Abstract
Study Objectives:
Increased cell injury would provide the type of change in constitution that would underlie sleep disruption as a risk factor for multiple diseases. The current study was undertaken to investigate cell injury and altered cell fate as consequences of sleep deprivation, which were predicted from systemic clues.
Design:
Partial (35% sleep reduction) and total sleep deprivation were produced in rats for 10 days, which was tolerated and without overtly deteriorated health. Recovery rats were similarly sleep deprived for 10 days, then allowed undisturbed sleep for 2 days. The plasma, liver, lung, intestine, heart, and spleen were analyzed and compared to control values for damage to DNA, proteins, and lipids; apoptotic cell signaling and death; cell proliferation; and concentrations of glutathione peroxidase and catalase.
Measurements and Results:
Oxidative DNA damage in totally sleep deprived rats was 139% of control values, with organ-specific effects in the liver (247%), lung (166%), and small intestine (145%). Overall and organ-specific DNA damage was also increased in partially sleep deprived rats. In the intestinal epithelium, total sleep deprivation resulted in 5.3-fold increases in dying cells and 1.5-fold increases in proliferating cells, compared with control. Two days of recovery sleep restored the balance between DNA damage and repair, and resulted in normal or below-normal metabolic burdens and oxidative damage.
Conclusions:
These findings provide physical evidence that sleep loss causes cell damage, and in a manner expected to predispose to replication errors and metabolic abnormalities; thereby providing linkage between sleep loss and disease risk observed in epidemiological findings. Properties of recovery sleep include biochemical and molecular events that restore balance and decrease cell injury.
Citation:
Everson CA, Henchen CJ, Szabo A, Hogg N. Cell injury and repair resulting from sleep loss and sleep recovery in laboratory rats. SLEEP 2014;37(12):1929-1940.
Keywords: apoptosis, cell injury, lipid peroxidation, oxidative stress, 8-hydroxydeoxyguanosine
INTRODUCTION
Abnormalities in the regulation and duration of sleep are known risk factors for diseases such as cardiovascular disease and cancer.1–4 The signs of sleep deficiency are insidious and historically the specific role of sleep deprivation in causing disease risk has proved difficult to pinpoint. Equally elusive are the physical sites and properties restored by recovery sleep. This lack of specificity resembles that of other deficiencies of basic biological requirements, for which compensatory changes can mask the effects of stringency and enable a long survival period with few outward signs.5,6 The purpose of the current investigation was to discover whether cell injury results from sleep deprivation, which would provide a plausible explanation for both the sense that sleep loss causes “wear and tear” and how disease risk may be conferred.
We hypothesized that sleep loss is a state of systemic cell injury because previous findings established that sleep deprivation results in uncompensated oxidative stress. This is manifested, in part, by decreases in liver glutathione and catalase activity without compensatory increases in other enzymatic antioxidants under study.7 The incomplete antioxidant protection would be expected to result in injured cells that then would either be repaired or die.8 From this viewpoint, several signs of sleep loss would be interpreted, not as inappropriate activation of the immune system or an immune-related dysfunction per se, but as responses to cell injury. These signs include increases in circulating proinflammatory cytokines (such as interleukin-1β and, often, interleukin-69–18), markers of cell stress or cytoprotection (e.g., inducible heme-oxygenase and heat shock proteins),19–24 changes in antioxidant enzymes,25,26 neutrophilia27–29 and neutrophil migration into tissues.21 DNA damage in the brain has been reported in laboratory rodents partially sleep deprived by the pedestal (or inverted flowerpot) technique,30 but the technique is well known to produce abundant nonspecific effects.31,32
The approach was to produce severe sleep loss in rats but avoid advanced morbidity, to discover changes to physical disposition that may eventually lead to disease. To measure cell injury we undertook fingerprinting of lipid, protein, and DNA damage. Measurements were taken in the plasma, liver, heart, lung, and intestine—where oxidative stress and markers of inflammation have been established7,21,33—as well as the spleen. The fate of injured cells—whether they died or may have been repaired—was assessed by quantifying apoptotic cells. (The other main form of cell death, necrosis, has not been found in any major organ of sleep deprived rats by brightfield microscopy.34,35) Cell proliferation, which would be expected if cell death were accelerated, was measured in the liver, spleen, and small intestine. The results indicate that sleep loss indeed causes increased cell injury. In the intestine, increased cell injury was associated with increased cell proliferation and cell death. The most pronounced cellular target of oxidative damage was DNA, which reflects either impairment of repair mechanisms or an increased rate of DNA damage that outstrips repair. The results also identify properties of restoration by sleep, which include downregulation of end-point damage to lipids, proteins, and DNA, and an absence of any increases in cell burdens, such as the production of new cells or an imbalance of DNA damage and repair.
METHODS
Animals and Experimental Conditions
Tissue specimens were allocated from 49 experiments in two long series of live animal studies conducted sequentially; only a small number of subjects can be studied at one time under the Bergmann-Rechtschaffen paradigm (described in the following paragraphs). Measurements made in each set of live animal experiments are enumerated in the supplemental material.
Protocols for animal care and use (Nos. 2560-01, -02, and -04) were approved by institutional animal care and use committees at The Medical College of Wisconsin and the Zablocki Veterans Administration Medical Center in accordance with procedures set forth in the Guide to the Care and Use of Laboratory Animals.36 Subjects in both sets of live animal experiments were male Sprague-Dawley rats (Harlan, Madison, WI), 23.5 (2.4 standard deviation [SD]) weeks old, which weighed 466 (37 SD) g at the time of surgery. Surgery was performed to implant macroelectrodes for recording electroencephalographic signals for the purpose of detecting sleep onset in those rats that would be totally deprived of sleep37 and for consistency of procedures across all treatment groups. Surgical procedures for cortical and muscle electrode placement and indwelling catheter implantation have been described previously.37 (Surgical anesthetics and analgesics are enumerated in the supplemental material.) All animals were allowed to recover from surgery for ≥ 7 days before the start of baseline conditions.
The rats were housed under conditions of constant ambient room light to minimize the circadian rhythm amplitude in control rats because sleep deprivation results in a dependent decrease in the amplitude, and possibly the phase, of the circadian rhythm.38,39 Rats were housed under an ambient temperature of 27°C–28°C, which is within the thermoneutral zone for rats,40 and fed ad libitum a purified diet, isocaloric to rat chow at 3.7 kcal/g (modified AIN-76A, Zeigler Brothers, Garners, PA). The different treatment conditions and their durations, described in the following paragraphs, are depicted in Figure S1 (supplemental material).
The Bergmann-Rechtschaffen experimental apparatus and method are described in detail elsewhere.37,41 In brief, two rats were housed on a large divided platform; each rat occupying one side. The platform could be rotated slowly at a speed of 3.3 rpm. Each rotation was brief, lasting 6 sec, which was sufficient to cause each rat to move in order to remain comfortably on the platform. Baseline conditions included an hourly rotation of the platform but there was no deliberate sleep restriction. Under these conditions, sleep occupies 50–61% of total time.34,41–44 Baseline controls were studied during 7 days of these conditions and compared with the treatment groups in the first set of live animal experiments. Total and partial sleep deprivation were produced for 10 days—a duration known to be sufficient for metabolic changes and mild neutrophilia to become manifest,33,43 but short enough to preclude the advanced morbidity that typically occurs by 18–26 days.34,41,42 To produce total sleep deprivation, the platform was rotated for 6 sec upon detection of sleep onset in one of the two paired rats. There otherwise was no ambulation requirement. Under these conditions sleep is largely prevented and only accumulates to < 10% of total time.34,41–43 Partial sleep deprivation was produced in the rat housed opposite to the totally sleep deprived rat because it experienced the ambulation requirements of the totally sleep deprived rat. Under these partial sleep deprivation conditions, sleep is heavily disrupted and occupies 38–44% of total time.34,41–43 Comparison controls in the second set of live animal experiments were subjected to the same amount of disk rotation time as were the partially and totally sleep deprived rats, but rotations of the housing platform were consolidated into periods that permitted lengthy opportunities to obtain uninterrupted sleep. Under these ambulation control conditions sleep occupied 51% of total time.44 In different groups of rats, recovery sleep was produced by reinstatement of baseline conditions after the 10-day period of total or partial sleep loss to permit a 2-day period of sleep ad libitum. Recovery sleep for 2 days in totally sleep deprived rats occupies 58–65% of total time, which includes dramatic rebounding of rapid eye movement (REM) sleep to amounts fourfold those observed during baseline.45,46 Two days of recovery sleep after partial sleep deprivation also results in rebound sleep averaging 66% of total time, which includes a 1.6-fold increase in REM sleep.44
Tissue harvest and necropsy procedures were conducted after completion of the planned durations of study. For exsanguination, rats were decapitated or placed under deep anesthesia for blood drawing via either cardiac puncture or indwelling catheter. Tissues were quickly dissected, divided, and either fast-frozen in liquid nitrogen or fixed for microscopy. The small intestine was sectioned into equal thirds in length and rinsed through three times with sterile saline, and subsections were fast-frozen, fixed in formalin, or frozen in embedding media. Fast-frozen specimens were stored at -80°C and later milled over dry ice to near powder consistency.
Signaling of Cell Death
Caspase-3, which is critical in the execution phase of apoptosis, was determined by measuring cleavage products by immunoblot. Tissue lysates were prepared from fast-frozen tissues and analyzed by methods described previously,21 using recombinant human caspase-3 as the standard (Sigma-Aldrich, St. Louis, MO), and rabbit anti-rat caspase-3 and mouse anti-rabbit IgG as primary and secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). The normalized intensity is the ratio of background-corrected intensities, expressed as a percentage of the standard per gel.
Localization and Quantification of Apoptotic Cell Death
Apoptotic cells were quantified by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL; ApopTag®, Millipore, formally Chemicon, Billerica, MA) to detect in situ DNA fragmentation by brightfield microscopy (Olympus BX51 microscope and DP71 camera, Center Valley, PA; Image-Pro Plus image analysis software, MediaCybernetics, Bethesda, MD). Brown and dense staining of condensed DNA within the cell was considered positive for late-stage cell damage/death. TUNEL-positive cells were counted at 400X magnification in 4 μm-thick sections of (1) frozen-embedded spleen (IHC Facility, University of Chicago, Chicago, IL) and (2) formalin-fixed, paraffin-embedded liver, jejunum, heart, and lung (IHCTech, Aurora, CO). The representative regions quantified were 1.6 mm2 of liver or an area that contained at least 1000 cells (0.20 mm2 of spleen and lung; 0.64 mm2 of heart). TUNEL-positive cells in these tissues were expressed as a proportion of the area. TUNEL-positive cells in the spleen were categorized by red or white pulp. In the jejunum, TUNEL-positive cells were counted between the crypt-villus junction and the villus tip in the basal, middle, and apical thirds of its length and categorized as epithelium or lamina propria. Positive cells in 10 villi were counted, unless fewer fully intact villi were available for study.
Lipid Peroxidation
Analysis of 8-iso-PGF2α (F2-isoprostane) in EDTA-plasma and fast-frozen liver were measured by mass spectrometry (Eicosanoid Core Laboratory, Vanderbilt University, Nashville, TN).47 Thiobarbituric acid-reactive substances (TBARS) were measured in heart and liver extracts according to the method of Ohkawa et al.,48 using malondialdehyde as the standard. Protein was determined by the Lowry method. The intra-assay coefficient of variation was < 6%.
Protein Damage
Lysate was prepared from fast-frozen aliquots of liver, heart, lung, jejunum, and spleen homogenized with Halt Protease Inhibitors in phosphate-buffered saline for the carbonyl assay and in Tissue Protein Extraction Reagent (both Thermo Scientific, Rockford, IL) for the nitrotyrosine assay. Protein carbonyls present in the sample were derivatized to dinitrophenylhydra-zone (DNP) and probed with an anti-DNP antibody, followed by an horseradish peroxidase-conjugated secondary antibody, using a commercial kit (Cell Biolabs, San Diego, CA). The concentration of carbonyl per specimen was calculated from a standard curve of protein carbonyls made of oxidized and reduced BSA and expressed in nmol/mg supernatant protein, relative to the control value per plate. Nitrotyrosine was determined by using a competitive enzyme-linked immunosorbent assay (ELISA) to measure 3-nitrotyrosine, a modification of tyrosine residues by peroxynitrite (Cell Biolabs, San Diego, CA). The concentration of nitrotyrosine was calculated from a standard curve of nitrated BSA and expressed relative to the control value per plate. Supernatant protein concentrations were determined by Lowry assay for carbonyl amounts and by Bradford assay for nitrotyrosine amounts.
DNA Damage
DNA was isolated from fast-frozen liver, heart, lung, spleen, and jejunum by using a commercially available kit (Gen Elute Mammalian Genomic DNA Miniprep kit, Sigma-Aldrich). The DNA concentration in the isolate was determined by absorbance at 260 nm. The DNA was denatured by incubation at 95°C, digested by separate incubations with alkaline phosphatase and nuclease P1 (both by Sigma-Aldrich), and stored at -20°C for 18–42 h. 8-hydroxydeoxyguanosine (8-OHdG) was determined by competitive ELISA (Cell Biolabs) with incubation of the secondary antibody for 24 h. Concentrations of 8-OHdG were calculated from a standard curve, expressed as concentration (pg 8-OHdG/μg DNA) of sample, relative to the control values per plate.
Antioxidants
Glutathione peroxidase activity
Assay procedures are those described in detail previously.7 Dilutions of homogenate supernatant were as follows: liver, 1:40; heart and spleen, 1:8; and jejunum and lung, 1:4. The reaction was started by the addition of tert-butyl hydroperoxide and measured every 15 sec for 5 min. The rate of the reaction was calculated over a 2-min, linear portion of absorbances. Glutathione peroxidase (GSHPx) activity per mg soluble protein was calculated from a standard curve prepared using GSHPx from bovine erythrocytes (Sigma-Aldrich) and expressed as a ratio of control values per plate. One unit of GSHPx activity will catalyze the oxidation of 1 μmol of reduced glutathione to oxidized glutathione per min at pH 7.0 and 25°C.
Catalase activity
The assay of catalase activity was based on the methods reported by Aebi49 with modifications by Karthikeyan et al.,50 which included centrifugation to remove nucleic acids and membrane components, as previously described.7 Tissues were homogenized at the following dilutions: 1:10 for liver, heart, lung, and spleen; 1:3 for small intestine. The supernatant for the liver was further diluted 1:40, while other supernatants were assayed without further dilution. The reaction was started with the addition of H2O2 and measured every second for 40 sec. The rate of the reaction was calculated from the linear portion of the curve lasting at least 30 sec. Catalase activity was determined from a standard curve using catalase from bovine liver (Sigma-Aldrich) and expressed as units per mg soluble protein relative to control values per plate. One unit of catalase decomposes 1 μmol H2O2 per min at pH 7.0 and 25°C.
Cell Proliferation
Rats in the second set of live animal experiments received injections of bromodeoxyuridine (BrdU; 50 mg/kg intraperitone-ally, Sigma-Aldrich) 90 min prior to tissue harvest procedures; BrdU is taken up by cells during the synthesis phase of the cell cycle. The administration was timed to optimize measurements of cell renewal in the jejunum.51–55 Formalin-fixed, paraffin-embedded 4-μm thick sections of the jejunum (midway between the pyloric valve and the ileocecal valve) were stained with anti-BrdU antibody (Novocastra, Leica Biosystems, Buffalo Grove, IL). Cells that exhibited positive staining were expressed as a proportion of total cells in 10 crypts. Antibody staining of Ki-67, a nuclear protein associated with cellular proliferation in all phases of the cell cycle except the resting (G0) phase,56 was used to capture the low cell renewal rate in frozen-embedded spleen (IHC Facility, University of Chicago, Chicago, IL) and in formalin-fixed, paraffin-embedded liver (IHCtech, Aurora, CO). Ki-67-positive cells were expressed as the percentage of positively stained area in both the splenic red and white pulp surveyed in areas of 0.04–0.21 mm2, and as the number of positive cells in a 0.32-mm2 survey region of the liver.
Data Analysis
The measurements were grouped by outcome measure and experimental treatment condition. Values were log-transformed for analysis, based on a preliminary finding that measurements were approximately lognormally distributed. The exception to this was the number of BrdU- and TUNEL-positive cells in the jejunum, which were expressed as a percentage of cells and a number of cells within a region, respectively. Values were then analyzed for each outcome measure using a linear model when only one tissue type was measured (e.g., ng/mL plasma isoprostane) and mixed-effects linear model with random animal-specific intercepts when multiple tissues were measured (e.g., 8-OHdG). Counts of TUNEL-positive cells in the jejunum were analyzed using Poisson regression with a random observation-specific effect to allow for overdispersion. The expectation was that many between-group differences would be consistent across organs, and therefore a multistep procedure was used to move from common effects to organ-specific effects. First, the average effect of treatment group on the outcome measure, across organs, was compared pairwise among treatment groups for an overall difference. Then, within-organ differences were compared to these averaged differences to determine the extent to which an individual organ(s) stood out. Finally, organ-specific differences were compared independently of the effect of treatment on other organs. A single-step adjustment for multiple tests, based on the multivariate t distribution, was performed over all the comparisons within one assay-based analysis group. Average differences and organ-specific differences among pairwise comparisons were considered significant at the 5% level after this adjustment for multiple comparisons. Further adjustment over assays was not performed as many of them provide complementary approaches to evaluate the same underlying process. Thus, specific statements of significance are not intended to be interpreted alone. Data are expressed as the geometric means and standard error (SE) for transformed data, and the arithmetic means and SE for cell counts.
RESULTS
An overview of the location and type of cell damage, cell death, and cell proliferation is provided in Table 1 and enumerated in the following paragraphs. The pairwise comparisons of outcome measurements are provided in Tables S1 and S2 (supplemental material).
Table 1.
Organ-specific indices of cell injury in partially and totally sleepdeprived rats during sleep deprivation and during recovery
Total Sleep Deprivation
Cell injury
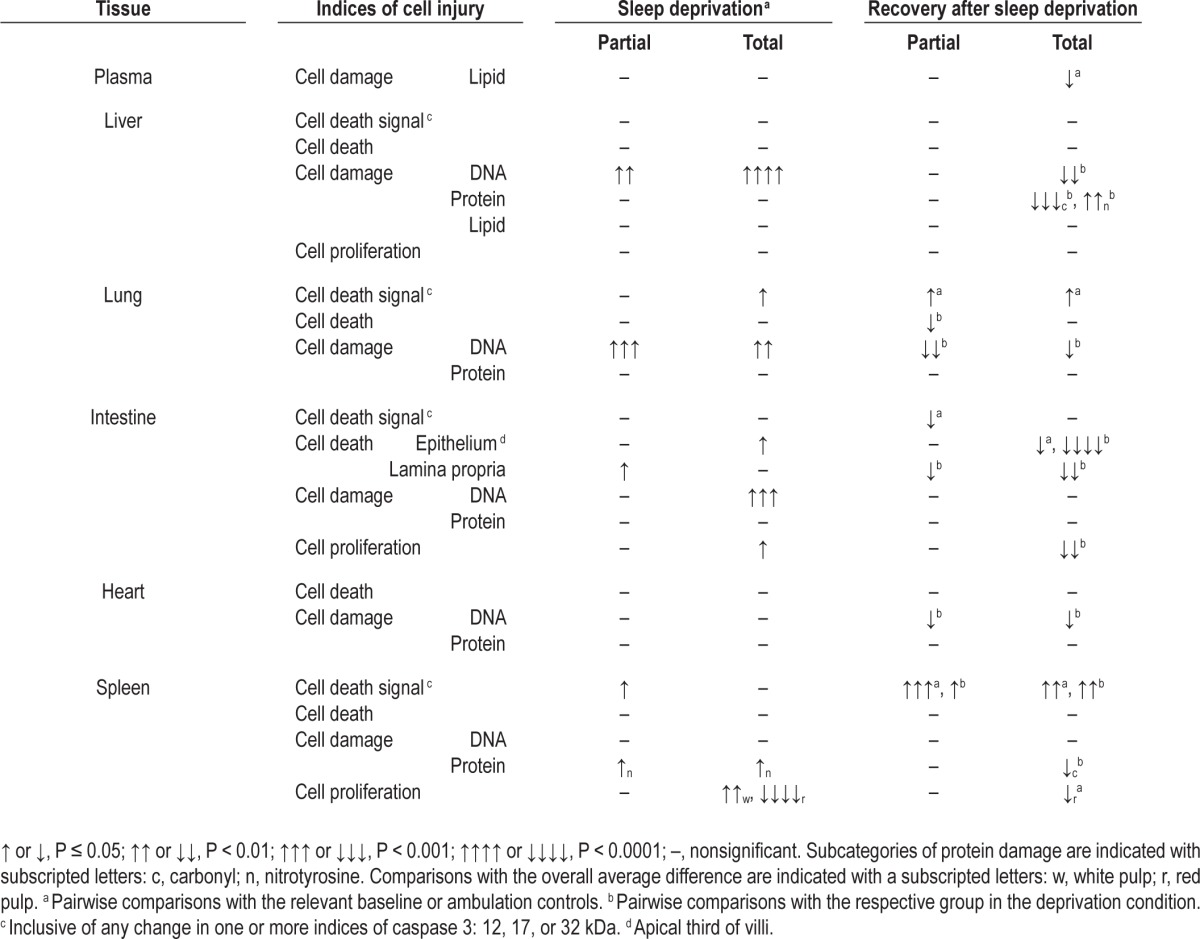
The outstanding feature of total sleep deprivation was a significant group effect for overall DNA damage by 8-OHdG determinations that were 139% of the ambulation control (AC) values (95% confidence interval [CI]: 1.17–1.66; P < 0.0001), indicating pervasive DNA damage. Organ-specific effects were most pronounced for the liver, jejunum, and lung, shown together with the heart in Figure 1. In pairwise comparisons with AC, 8-OHdG in totally sleep deprived rats was 247% in the liver (95% CI: 1.63–3.75, P < 0.0001), 145% in the jejunum (95% CI: 1.17–1.80, P = 0.00016), and 166% in the lung (95% CI: 1.16–2.38, P = 0.0021). Measurements of 8-OHdG in the heart and the spleen were not statistically significantly different from AC.
Figure 1.
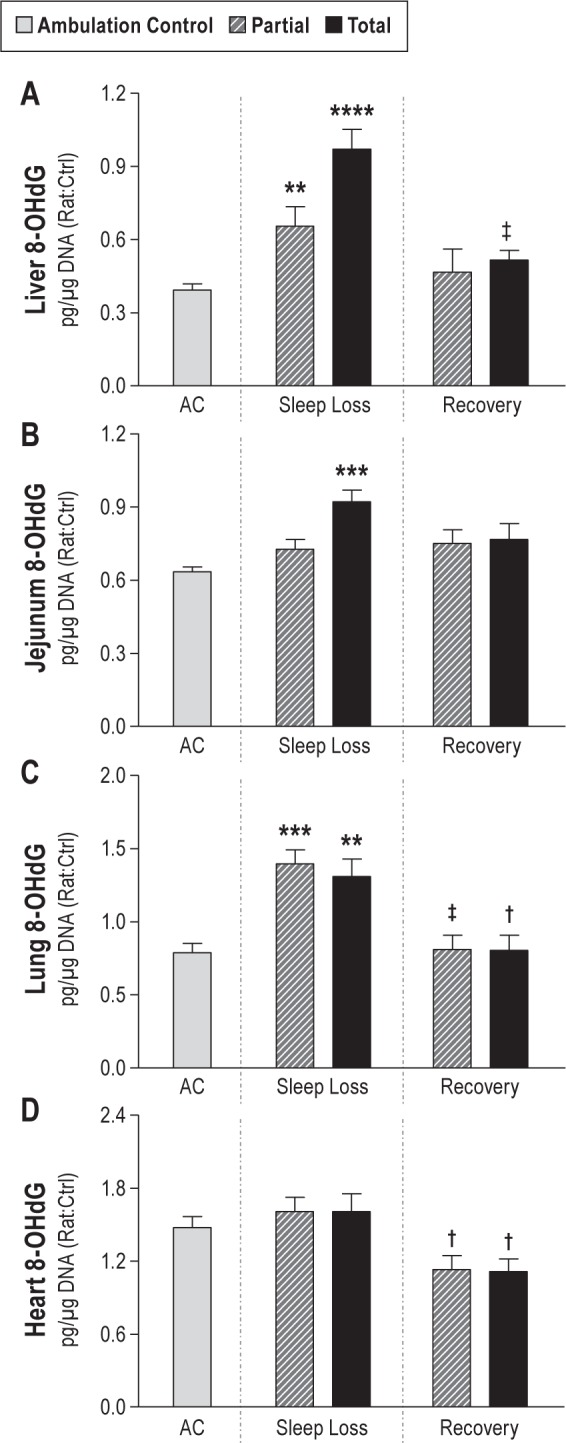
8-hydroxydeoxyguanosine (8-OHdG) concentrations indicative of DNA damage in ambulation controls (ACs; gray bar, n = 11) and in partially (hatched bar) and totally (solid bar) sleep deprived rats during 10 days of sleep loss (n = 8–11 per group), and after 2 days of recovery sleep after sleep loss (n = 6–7 per group). Values are shown for the liver (A), jejunum (B), lung (C), and heart (D) concentrations of 8-OHdG in pg/μg DNA, normalized to intra-assay controls (Ctrl) and expressed as the geometric means ± standard error. ** P < 0.01, *** P < 0.001, and **** P < 0.0001 for the comparison between deprivation and AC conditions. † P < 0.05 and ‡ P < 0.01 for the comparison between recovery and deprivation conditions.
Differences in carbonyl concentrations did not reach statistical significance in pairwise comparisons between AC rats and totally sleep deprived rats. However, increased carbonyl concentrations were observed in the livers of several individual animals in the totally sleep deprived group. Nitrotyrosine concentrations during deprivation were significantly higher only in the spleens of totally sleep deprived rats―measured at 273% of AC values (95% CI: 1.11–6.70, P = 0.021).
Lipid damage was not found. No differences in TBARS concentration in the heart or the liver of totally sleep deprived rats were observed. Plasma isoprostane concentration, considered a more sensitive marker for lipid peroxidation than TBARS, did not exceed that of baseline control rats during total sleep loss (Figure 2).
Figure 2.
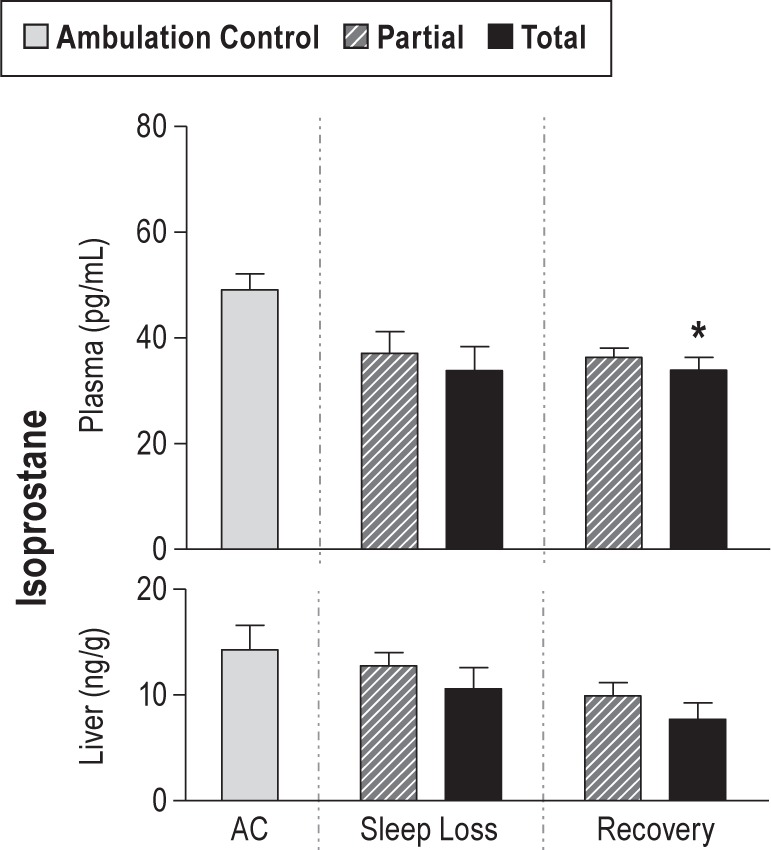
Mean concentrations of isoprostane (lipid damage) by experimental conditions in ambulation control (AC; gray bar, n = 6) and in partially (hatched bar) and totally (solid bar) sleep deprived rats during 10 days of sleep loss (n = 4–7 per group), and after 2 days of recovery sleep after sleep loss (n = 6 per group). Values are shown for plasma and liver, expressed as the geometric means ± standard error. * P < 0.05 for the comparison with ambulation controls.
Cell death signaling and cell death quantification
Caspase-3, measured at 12, 17, or 32 kDa, was not statistically significant for an overall effect, or as an average difference across organs. Organ-specific effects were limited to the lung where the active small subunit (12 kDa) was increased in the totally sleep deprived rats to 171% of the level observed in baseline controls (95% CI: 1.06–2.76, P = 0.024). It may be noted that the active large 17 kDa subunit of caspase-3 in the spleen tended to be increased in totally sleep deprived rats to 159% of baseline control (NS); a similar percentage change in partially sleep deprived rats was statistically significant.
Cell death and renewal
Despite significant DNA damage and the detection of caspase-3 fragments in the lung, discussed previously, no statistically significant increases in dead cells were found in organ-specific comparisons of the liver, lung, heart, or spleen. The changes in the intestine, however, were marked. Representative micrographs of TUNEL-positive staining are shown in Figure 3. Statistically significant increases in the number of TUNEL-positive epithelial cells were exclusive to totally sleep deprived rats and limited to the apical ends of the villi, and 5.3-fold those of ACs (95% CI: 1.24–23.03, P = 0.015). Cell death in the intestinal lamina propria of totally sleep deprived rats averaged 7.8-fold those of AC values, although statistical significance was not attained here (95% CI: 0.88–68.77, P = 0.076). Associated with the dramatic increases in cell death during the sleep deprivation period, the jejunal epithelium showed a striking increase in the percentage of replicating cells in the crypts; 146% of AC values (38% in sleep deprived rats versus 26% in AC rats, 95% CI: 1.04–22.9 difference, P = 0.026). A representative micrograph of BrdU labeling and the quantification of labeled cells are shown in Figure 4.
Figure 3.
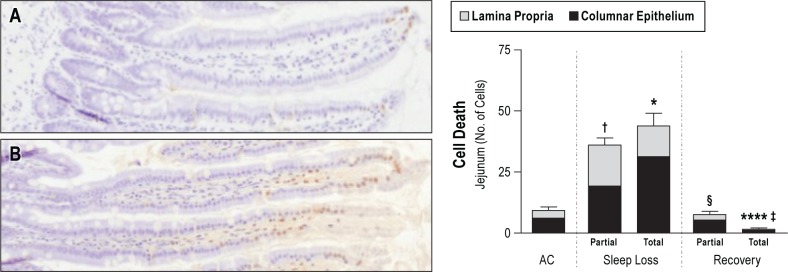
The number of dead cells in the jejunum. Left: representative micrographs (40X magnification) illustrate the distribution of terminal deoxynucleotidyl transferase dUTP nick end labeling-positive cells localized mainly in the apical third of a villus in an ambulation control (A) and a sleep deprived (B) rat. Right: results of quantitative analysis in ambulation control (AC) rats (n = 7) and in partially and totally sleep deprived rats during 10 days of sleep loss (n = 7–11 per group), and after 2 days of recovery sleep after sleep loss (n = 5–6 per group). The bars are the total number of dead cells in the apical third of the villi in the jejunum, further subcategorized as among the columnar epithelium or in the lamina propria. For comparison of differences from AC, * P < 0.05 for a difference in dead cell numbers in the epithelium of total sleep loss condition and † P < 0.05 for a difference in dead cell number in the lamina propria of partially sleep deprived rats. For comparison with the respective sleep deprivation condition, **** P < 0.0001 for a difference in dead cell number in the epithelium and § P < 0.05 and ‡ P < 0.01 for a difference in dead cell numbers in the lamina propria.
Figure 4.
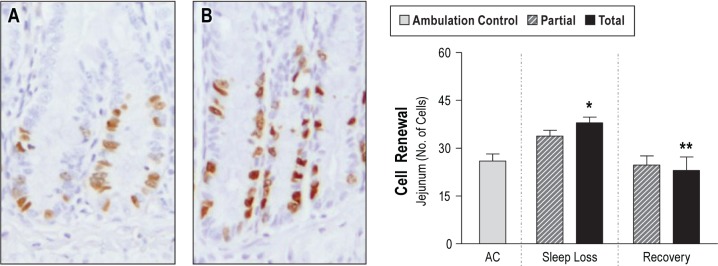
Replication of cells affected by sleep loss. Left: representative micrographs (100X magnification) illustrate bromodeoxyuridine (BrdU) incorporation into proliferating cells in the crypts of jejunum in an ambulation control (A) and a sleep deprived rat (B). Right: results of quantitative analysis results in ambulation control (AC) rats (gray bar, n = 7) and in partially (hatched bar) and totally (solid bar) sleep deprived rats during 10 days of sleep loss (n = 7–9 per group), and after 2 days of recovery sleep after sleep loss (n = 6–7 per group). Values shown are the number of positively labeled cells relative to the total number of crypt cells, expressed as the mean ± standard error. * P < 0.05 for the comparison of differences between sleep deprivation and AC and ** P < 0.01 for the comparison between recovery and sleep deprivation.
Total cell proliferation in the spleen did not differ significantly from that of ambulation controls during sleep deprivation. However, in totally sleep deprived rats, the red and white pulp behaved differently; cell proliferation in the red pulp decreased fourfold and that in the white pulp increased 2.49-fold compared to the average change (red pulp, 25%, 95% CI: 0.11–0.53, P < 0.0001; white pulp, 249%, 95% CI: 1.15–5.39, P = 0.0087).
Antioxidant imbalance
A group effect for GSHPx was significant (P < 0.0001). The liver varied significantly from the average difference across organs in pairwise comparisons. Total sleep deprivation values of liver GSHPx were 29% lower than in AC rats (95% CI: 0.56–0.89, P < 0.001). Values for liver catalase tended to show a pattern similar to that of liver GSHPx, but the pairwise comparisons were not statistically significant.
Partial Sleep Deprivation
Cell injury
The outstanding feature of partial sleep deprivation resembled that of totally sleep deprived rats―a global increase in DNA damage as measured by 8-OHdG concentrations that were 127% of AC values (95% CI: 1.07–1.49; P = 0.0002). As shown in Figure 1, organs in which statistical significance was attained were the liver and the lung; values were 167% and 177% of those of AC rats, respectively (liver, 95% CI: 1.12–2.47, P < 0.006; lung, 95% CI: 1.26–2.49, P = 0.0002). Carbonyl concentrations were not significantly different between partially sleep deprived and AC rats. Nitrotyrosine concentrations during partial sleep deprivation were significantly higher only in the spleen―measured at 234% of AC values (95% CI: 1.00–5.48, P = 0.05), which is similar to that observed in totally sleep deprived rats.
As was the case for total sleep deprivation, partial sleep deprivation was not associated with lipid damage, as measured by isoprostane (Figure 2) or TBARS.
Cell death signaling and cell death quantification
Similar to totally sleep deprived rats, partially sleep deprived rats did not show an overall effect of treatment on cell death signaling by caspase-3 measured at 12, 17, or 32 kDa, or in the average differences across organs in pairwise comparisons. Organ-specific effects were detected only once by means of a statistically significant level of the active large subunit (17 kDa) of caspase-3 in the spleens of partially sleep deprived rats; 178% of that of the baseline controls (95% CI: 1.05–3.04, P = 0.029).
Cell death and renewal
In similar manner to the totally sleep deprived group, cell death was not found in the liver, lung, heart, or spleen. Cell death was detected in the lamina propria (Figure 3), for which values in partially sleep deprived rats were 9.5-fold of AC values (95% CI: 1.28–70.76, P = 0.019). In contrast to the totally sleep deprived group, cell death in the epithelial cells of the jejunum was not statistically significantly different from ACs for partially sleep deprived rats. Cell proliferation was not significantly different from ACs in the jejunal crypts (Figure 4), liver, or spleen.
Antioxidant imbalance
Partial sleep deprivation values of liver GSHPx, were significantly lower than in AC rats by 40% (95% CI: 0.49–0.75, P < 0.0001), whereas catalase values were not different.
Recovery Sleep after Partial and Total Sleep Deprivation
Cell injury
In the recovery conditions, cell damage appeared to cease and, in many comparisons, was below normal (Figure 1). 8-OHdG levels were decreased by 19% and 26% from deprivation levels in partial and total sleep deprivation conditions, respectively (95% CI: partial sleep deprivation versus recovery sleep, 0.66–0.98, P = 0.021; total sleep deprivation versus recovery sleep, 0.61–0.91, P < 0.0001). Carbonyl concentrations in recovering totally sleep deprived rats were 57% of deprivation values across organs (95% CI: 0.39–0.84, P < 0.0002). Nitrotyrosine concentrations during recovery from partial and total sleep deprivation were not different from ACs. The lowest average values for lipid peroxidation were observed during the recovery period in totally sleep deprived rats when plasma and liver isoprostanes were only 69% and 54% of baseline control values (plasma, 95% CI: 0.48–0.99, P < 0.04; liver, 95% CI: 0.28–1.04, P = 0.076, not significant [NS]), as shown in Figure 2. Values for lipid peroxidation in recovering partially sleep deprived rats were not different from those of baseline controls.
Organ-specific values for 8-OHdG during recovery were significantly decreased from deprivation levels in the heart by 30% and 31% in partially and totally sleep deprived rats, respectively (95% CI: partial sleep deprivation versus recovery, 0.50–0.99, P = 0.04; total sleep deprivation versus recovery, 0.49–0.98, P < 0.04). Compared with deprivation levels, 8-OHdG was decreased to 53% in the liver (95% CI: 0.33–0.85, P = 0.0035) and 61% in the lung (95% CI: 0.41–0.92, P = 0.011) in recovering totally sleep deprived rats, and 58% in the lung (95% CI: 0.39–0.87, P = 0.0038) in partially sleep deprived rats. Other 8-OHdG values during the recovery period were not different from those of controls.
Organ-specific values for carbonyl were especially low in the liver and the spleen; 21% and 43% of total sleep deprivation values (95% CI: liver, 0.08–0.52, P < 0.0002; spleen, 0.20–0.93, P = 0.027). There were no organ-specific differences in nitrotyrosine concentrations between recovery sleep and AC. However, the nitrotyrosine concentration in the liver was significantly increased to 310% of the deprivation value during recovery in totally sleep deprived rats, which may reflect suppression of nitrated proteins in this organ during the deprivation period (recovery vs. total sleep deprivation, 95% CI: 1.44–6.66, P = 0.0012).
Cell death signaling
Organ-specific differences in procaspase-3 (32 kDa) were limited to the jejuna of recovering partially sleep deprived rats, which had levels that were 57% of those of the baseline controls (95% CI: 0.33–0.97, P = 0.034), whereas the recovery values for totally sleep deprived rats were 76% of the baseline control (NS). Compared with baseline controls, the levels of the active small subunit of caspase-3 (12 kDa) during recovery were 164% in the lung in totally sleep deprived rats (95% CI: 1.03–2.59, P = 0.032), and 246% and 234% in the spleen in both partially and totally sleep deprived rats, respectively (partial, 95% CI: 1.42–4.25, P < 0.0006; total, 95% CI: 1.35–4.04, P = 0.001).
Cell death and renewal
Recovery treatment in totally sleep deprived rats resulted in a marked reduction of TUNEL-positive cells in both the apical thirds of the villi and the total area of lamina propria, each to 2% of deprivation values (95% CI: 0.00–0.18, P < 0.0001; 95% CI: 0.00–0.37, P = 0.002, respectively, Figure 3). Recovery values for TUNEL-positive cells in the lung, shown in Figure 5, were only 9% and 13% of deprivation values in partially and totally sleep deprived rats, respectively (95% CI: recovery versus partial sleep deprivation, 0.01–0.74, P = 0.018; recovery versus total sleep deprivation, 0.02–1.11, NS, P = 0.068). The proliferation of jejunal crypt cells decreased from the deprivation to the recovery period by 40% in totally sleep deprived rats (-14.9 percentage points, 95% CI: -25.82 to -3.96 difference, P = 0.0036), and tended to decrease in the partially sleep deprived rats (-27%, NS), as shown in Figure 4.
Figure 5.
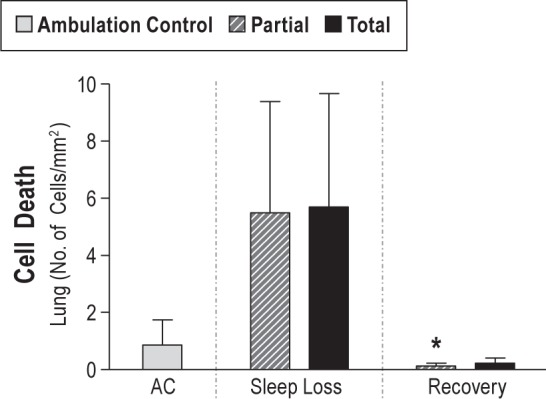
Dead cells in the lung in ambulation control (AC) rats (gray bar, n = 8) and in partially (hatched bar) and totally (solid bar) sleep deprived rats during 10 days of sleep loss (n = 8–11 per group), and after 2 days of recovery sleep after sleep loss (n = 6–7 per group). Values are expressed as the geometric mean ± standard error of positive cells relative to the area measured. * P < 0.05 compared with the respective sleep deprivation condition.
During recovery, as for the deprivation period, total replicating cells in the spleen did not differ significantly between total sleep deprivation and control, but those in the red pulp were decreased by 2.44-fold compared to the average change (41%, 95% CI: 0.18–0.92, P = 0.018), leaving most replicating cells attributable to the white pulp. Differences in the proportion of replicating cells attributable to red versus white pulp were not detected in partially sleep deprived rats. No group differences in cell renewal were detected in the liver of recovering totally or partially sleep deprived rats.
Antioxidant imbalance
Recovery values of liver GSHPx were significantly higher than deprivation values―155% and 142% in partially and totally sleep deprived groups, respectively (95% CI: partial, 1.19–2.00, P = 0.0002; total, 1.10–1.84, P = 0.003)―representing a return to control levels. Values for liver catalase tended to show patterns similar to those of liver GSHPx, but the difference from deprivation values was not statistically significant.
DISCUSSION
The current outcomes indicate that DNA is a cellular target of damage resulting from sleep deprivation, manifested by widespread increases in 8-OHdG, a DNA base nonspecific oxidation product. The liver, lung, and jejunum were especially affected. DNA damage in the livers of totally and partially sleep deprived rats, for example, was increased 2.5- and 1.7-fold that of ACs, respectively. Increased DNA damage probably reflects either compromised repair mechanisms or insufficient repair for an increased rate of damage.57–59 The oxidative DNA damage did not appear to result in detectable increases in p53-dependent apoptosis (intrinsic pathway),60 except in the intestine, discussed in the next paragraph. Cell death signaling lacked correspondence among caspase cleavage products and among organs, and therefore also did not indicate a marked increase in cell death. These outcomes suggest that other mechanisms, such as growth arrest of damaged cells until repair is completed, may be induced to mediate oxidative DNA damage before cell death is triggered.57
The changes in oxidative DNA damage observed in this study are of a magnitude thought to be biologically relevant.57–59 A 50% increase in urinary 8-OHdG in smokers, for example, is assumed to reflect a 50% increase in the rate of oxidative damage from smoking.61 The current outcomes are within the same order of magnitude observed in other disease-associated oxidative stress conditions. For example, Matsui et al. showed that cancerous breast tissue contains 8-OHdG that is 154% that of noncancerous breast tissue.62 In rats exposed to diesel exhaust, the lung concentration of 8-OHdG was 300% of values of age-matched controls, and preceded the development of tumors months after the exposure had ended.63 Lymphocyte 8-OHdG levels in individuals with autoimmune and recurrent inflammatory diseases (i.e., systemic lupus erythematosus, rheumatoid arthritis, vasculitis, and Behçet's disease) are 115–195% of values observed in nonautoimmune controls.64 As these examples illustrate, the associations between DNA lesions and disease processes are serious and diverse and have the potential for latent outcomes. Increased DNA damage is considered one of the necessary events predisposing an individual toward disease―such as cancer8,59,61; atherosclerosis65–67; diabetes or its complications68,69―and the progression of disease, such as colitis.70,71 Furthermore, several other DNA bases that were not measured may be oxidized in ways that contribute to mutagenicity.72
The intestine has the highest epithelial cell turnover rate in the body and is continuously moving at a speed of 5 to 10 μm/h.73 Complete intestinal epithelium turnover occurs in 3 days in rodents and in 5–7 days in humans.74 The current results show that this intense and constant turnover was accelerated by sleep loss. The normal pattern of cell turnover consists of proliferation of cells in the crypts, differentiation of progenitor cells in the crypt-to-villus transition, and migration of cells to the villus apex where they are shed into the lumen and the cellular components are resorbed.74,75 The proportion of crypt cells replicating in totally sleep deprived rats was 146% that of ACs, indicative of an accelerated generation time. Epithelial cell death near the villus apex in totally sleep deprived rats was more than fivefold that of ACs, suggesting accelerated migration and death rates. Also detected in partially sleep deprived rats was a significant number of apoptotic cells in the lamina propria, which is the major effector site of the gut-associated lymphoid tissue and the “graveyard” for activated lymphocytes.76 Although our determinations did not identify the cell type affected, alterations in the lamina propria may have immune implications. The dramatic increases in cell production and destruction observed in the intestine signify increased metabolic burdens; even under normal conditions the digestive tract accounts for an estimated 15–20% of daily energy expenditure requirements.77,78 The high concentration of oxidative DNA damage in association with increased cell production and death, as demonstrated in this study, should increase the frequency of replication errors and produce genetic instability and epigenetic changes.79 Such changes have been hypothesized to explain why tissues with rapid cell turnover are prone to cancer.80
Lipid peroxidation in the liver and in circulation were evaluated by measuring isoprostane, considered a highly sensitive and specific marker of lipid damage that is unaffected by diet.81,82 Instead of the expected increases, isoprostane concentrations tended to decrease. The results of TBARS assays were concordant with those of the isoprostane analysis, finding no detectable end-point damage to lipid as an oxidative stress target during sleep loss. Glutathione peroxidase, which is the principal scavenger of H2O2 under most circumstances,8 was significantly decreased in the livers of sleep deprived rats, which suggests potentially increased susceptibility to damage if additional insults would be encountered from, for instance, co-morbidities. It remains possible that damage to membrane lipids occurred at a low level or that lipid peroxidation was not detected in this initial survey of end points of irreversible damage. Also, lipid peroxidation is low in experimental models of chronic calorie restriction, characterized by a decrease in the availability of peroxidizable fatty acids and low levels of the hormones insulin, triiodothyronine, and growth hormone.83,84 Most of these same signs are found in sleep deprived rats: a progressively deep negative energy balance indicating calorie deficiency, central hypothyroidism, and abolishment of growth hormone secretory bursts.7,41,42,85–87 (Insulin levels may remain elevated in sleep deprived rats because of increased food consumption.) Therefore, this resemblance between sleep deprivation and experimental calorie restriction allows us to speculate that the outcome of low lipid peroxidation during oxidative damage may plausibly be related to normal lipid degradation during autophagy to produce energy. Comparison data are limited, but include a 30% decrease in the expression of liver phosphatidylcholine, a phospholipid that is considered susceptible to peroxidation, in rats deprived of sleep for 5 days,19 and a decrease in glutathione peroxidase in insomnia patients.88 These latter studies included reports of increased TBARS, not observed here, but the well-known caveats with the assay procedure tend to limit its utility as a sole measure of lipid peroxidation.8
The lack of evidence for significant lipid peroxidation or carbonyl concentrations during the sleep deprivation condition in the present results are generally consistent with those reported by Gopalakrishnan et al. who studied sleep deprivation in rats under a similar paradigm, but without ambulation controls or recovery period comparisons.89 They did not find differences between partial and total sleep deprivation treatments in the amount of TBARS or carbonyl in the muscle, liver, or brain cortex, or in the amount of superoxide dismutase in the brain cortex.
In the current study, carbonyls and nitrotyrosine did not vary together, even though both are fingerprints of protein damage. Divergent effects were most readily apparent in the liver during the recovery period when carbonyls were only 21%, and nitrotyrosine levels were 310%, of total sleep deprivation levels, both of which were returns to baseline levels. The carbonyl assay mainly measures the residues of amino acids―particularly histidine, arginine, lysine, and proline―that have been oxidatively modified by reactive oxygen species, whereas the nitrotyrosine assay reflects the nitric oxide-related attack on tyrosine by peroxynitrite and/or reactive nitrogen species.8 The lymphoid tissues may respond differently to sleep loss than do other tissues; splenic responses to sleep loss included increased nitrotyrosine, increased large subunit caspase-3, and relative proliferation of cells in the white pulp. Further studies will be required to establish the functional implications of these differences, but they presumably point to potentially important physiological actions associated with homeostasis and recuperation, such as nitric oxide-mediated events that might have been suppressed by sleep loss.
Disruption of sleep is well known to result in dependent decreases in circadian rhythm amplitude and phase38,39; this may be expected to contribute to the outcomes. Likewise, disruption of the circadian rhythm (e.g., jetlag) is well known to result in dependent disruption of sleep. As has been historically practiced, all rats in the current investigation were studied under constant, standard room illumination to reduce the robustness of the circadian rhythm amplitude in control rats. In addition, the schedules of ambulation requirements, as well as the availability of food and water ad libitum, comprised a constant routine with few clues for entrainment except for daily husbandry. The constancy of these conditions across treatment groups suggests that the outcomes are mainly related to the sleep deprivation, which is the primary intervention and the one on which circadian rhythm influences would be dependent and potentially modulating influences. Recent studies have shown that the circadian rhythms of gene expression in peripheral tissues respond to sleep loss, and that the patterns change depending on the amount, duration, and history of sleep loss.90,91 Functional annotation revealed that up-regulated genes induced after restricted sleep are predominantly associated with cellular responses to oxidative stress and reactive oxygen species, and with metabolism.90,91 The properties of recovery sleep observed in the current study also appear primarily dependent on reinstated sleep because all other experimental conditions were kept constant.
Recovery sleep was striking in both an overall quiescence with regard to oxidative damage and a remission of sleep loss effects. For example, cell death in the lung was significantly decreased to only 9% of deprivation values in partially sleep deprived rats, and showed a strong tendency to be decreased in totally sleep deprived rats. Cell death in each the intestinal epithelium and the lamina propria during recovery was only 2% of total sleep deprivation values. Cell proliferation in the intestinal crypts of recovering partially and totally sleep deprived rats returned toward normal: means were 5% and 11% below the value for ACs and 27% and 39% below deprivation values, respectively. In other tissues, the number of apoptotic cells during recovery sleep was normal; no increases were found. During recovery, 8-OHdG values for all tissues studied were the same as those of ACs and/or were significantly decreased from deprivation levels. Lipid peroxidation, as measured by plasma isoprostane concentration, was 31% lower during recovery in totally sleep deprived rats than in baseline controls. Concentrations of the antioxidant glutathione peroxidase were normalized. Concentrations of carbonyls and nitrotyrosine were at control levels. Therefore, the susceptibilities and vulnerabilities posed by sleep loss appear to have been ameliorated: (1) the balance between DNA damage and repair was restored, (2) cell turnover in the intestine was low, (3) damage to lipid and protein targets of oxidative stress were minimal or normal, and (4) the extra metabolic burdens associated with cell damage and cell renewal were abolished. These properties appear to be among those that underlie restoration by sleep.
The main limitations to the outcomes are as follows. First, sleep physiology and behavior in rats and other mammals are comparable and therefore believed to serve the same fundamental functions. However, the outcomes of studies on other species may be different, based on several factors: the parameters measured, tissue studied, species-specific effects, and stringency of sleep loss. Future studies may be needed to find the common denominators among species and outcome measurements. Second, it may be argued that the stringency of prolonged sleep deprivation in the current study is not relevant to real life. However, comparable stringency indeed occurs in subsets of the human population, such as the critically ill and individuals functioning under chronically atypical sleep-work schedules.92,93 Furthermore, stringency during sleep loss is necessary to produce observable functional deficits that may point to functions that take place more effectively or efficiently during sleep, because studies of the replete state of sleep have not revealed its functions. Third, as in all analyses, the lack of statistical significance should not be interpreted as a lack of effect, but may be a type II error because of limitations in measurement, sample size and variability, or statistical approach. Nonetheless, the consistent pattern of changes in multiple related assays and across multiple organs imply that our qualitative conclusions are likely robust. Fourth, negative findings would not rule out the occurrence of potentially important changes in biochemical pathways that did not result in the end-point products measured here, or those that have not yet evolved at the time of observation.
In conclusion, severe deficiency of sleep is known to affect health and longevity, but appears to do so in insidious ways and by nebulous processes. The current outcomes provide evidence that the pathophysiology of inadequate sleep involves specific cell damage; previously, this had been inferred from systemic clues. Especially pronounced in this study was oxidative DNA damage in the liver, lung, and small intestine. The damage was not associated with increased apoptosis except in the intestine, where cell renewal was accelerated. Evidence of DNA damage can precede evidence of protein and lipid damage,8 which might eventually contribute to the decline in health that occurs during the second half of the typical survival period in rats34,41,42 or to comorbid conditions in humans.1,3,94 We speculate that these outcomes may be borne out in humans, whose overall physiological responses to deprivation of any basic biological need are comparable to those of rats, aside from the wide variability in tolerance and expected survival time.10 The evidence presented here provides the types of subtle, subclinical changes that can help explain (1) the lack of clinical signs by which one could identify a state of deficient sleep, and (2) why a serious deficit in sleep may promote multifarious disease processes. Increased and obligatory demands for repair and replacement of cells, as well as clearance of debris, furthermore would constitute oxidative burdens with metabolic and immune-related implications. The finding that imbalanced systems were restored by recovery sleep adds emphasis to increasing awareness that sleep affects a vast array of biomolecules and pathways.
DISCLOSURE STATEMENT
This was not an industry supported study. Research support was provided by the National Heart, Lung, and Blood Institute through Grants HL080744 and HL086447 and by the National Center for Advancing Translational Sciences, Grant 8UL1TR000055. Additional support and facilities were provided by the Medical College of Wisconsin, Department of Neurology, and the Department of Veterans Affairs. Dr. Hogg is Editor-in-Chief of the journal Nitric Oxide. The other authors have indicated no financial conflicts of interest. The work was performed at the Zablocki Veterans Administration Medical Center and The Medical College of Wisconsin.
ACKNOWLEDGMENTS
The authors thank Ginger Milne and the late Jason D. Morrow, Vanderbilt University Eicosanoid Core, for F2-isoprostane analysis; Anne Folley, Nichole Nellessen Miller, Christa Thalacker for technical assistance; and Elizabeth Brown and Nathan Whitaker for editing. Portions of this work on cell regeneration and death were presented at the 26th and 28th annual meetings of the Associated Professional Sleep Societies in 2012 and 2014, and the Gordon Research Conference, Sleep Regulation and Function, in 2014.
SUPPLEMENTAL MATERIAL
Tissue Allocation
Tissue specimens from the first set of live animal experiments, composed of 52 rats, had been preserved for further study from rats in which the antioxidants and immune-related variables were assayed and reported previously.1,2 The following measurements were made in this set of specimens: cell death signaling, cell death and cell proliferation in the spleen, and markers of lipid damage in the plasma, liver, and heart. A subset of spleens from this first set of tissues was analyzed together with those of the second set of experiments to provide sufficient quantities. Rats in the second set of experiments, composed of 46 rats, had been implanted with indwelling catheters for the collection of blood, as performed in prior studies.3–7 A subset was also implanted with saline-filled osmotic minipumps to serve as comparisons for other pharmacological studies. There were no indications that the implants affected food intake or body weight. Tissue specimens from this second series of live animal experiments were analyzed for cell death in the liver, heart, lung, and small intestine; cell proliferation in the liver and small intestine; and DNA and protein damage and antioxidant activities in all organs under study.
Surgical Analgesics and Anesthetics Administered
After brief inhalation anesthesia with halothane or isoflurane, a surgical plane of anesthesia and analgesia was obtained by one of the following combinations of injectable drugs: (1) ketamine·HCl (100 mg/kg intraperitoneally [ip]), xylazine·HCl (10 mg/kg intramuscularly [im]), and atropine sulfate (0.04 mg/ kg im), with supplementary doses of ketamine·HCl (10 mg/kg ip) administered as needed, or (2) ketamine·HCl (40 mg/kg ip), medetomidine·HCl (0.25 mg/kg ip), or dexmedetomidine·HCl (0.23 mg/kg ip), with supplementary doses of (dex) medetomidine·HCl (0.1 mg/kg ip) administered as needed. Atipamezole·HCl was administered ip in a volume equal to the total administered volume of (dex)medetomidine to reverse anesthesia at the conclusion of surgery.
For a subset of rats, brief anesthesia was induced with isoflurane for saline-filled pump placement (Alzet, Model 2ML1, Durect Corp., Cupertino, CA) after 7 days of baseline conditions and replaced after 5–6 experimental days.
Timeline illustrating the experimental schedules. All rats were allotted at least 7 days to recover from surgery and acclimate to Bergmann-Rechtschaffen apparatuses, as well as 7 days of baseline conditions. Baseline control rats were studied at the end of the baseline period. Ambulation controls and partially and totally sleep deprived rats were studied after 10 experimental days. Recovery rats were studied after 2 days of sleep ad libitum after 10 experimental days.
Pairwise comparisons of the outcome measures among experimental treatments.
Values for outcome measurements grouped by pairwise comparison.
REFERENCES
- 1.2011. National Sleep Disorders Research Plan: US Department of Health and Human Services, NIH Publication No. 11-7820.
- 2.Altman NG, Izci-Balserak B, Schopfer E, et al. Sleep duration versus sleep insufficiency as predictors of cardiometabolic health outcomes. Sleep Med. 2012;13:1261–70. doi: 10.1016/j.sleep.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 4.von Ruesten A, Weikert C, Fietze I, Boeing H. Association of sleep duration with chronic diseases in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. PLoS ONE. 2012;7(1):e30972. doi: 10.1371/journal.pone.0030972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson CM. Philadelphia, PA: P. Blakiston's Son and Co.; 1925. The Effects of Inanition and Malnutrition Upon Growth and Structure. [Google Scholar]
- 6.Morgulis S. Fasting and Undernutrition. New York: Dutton; 1923. [Google Scholar]
- 7.Everson CA, Laatsch CD, Hogg N. Antioxidant defense responses to sleep loss and sleep recovery. Am J Physiol Regul Integr Comp Physiol. 2005;288:374–83. doi: 10.1152/ajpregu.00565.2004. [DOI] [PubMed] [Google Scholar]
- 8.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 3rd ed. Oxford: Oxford University Press; 1999. [Google Scholar]
- 9.Altemus M, Rao B, Dhabhar FS, Ding W, Granstein RD. Stress-induced changes in skin barrier function in healthy women. J Invest Dermatol. 2001;117:309–17. doi: 10.1046/j.1523-1747.2001.01373.x. [DOI] [PubMed] [Google Scholar]
- 10.Everson CA. Clinical manifestations of prolonged sleep deprivation. In: Schwartz WJ, editor. Sleep Science: Integrating Basic Research and Clinical Practice. Basel: Karger; 1997. pp. 34–59. [Google Scholar]
- 11.Frey DJ, Fleshner M, Wright KP., Jr The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21:1050–7. doi: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–52. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu J, Chen Z, Gorczynski CP, et al. Sleep-deprived mice show altered cytokine production manifest by perturbations in serum IL-1ra, TNFa, and IL-6 levels. Brain Behav Immun. 2003;17:498–504. doi: 10.1016/j.bbi.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. JACC. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 15.Moldofsky H, Lue FA, Davidson JR, Gorczynski R. Effects of sleep deprivation on human immune functions. FASEB J. 1989;3:1972–7. doi: 10.1096/fasebj.3.8.2785942. [DOI] [PubMed] [Google Scholar]
- 16.Shearer WT, Reuben JM, Mullington JM, et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–70. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- 17.van Leeuwen WM, Lehto M, Karisola P, et al. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS ONE. 2009;4(2):e4589. doi: 10.1371/journal.pone.0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–26. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 19.Chang HM, Mai FD, Chen BJ, et al. Sleep deprivation predisposes liver to oxidative stress and phospholipid damage: a quantitative molecular imaging study. J Anat. 2008;212:295–305. doi: 10.1111/j.1469-7580.2008.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cirelli C, Faraguna U, Tononi G. Changes in brain gene expression after long-term sleep deprivation. J Neurochem. 2006;98:1632–45. doi: 10.1111/j.1471-4159.2006.04058.x. [DOI] [PubMed] [Google Scholar]
- 21.Everson CA, Thalacker CD, Hogg N. Phagocyte migration and cellular stress induced in liver, lung, and intestine during sleep loss and sleep recovery. Am J Physiol Regul Integr Comp Physiol. 2008;295:2067–74. doi: 10.1152/ajpregu.90623.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo JS, Chau JF, Shen XZ, Cho CH, Luk JM, Koo MW. Over-expression of inducible heat shock protein 70 in the gastric mucosa of partially sleep-deprived rats. Scand J Gastroenterol. 2004;39:510–5. doi: 10.1080/00365520410004523. [DOI] [PubMed] [Google Scholar]
- 23.Naidoo N, Ferber M, Master M, Zhu Y, Pack AI. Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. J Neurosci. 2008;28:6539–48. doi: 10.1523/JNEUROSCI.5685-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terao A, Steininger TL, Hyder K, et al. Differential increase in the expression of heat shock protein family members during sleep deprivation and during sleep. Neuroscience. 2003;116:187–200. doi: 10.1016/s0306-4522(02)00695-4. [DOI] [PubMed] [Google Scholar]
- 25.Lungato L, Marques MS, Pereira VG, et al. Sleep deprivation alters gene expression and antioxidant enzyme activity in mice splenocytes. Scand J Immunol. 2013;77:195–9. doi: 10.1111/sji.12029. [DOI] [PubMed] [Google Scholar]
- 26.Ramanathan L, Gulyani S, Nienhuis R, Siegel JM. Sleep deprivation decreases superoxide dismutase activity in rat hippocampus and brainstem. Neuroreport. 2002;13:1387–90. doi: 10.1097/00001756-200208070-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinges DF, Douglas SD, Zaugg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93:1930–9. doi: 10.1172/JCI117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleitman N. Sleep and Wakefulness. Chicago, IL: University of Chicago Press; 1963. [Google Scholar]
- 29.Kuhn E, Brodan V, Brodanova M, Rysanek K. Metabolic reflection of sleep deprivation. Act Nerv Super (Praha) 1969;11:165–74. [PubMed] [Google Scholar]
- 30.Andersen ML, Ribeiro DA, Bergamaschi CT, et al. Distinct effects of acute and chronic sleep loss on DNA damage in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:562–7. doi: 10.1016/j.pnpbp.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Mendelson WB, Guthrie RD, Frederick G, Wyatt R.J. The flower pot technique of rapid eye movement (REM) sleep deprivation. Pharmacol Biochem Behav. 1974;2:553–6. doi: 10.1016/0091-3057(74)90018-5. [DOI] [PubMed] [Google Scholar]
- 32.Suchecki D, Tufik S. Social stability attenuates the stress in the modified multiple platform method for paradoxical sleep deprivaiton in the rat. Physiol Behav. 2000;68:309–16. doi: 10.1016/s0031-9384(99)00181-x. [DOI] [PubMed] [Google Scholar]
- 33.Everson CA. Clinical assessment of blood leukocytes, serum cytokines, and serum immunoglobulins as responses to sleep deprivation in laboratory rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:1054–63. doi: 10.1152/ajpregu.00021.2005. [DOI] [PubMed] [Google Scholar]
- 34.Everson CA. Sustained sleep deprivation impairs host defense. Am J Physiol Regul Integr Comp Physiol. 1993;265:1148–54. doi: 10.1152/ajpregu.1993.265.5.R1148. [DOI] [PubMed] [Google Scholar]
- 35.Gilliland M, Wold L, Wollmann R, Eschenbach K, Rechtschaffen A. Pathology in sleep deprived rats is not reflected in histologic abnormalities (Abstract) Sleep Res. 1984;13:190. [Google Scholar]
- 36.National Research Council of the National Academies. 8th ed. Washington, DC: The National Academies Press; 2011. Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 37.Bergmann BM, Kushida CA, Everson CA, Gilliland MA, Obermeyer W, Rechtschaffen A. Sleep deprivation in the rat: II. Methodology. Sleep. 1989;12:5–12. doi: 10.1093/sleep/12.1.5. [DOI] [PubMed] [Google Scholar]
- 38.Mistlberger RE, Belcourt J, Antle MC. Circadian clock resetting by sleep deprivation without exercise in Syrian hamsters: dark pulses revisited. J Biol Rhythms. 2002;17:227–37. doi: 10.1177/07430402017003006. [DOI] [PubMed] [Google Scholar]
- 39.Tsai LL, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: XVI. Effects in a light-dark cycle. Sleep. 1992;15:537–44. doi: 10.1093/sleep/15.6.537. [DOI] [PubMed] [Google Scholar]
- 40.Romanovsky AA, Ivanov AI, Shimansky YP. Ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol. 2002;92:2667–79. doi: 10.1152/japplphysiol.01173.2001. [DOI] [PubMed] [Google Scholar]
- 41.Everson CA, Wehr TA. Nutritional and metabolic adaptations to prolonged sleep deprivation in the rat. Am J Physiol Regul Integr Comp Physiol. 1993;264:376–87. doi: 10.1152/ajpregu.1993.264.2.R376. [DOI] [PubMed] [Google Scholar]
- 42.Everson CA, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat. III. Total sleep deprivation. Sleep. 1989;12:13–21. doi: 10.1093/sleep/12.1.13. [DOI] [PubMed] [Google Scholar]
- 43.Everson CA, Smith CB, Sokoloff L. Effects of prolonged sleep deprivation on local rates of cerebral energy metabolism in freely moving rats. J Neurosci. 1994;14:6769–78. doi: 10.1523/JNEUROSCI.14-11-06769.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Everson CA, Szabo A. Recurrent restriction of sleep and inadequate recuperation induce both adaptive changes and pathological outcomes. Am J Physiol Regul Integr Comp Physiol. 2009;297:1430–40. doi: 10.1152/ajpregu.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Everson CA, Gilliland MA, Kushida CA, et al. Sleep deprivation in the rat: IX. Recovery. Sleep. 1989;12:60–7. [PubMed] [Google Scholar]
- 46.Rechtschaffen A, Bergmann BM, Gilliland MA, Bauer K. Effects of method, duration, and sleep stage on rebounds from sleep deprivation in the rat. Sleep. 1999;22:11–31. doi: 10.1093/sleep/22.1.11. [DOI] [PubMed] [Google Scholar]
- 47.Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoc. 2007;2:221–6. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- 48.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 49.Aebi H. Catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York: Academic Press; 1974. pp. 673–84. [Google Scholar]
- 50.Karthikeyan K, Bai BR, Gauthaman K, Sathish KS, Devaraj SN. Cardioprotective effect of the alcoholic extract of Terminalia arjuna bark in an in vivo model of myocardial ischemic reperfusion injury. Life Sci. 2003;73:2727–39. doi: 10.1016/s0024-3205(03)00671-4. [DOI] [PubMed] [Google Scholar]
- 51.Drucker DJ, Ehrlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci U S A. 1996;93:7911–6. doi: 10.1073/pnas.93.15.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kotani J, Usami M, Nomura H, et al. Enteral nutrition prevents bacterial translocation but does not improve survival during acute pancreatitis. Arch Surg. 1999:287–92. doi: 10.1001/archsurg.134.3.287. [DOI] [PubMed] [Google Scholar]
- 53.Renes IB, Verburg M, Bulsing NP, et al. Protection of the Peyer's patch-associated crypt and villus epithelium against methotrexate-induced damage is based on its distinct regulation of proliferation. J Pathol. 2002;198:60–8. doi: 10.1002/path.1183. [DOI] [PubMed] [Google Scholar]
- 54.Taminiau JA, Gall DG, Hamilton JR. Response of the rat small-intestine epithelium to methotrexate. Gut. 1980;21:486–92. doi: 10.1136/gut.21.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toyoda K, Nishikawa A, Furukawa F, Kawanishi T, Hayashi Y, Takahashi M. Cell proliferation induced by laxatives and related compounds in the rat intestine. Cancer Lett. 1994;83:43–9. doi: 10.1016/0304-3835(94)90297-6. [DOI] [PubMed] [Google Scholar]
- 56.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 57.Hakem R. DNA-damage repair; the good, the bad, and the ugly. EMBO J. 2008;27:589–605. doi: 10.1038/emboj.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Halliwell B. Effect of diet on cancer development: is oxidative DNA damage a biomarker? Free Radic Biol Med. 2002;32:968–74. doi: 10.1016/s0891-5849(02)00808-0. [DOI] [PubMed] [Google Scholar]
- 59.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2' -deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health Part C Environ Carcinog Ecotoxicol Rev. 2009;27:120–39. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 60.Chipuk JE, Green DR. Dissecting p53-dependent apoptosis. Cell Death Differ. 2006;13:994–1002. doi: 10.1038/sj.cdd.4401908. [DOI] [PubMed] [Google Scholar]
- 61.Loft S, Poulsen HE. Cancer risk and oxidative DNA damage in man. J Mol Med. 1996;74:297–312. doi: 10.1007/BF00207507. [DOI] [PubMed] [Google Scholar]
- 62.Matsui A, Ikeda T, Enomoto K, et al. Increased formation of oxidative DNA damage, 8-hydroxy-2'-deoxyguanosine, in human breast cancer tissue and its relationship to GSTP1 and COMT genotypes. Cancer Lett. 2000;151:87–95. doi: 10.1016/s0304-3835(99)00424-3. [DOI] [PubMed] [Google Scholar]
- 63.Iwai K, Adachi S, Takahashi M, et al. Early oxidative DNA damages and late development of lung cancer in diesel exhaust-exposed rats. Environ Res. 2000;84:255–64. doi: 10.1006/enrs.2000.4072. [DOI] [PubMed] [Google Scholar]
- 64.Bashir S, Harris G, Denman MA, Blake DR, Winyard PG. Oxidative DNA damage and cellular sensitivity to oxidative stress in human autoimmune diseases. Ann Rheum Dis. 1993;52:659–66. doi: 10.1136/ard.52.9.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gray K, Bennett M. Role of DNA damage in atherosclerosis--bystander or participant? Biochem Pharmacol. 2011;82:693–700. doi: 10.1016/j.bcp.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 66.Mahmoudi M, Mercer J, Bennett M. DNA damage and repair in atherosclerosis. Cardiovasc Res. 2006;71:259–68. doi: 10.1016/j.cardiores.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 67.Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012;111:245–59. doi: 10.1161/CIRCRESAHA.111.261388. [DOI] [PubMed] [Google Scholar]
- 68.Feng B, Ruiz MA, Chakrabarti S. Oxidative-stress-induced epigenetic changes in chronic diabetic complications. Can J Physiol Pharmacol. 2013;91:213–20. doi: 10.1139/cjpp-2012-0251. [DOI] [PubMed] [Google Scholar]
- 69.Kawasaki E, Abiru N, Eguchi K. Prevention of type 1 diabetes: from the view point of beta cell damage. Diabetes Res Clin Pract. 2004;66:27–32. doi: 10.1016/j.diabres.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 70.Jena G, Trivedi PP, Sandala B. Oxidative stress in ulcerative colitis: an old concept but a new concern. Free Radic Res. 2012;46:1339–45. doi: 10.3109/10715762.2012.717692. [DOI] [PubMed] [Google Scholar]
- 71.Okayasu I. Development of ulcerative colitis and its associated colorectal neoplasia as a model of the organ-specific chronic inflammation-carcinoma sequence. Pathol Int. 2012;62:368–80. doi: 10.1111/j.1440-1827.2012.02807.x. [DOI] [PubMed] [Google Scholar]
- 72.Berquist BR, Wilson DM., 3rd Pathways for repairing and tolerating the spectrum of oxidative DNA lesions. Cancer Lett. 2012;327:61–72. doi: 10.1016/j.canlet.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heath JP. Epithelial cell migration in the intestine. Cell Biol Int. 1996;20:139–46. doi: 10.1006/cbir.1996.0018. [DOI] [PubMed] [Google Scholar]
- 74.Klein RM, McKenzie JC. The role of cell renewal in the ontogeny of the intestine. I. Cell proliferation patterns in adult, fetal, and neonatal intestine. J Pediatr Gastroenterol Nutr. 1983;2:10–43. doi: 10.1097/00005176-198302010-00004. [DOI] [PubMed] [Google Scholar]
- 75.Creamer B, Shorter RG, Bamforth J. The turnover and shedding of epithelial cells. I. The turnover in the gastro-intestinal tract. Gut. 1961:110–8. doi: 10.1136/gut.2.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dotan I, Mayer L. Mucosal immunity. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran's Gastrointestinal and Liver Disease. 9th ed. Philadelphia, PA: Saunders; 2010. pp. 21–30. [Google Scholar]
- 77.Casado J, Fernandez-Lopez JA, Esteve M, Rafecas I, Argiles JM, Alemany M. Rat splanchnic net oxygen consumption, energy implications. J Physiol (Lond) 1990;431:557–69. doi: 10.1113/jphysiol.1990.sp018347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferrell CL. Contribution of visceral organs to animal energy expenditures. J Anim Sci. 1988;66:23–34. [Google Scholar]
- 79.Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis. 2003;24:353–62. doi: 10.1093/carcin/24.3.353. [DOI] [PubMed] [Google Scholar]
- 80.Rizk P, Barker N. Gut stem cells in tissue renewal and disease: methods, markers, and myths. Wiley Interdiscip Rev Syst Biol Med. 2012;4:475–96. doi: 10.1002/wsbm.1176. [DOI] [PubMed] [Google Scholar]
- 81.Gopaul NK, Halliwell B, Anggard EE. Measurement of plasma F2-isoprostanes as an index of lipid peroxidation does not appear to be confounded by diet. Free Radic Res. 2000;33:115–27. doi: 10.1080/10715760000300671. [DOI] [PubMed] [Google Scholar]
- 82.Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28:505–13. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 83.Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev. 2007;87:1175–213. doi: 10.1152/physrev.00047.2006. [DOI] [PubMed] [Google Scholar]
- 84.Sanz A, Pamplona R, Barja G. Is the mitochondrial free radical theory of aging intact? Antioxid Redox Signal. 2006;8:582–99. doi: 10.1089/ars.2006.8.582. [DOI] [PubMed] [Google Scholar]
- 85.Everson CA, Crowley WR. Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats. Am J Physiol Endocrinol Metab. 2004;286:1060–70. doi: 10.1152/ajpendo.00553.2003. [DOI] [PubMed] [Google Scholar]
- 86.Everson CA, Nowak TSJ. Hypothalamic thyrotropin-releasing hormone mRNA responses to hypothyroxinemia induced by sleep deprivation. Am J Physiol Endocrinol Metab. 2002;283:85–93. doi: 10.1152/ajpendo.00558.2001. [DOI] [PubMed] [Google Scholar]
- 87.Everson CA, Reed HL. Pituitary and peripheral thyroid hormone responses to thyrotropin-releasing hormone during sustained sleep deprivation in freely moving rats. Endocrinology. 1995;136:1426–34. doi: 10.1210/endo.136.4.7895653. [DOI] [PubMed] [Google Scholar]
- 88.Gulec M, Ozkol H, Selvi Y, et al. Oxidative stress in patients with primary insomnia. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:247–51. doi: 10.1016/j.pnpbp.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 89.Gopalakrishnan A, Ji L, Cirelli C. Sleep deprivation and cellular responses to oxidative stress. Sleep. 2004;27:27–35. doi: 10.1093/sleep/27.1.27. [DOI] [PubMed] [Google Scholar]
- 90.Barclay JL, Husse J, Bode B, et al. Circadian desynchrony promotes metabolic disruption in a mouse model of shiftwork. PLoS ONE. 2012;7(5):e37150. doi: 10.1371/journal.pone.0037150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moller-Levet CS, Archer SN, Bucca G, et al. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proc Natl Acad Sci U S A. 2013;110:E1132–41. doi: 10.1073/pnas.1217154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Orr WC, Stahl ML. Sleep disturbances after open heart surgery. Am J Cardiol. 1977;39:196–201. doi: 10.1016/s0002-9149(77)80191-4. [DOI] [PubMed] [Google Scholar]
- 93.Soutiere SE, Shemery A, Steele CT, Quatroche A, Dyche J. Groton, CT: 2013. Scientific rationale for the “All Hands Awake” 24-hour watchstanding schedule. Document NSMRL/50513/TR-2013. [Google Scholar]
- 94.Luyster FS, Strollo PJ, Zee PC, Walsh JK. Boards of Directors of the American Academy of Sleep Medicine and the Sleep Research Society. Sleep: a health imperative. Sleep. 2012;35:727–34. doi: 10.5665/sleep.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
REFERENCES
- 1.Everson CA, Laatsch CD, Hogg N. Antioxidant defense responses to sleep loss and sleep recovery. Am J Physiol Regul Integr Comp Physiol. 2005;288:374–83. doi: 10.1152/ajpregu.00565.2004. [DOI] [PubMed] [Google Scholar]
- 2.Everson CA, Thalacker CD, Hogg N. Phagocyte migration and cellular stress induced in liver, lung, and intestine during sleep loss and sleep recovery. Am J Physiol Regul Integr Comp Physiol. 2008;295:2067–74. doi: 10.1152/ajpregu.90623.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Everson CA, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat. III. Total sleep deprivation. Sleep. 1989;12:13–21. doi: 10.1093/sleep/12.1.13. [DOI] [PubMed] [Google Scholar]
- 4.Everson CA, Crowley WR. Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats. Am J Physiol Endocrinol Metab. 2004;286:1060–70. doi: 10.1152/ajpendo.00553.2003. [DOI] [PubMed] [Google Scholar]
- 5.Everson CA, Reed HL. Pituitary and peripheral thyroid hormone responses to thyrotropin-releasing hormone during sustained sleep deprivation in freely moving rats. Endocrinology. 1995;136:1426–34. doi: 10.1210/endo.136.4.7895653. [DOI] [PubMed] [Google Scholar]
- 6.Everson CA, Smith CB, Sokoloff L. Effects of prolonged sleep deprivation on local rates of cerebral energy metabolism in freely moving rats. J Neurosci. 1994;14:6769–78. doi: 10.1523/JNEUROSCI.14-11-06769.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everson CA, Wehr TA. Nutritional and metabolic adaptations to prolonged sleep deprivation in the rat. Am J Physiol Regul Integr Comp Physiol. 1993;264:R376–87. doi: 10.1152/ajpregu.1993.264.2.R376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Timeline illustrating the experimental schedules. All rats were allotted at least 7 days to recover from surgery and acclimate to Bergmann-Rechtschaffen apparatuses, as well as 7 days of baseline conditions. Baseline control rats were studied at the end of the baseline period. Ambulation controls and partially and totally sleep deprived rats were studied after 10 experimental days. Recovery rats were studied after 2 days of sleep ad libitum after 10 experimental days.
Pairwise comparisons of the outcome measures among experimental treatments.
Values for outcome measurements grouped by pairwise comparison.


