of cardiovascular-related health factors, other factors may mediate cardiovascular health that were not measured and that are contributing to our observational findings. Another limitation is that though the between-group differences are significant, the magnitude of the effect sizes found are low, in particular when adding the additional covariates into each model. Finally, we compared four measures of CAR across six measures of cognition, raising the concern of a chance finding. However, we believe that our findings are reliable because our results were consistent for executive function across four models, incrementally adjusting for a number of factors. Thus, the current findings suggest that CARs could be an early indicator of executive function declines in the future and may serve as a biomarker for developing and expanding interventions related to sleep to improve healthy aging.
DISCLOSURE STATEMENT
This was not an industry supported study. The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. This research was funded by National Institute on Aging (NIA) under the following grant numbers: K24 AG031155, R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, and R01 AG027576 and R01 AG026720. Dr. Ancoli-Israel has been a consultant or on the advisory board of Astra Zeneca, Ferring Pharmaceuticals Inc., GlaxoSmithKline, Hypnocore, Johnson & Johnson, Merck, NeuroVigil, Inc., Orphagen Pharmaceuticals, Pfizer, Philips, Purdue Pharma LP, Sanofi-Aventis, Somaxon, and had a grant from Litebook, Inc. Dr. Redline has received funding from ResMed foundation and ResMed Inc, and equipment from both ResMed Inc and Philips-Respironics for use in clinical trials. The other authors have indicated no financial conflicts of interest. The data collection for this longitudinal observational study was performed through sites at: Baltimore, MD; Minneapolis, MN; Portland, OR; and Monongahela Valley, PA. The analyses for this study were performed at the Memory and Aging Center, University of California, San Francisco, CA. No off-label drugs were used in this study
ACKNOWLEDGMENTS
The authors thank Dr John Neuhaus and Dr Joaquin Anguera for their advice and input.
REFERENCES
- 1.Bliwise DL, King AC, Harris RB, Haskell WL. Prevalence of self-reported poor sleep in a healthy population aged 50-65. Soc Sci Med. 1992;34:49–55. doi: 10.1016/0277-9536(92)90066-y. [DOI] [PubMed] [Google Scholar]
- 2.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 3.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 4.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–10. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 5.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep. 2011;34:1347–56. doi: 10.5665/SLEEP.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naismith SL, Rogers NL, Hickie IB, Mackenzie J, Norrie LM, Lewis SJ. Sleep well, think well: sleep-wake disturbance in mild cognitive impairment. J Geriatr Psychiatry Neurol. 2010;23:123–30. doi: 10.1177/0891988710363710. [DOI] [PubMed] [Google Scholar]
- 7.Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TH. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J Gerontol B Psychol Sci Soc Sci. 2009;64:180–7. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oosterman JM, van Someren EJ, Vogels RL, Van Harten B, Scherder EJ. Fragmentation of the rest-activity rhythm correlates with age-related cognitive deficits. J Sleep Res. 2009;18:129–35. doi: 10.1111/j.1365-2869.2008.00704.x. [DOI] [PubMed] [Google Scholar]
- 9.Duffy JF, Czeisler CA. Age-related change in the relationship between circadian period, circadian phase, and diurnal preference in humans. Neurosci Lett. 2002;318:117–20. doi: 10.1016/s0304-3940(01)02427-2. [DOI] [PubMed] [Google Scholar]
- 10.Hofman MA, Swaab DF. Living by the clock: the circadian pacemaker in older people. Ageing Res Rev. 2006;5:33–51. doi: 10.1016/j.arr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Kripke DF, Youngstedt SD, Elliott JA, et al. Circadian phase in adults of contrasting ages. Chronobiol Int. 2005;22:695–709. doi: 10.1080/07420520500180439. [DOI] [PubMed] [Google Scholar]
- 12.Czeisler CA, Dumont M, Duffy JF, et al. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet. 1992;340:933–6. doi: 10.1016/0140-6736(92)92817-y. [DOI] [PubMed] [Google Scholar]
- 13.Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516:611–27. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turek FW, Penev P, Zhang Y, van Reeth O, Zee P. Effects of age on the circadian system. Neurosci Biobehav Rev. 1995;19:53–8. doi: 10.1016/0149-7634(94)00030-5. [DOI] [PubMed] [Google Scholar]
- 15.Yoon IY, Kripke DF, Elliott JA, Youngstedt SD, Rex KM, Hauger RL. Age-related changes of circadian rhythms and sleep-wake cycles. J Am Geriatr Soc. 2003;51:1085–91. doi: 10.1046/j.1532-5415.2003.51356.x. [DOI] [PubMed] [Google Scholar]
- 16.Gehrman P, Marler M, Martin JL, Schochat T, Corey-Bloom J, Ancoli-Israel S. The relationship between dementia severity and rest/activity circadian rhythms. Neuropsychiatr Dis Treat. 2005;1:155–63. doi: 10.2147/nedt.1.2.155.61043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ancoli-Israel S, Klauber MR, Jones DW, et al. Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep. 1997;20:18–23. [PubMed] [Google Scholar]
- 18.Satlin A, Teicher MH, Lieberman HR, Baldessarini RJ, Volicer L, Rheaume Y. Circadian locomotor activity rhythms in Alzheimer's disease. Neuropsychopharmacology. 1991;5:115–26. [PubMed] [Google Scholar]
- 19.Paudel ML, Taylor BC, Ancoli-Israel S, et al. Rest/activity rhythms and mortality rates in older men: MrOS Sleep Study. Chronobiol Int. 2010;27:363–77. doi: 10.3109/07420520903419157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tranah GJ, Blackwell T, Ancoli-Israel S, et al. Circadian activity rhythms and mortality: the study of osteoporotic fractures. J Am Geriatr Soc. 2010;58:282–91. doi: 10.1111/j.1532-5415.2009.02674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gehrman P, Marler M, Martin JL, Shochat T, Corey-Bloom J, Ancoli-Israel S. The timing of activity rhythms in patients with dementia is related to survival. J Gerontol A Biol Sci Med Sci. 2004;59:1050–5. doi: 10.1093/gerona/59.10.m1050. [DOI] [PubMed] [Google Scholar]
- 22.Tranah GJ, Blackwell T, Stone KL, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70:722–32. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cummings SR, Black DM, Nevitt MC, et al. Appendicular bone density and age predict hip fracture in women. JAMA. 1990;263:665–8. [PubMed] [Google Scholar]
- 24.Vogt MT, Rubin DA, Palermo L, et al. Lumbar spine listhesis in older African American women. Spine. 2003;3:255–61. doi: 10.1016/s1529-9430(03)00024-x. [DOI] [PubMed] [Google Scholar]
- 25.Marler MR, Gehrman P, Martin JL, Ancoli-Israel S. The sigmoidally transformed cosine curve: a mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat Med. 2006;25:3893–904. doi: 10.1002/sim.2466. [DOI] [PubMed] [Google Scholar]
- 26.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 27.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 28.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–8. [PubMed] [Google Scholar]
- 29.Delis DC, Freeland J, Kramer JH, Kaplan E. Integrating clinical assessment with cognitive neuroscience: construct validation of the California Verbal Learning Test. J Consult Clin Psychol. 1988;56:123–30. doi: 10.1037//0022-006x.56.1.123. [DOI] [PubMed] [Google Scholar]
- 30.Reitan RM. Validity of the Trail Making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–6. [Google Scholar]
- 31.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–11. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 32.Sheikh JI, Yesavage JA. New York: The Haworth Press, Inc; 1986. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clinical gerontology: a guide to assessment and intervention; pp. 165–73. [Google Scholar]
- 33.Blom G. New York: Wiley; 1958. Statistical estimates and transformed beta-variables. [Google Scholar]
- 34.Paavilainen P, Korhonen I, Lötjönen J, et al. Circadian activity rhythm in demented and non-demented nursing-home residents measured by telemetric actigraphy. J Sleep Res. 2005;14:61–8. doi: 10.1111/j.1365-2869.2004.00433.x. [DOI] [PubMed] [Google Scholar]
- 35.Motohashi Y, Maeda A, Wakamatsu H, Higuchi S, Yuasa T. Circadian rhythm abnormalities of wrist activity of institutionalized dependent elderly persons with dementia. J Gerontol A Biol Sci Med Sci. 2000;55:740–3. doi: 10.1093/gerona/55.12.m740. [DOI] [PubMed] [Google Scholar]
- 36.Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. Alterations in the circadian rest-activity rhythm in aging and Alzheimer's disease. Biol Psychiatry. 1990;27:563–72. doi: 10.1016/0006-3223(90)90523-5. [DOI] [PubMed] [Google Scholar]
- 37.Lo RY, Jagust WJ. Vascular burden and Alzheimer disease pathologic progression. Neurology. 2012;79:1349–55. doi: 10.1212/WNL.0b013e31826c1b9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marra C, Ferraccioli M, Vita MG, Quaranta D, Gainotti G. Patterns of cognitive decline and rates of conversion to dementia in patients with degenerative and vascular forms of MCI. Curr Alzheimer Res. 2011;8:24–31. doi: 10.2174/156720511794604552. [DOI] [PubMed] [Google Scholar]
- 39.Mishima K, Okawa M, Satoh K, Shimizu T, Hozumi S, Hishikawa Y. Different manifestations of circadian rhythms in senile dementia of Alzheimer's type and multi-infarct dementia. Neurobiol Aging. 1997;18:105–9. doi: 10.1016/s0197-4580(96)00167-4. [DOI] [PubMed] [Google Scholar]
- 40.Janszky I, Ahnve S, Ljung R, et al. Daylight saving time shifts and incidence of acute myocardial infarction--Swedish Register of Information and Knowledge About Swedish Heart Intensive Care Admissions (RIKSHIA) Sleep Med. 2012;13:237–42. doi: 10.1016/j.sleep.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 41.Chan CM, Chen WL, Kuo HY, Huang CC, Shen YS, Choy CS, Chen JH. Circadian variation of acute myocardial infarction in young people. Am J Emerg Med. 2012;30:1461–5. doi: 10.1016/j.ajem.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 42.Founier S, Eeckhout E, Mangiacapra F, et al. Circadian variations of ischemic burden among patients with myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J. 2012;163:208–13. doi: 10.1016/j.ahj.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 43.Kawachi I, Colditz GA, Stampfer MJ, Willett WC, et al. Prospective study of shift work and risk of coronary heart disease in women. Circulation. 1995;92:3178–82. doi: 10.1161/01.cir.92.11.3178. [DOI] [PubMed] [Google Scholar]
- 44.Paudel ML, Taylor BC, Ancoli-Israel S, et al. Rest/activity rhythms and cardiovascular disease in older men. Chronobiol Int. 2011;28:258–66. doi: 10.3109/07420528.2011.553016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pimenta AM, Kac G, Souza RR, Ferreira LM, Silqueira SM. Night-shift work and cardiovascular risk among employees of a public university. Rev Assoc Med Bras. 2012;58:168–77. [PubMed] [Google Scholar]
- 46.Ahnve S, Theorell T, Akerstedt T, Fröberg JE, Halberg F. Circadian variations in cardiovascular parameters during sleep deprivation. A noninvasive study of young healthy men. Eur J Appl Physiol Occup Physiol. 1981;46:9–19. doi: 10.1007/BF00422170. [DOI] [PubMed] [Google Scholar]
- 47.Takeda N, Maemura K. Circadian clock and vascular disease. Hypertens Res. 2010;33:645–51. doi: 10.1038/hr.2010.68. [DOI] [PubMed] [Google Scholar]