Abstract
Study Objectives:
Forty-one percent of shift workers report dozing while driving. This study tested whether armodafinil improves driving simulator performance in subjects with shift work disorder (SWD). A primary outcome was performance late in the shift when workers are typically driving home.
Design:
Randomized, double-blind, crossover. During each 12-h test session (21:30-09:30), subjects were kept awake except for multiple sleep latency testing (MSLT: 01:30, 03:30, 05:30, and 07:30). Subjective sleepiness (Karolinska Sleepiness Scale, KSS), driving performance, and cognitive performance (digit symbol substitution test and creativity on the Remote Associates Test, RAT) were evaluated during the night shift and commute home times.
Setting:
Hospital-based sleep research laboratory.
Participants:
Twenty night workers (age: 42.7 ± 8.7 y, 17 F) with excessive sleepiness (≥ 10 on the Epworth Sleepiness Scale), meeting International Classification of Sleep Disorders, Second Edition (ICSD-2) criteria for SWD, and having no other medical conditions.
Interventions:
Armodafinil (150 mg) or placebo at (23:45 h) on counterbalanced nights separated by 7-14 days.
Measurement and Results:
Primary endpoints were driving simulator performance (standard deviation of lateral position (SDLP) and off-road deviations) with four sessions starting 3.25 h after drug administration, objective sleepiness (MSLT; 1.75 to 7.75 h post-drug), and creativity (5 h post-drug). Significant effects of drug were observed for each driving measure (P < 0.05). Armodafinil significantly improved SDLP for simulator sessions at 05:30, 07:30, and 09:30, and off-road deviations at 7 h, 15 min and 9 h, 15 min post-drug (P < 0.05). Armodafinil also improved objective sleepiness from 3.7 ± 0.6 min to 9.7 ± 5.2 min (P < 0.001) and RAT score from 8.75 ± 4.9 to 11.25 ± 6.0 (P < 0.005).
Conclusions:
Armodafinil 150 mg early in the night shift improves driving simulator performance in shift work disorder. Effects on sleepiness, cognition, and driving were found up to 9.5 h post-ingestion, during the critical time when many night workers are driving home.
Citation:
Drake C, Gumenyuk V, Roth T, Howard R. Effects of armodafinil on simulated driving and alertness in shift work disorder. SLEEP 2014;37(12):1987-1994.
Keywords: armodafinil, driving, shift work disorder, alertness, creativity
INTRODUCTION
It has been estimated that more than 17% of US wage and salary workers (21 million) work shifts that require maintaining alertness and performance during times of night that are normally reserved for sleep.1 Regular night shift workers are typically exposed to both a homeostatic and a circadian challenge because of the accumulation of a sleep debt in conjunction with the necessity of maintaining work performance at an adverse circadian phase. A convergence of evidence has accumulated over the past several decades demonstrating the significant negative effect that night shift work has on human health, productivity, and safety.2–5 Because of the high proportion of shift workers involved in safety-sensitive operations including transportation, one of the most well-documented and long-established effects of night shift work is an increased risk of accidents.6,7 Importantly, it is the subset of night workers who fail to realign their internal circadian timing to match the shift-work–imposed sleep-wake schedule that are at greatest risk for impairments in alertness, safety and performance,8 and who are in most need of interventions to mitigate such risk.4,6
The severity of the combined circadian and homeostatic challenge of shift work becomes evident when considering links to drowsy driving and increased risk of automotive accidents in community-based studies.9–11 A study of rotating shift nurses showed a twofold increased risk of automotive accidents relative to day workers.12 The increased risk of driving impairment associated with night shift work has also been demonstrated in well-controlled laboratory environments using driving simulators.13–16 Both laboratory13,15 and field17,18 studies show that driving at night or home from the night shift are times of particularly high risk because of their proximity with the circadian nadir in alertness. The increased risk of accidents during the commute following a night shift is likely caused by the interactive effects of both circadian misalignment and increased homeostatic sleep pressure from extended wakefulness19 and their accumulated effects on increasing risk over multiple night shifts, particularly in the morning hours.20–22 One recent study of night and rotating nurses showed that the risk for hazardous driving events was eight times higher during the commute home from a night shift compared to the pre-shift commute.17
Aside from increased risk of accidents, studies have also shown impairment in productivity among shift workers.23 In controlled laboratory studies of simulated shift work, circadian misalignment has been shown to impair learning and performance.24 In a study of night shift workers, impairments in performance were identified as early as the first night of work.25 Consistent with these findings, studies have also suggested specific cognitive deficits can be linked to one's inability to adapt to night shift work despite several years on a shift schedule. For example, one study that measured auditory event-related potentials to assess cortical responses to auditory stimuli showed impairment in sensory memory and attention function in individuals with shift work disorder (SWD) compared to asymptomatic night workers.26 Baseline impairments in measures of creativity have also been demonstrated in individuals on simulated shift work schedules.27
Multiple treatments have been successfully used to reduce excessive sleepiness associated with shift work.28 Both animal and human studies of the negative effect of circadian misalignment on health outcomes demonstrate the need for circadian interventions such as appropriately timed bright light exposure aimed at realignment of the circadian system to match the shift work schedule.29 But it is also important to identify countermeasures that acutely reduce the negative effects of SWD on safety and productivity. Pharmacological treatments including methylphenidate, caffeine, amphetamines, and modafinil have been shown to improve driving simulator30–33 and real-world34 performance. However, sleep related studies have been performed in day workers with sleep disorders such as obstructive sleep apnea35–37 and during acute sleep deprivation in healthy day workers with limited nocturnal assessment.33 No studies have investigated the effects of stimulants on driving in patients with SWD.
Armodafinil, a longer-lasting R-isomer of modafinil, is approved for the treatment of SWD and has been shown to improve nocturnal alertness, memory, and attention in SWD.38 The pharmacodynamic profile of armodafinil suggests it may have benefits on driving performance later in the night shift, including typical commute times.39 However, the effects of armodafinil on driving performance have not been investigated in shift workers. Such data, especially at the time of the commute home, is important, as epidemiological studies have shown that shift workers who fail to adapt to the night work schedule (i.e., continue to report excessive sleepiness) are at greater risk of automotive and industrial accidents.11,40 Furthermore, given a failure to adjust their circadian rhythms to the night work schedule, patients with SWD are likely to be commuting home from the night shift at or near their circadian nadir in alertness.8
The current study tested the effects of armodafinil 150 mg on driving performance in a sample of regular night workers with SWD. A second goal of this study was to determine whether armodafinil 150 mg improved cognitive function, including a creativity task during night shift hours. Finally, measurement of the effects of armodafinil on objective sleepiness using the MSLT was done to determine whether improved alertness mediated effects on driving performance and creativity.
METHODS
Subjects
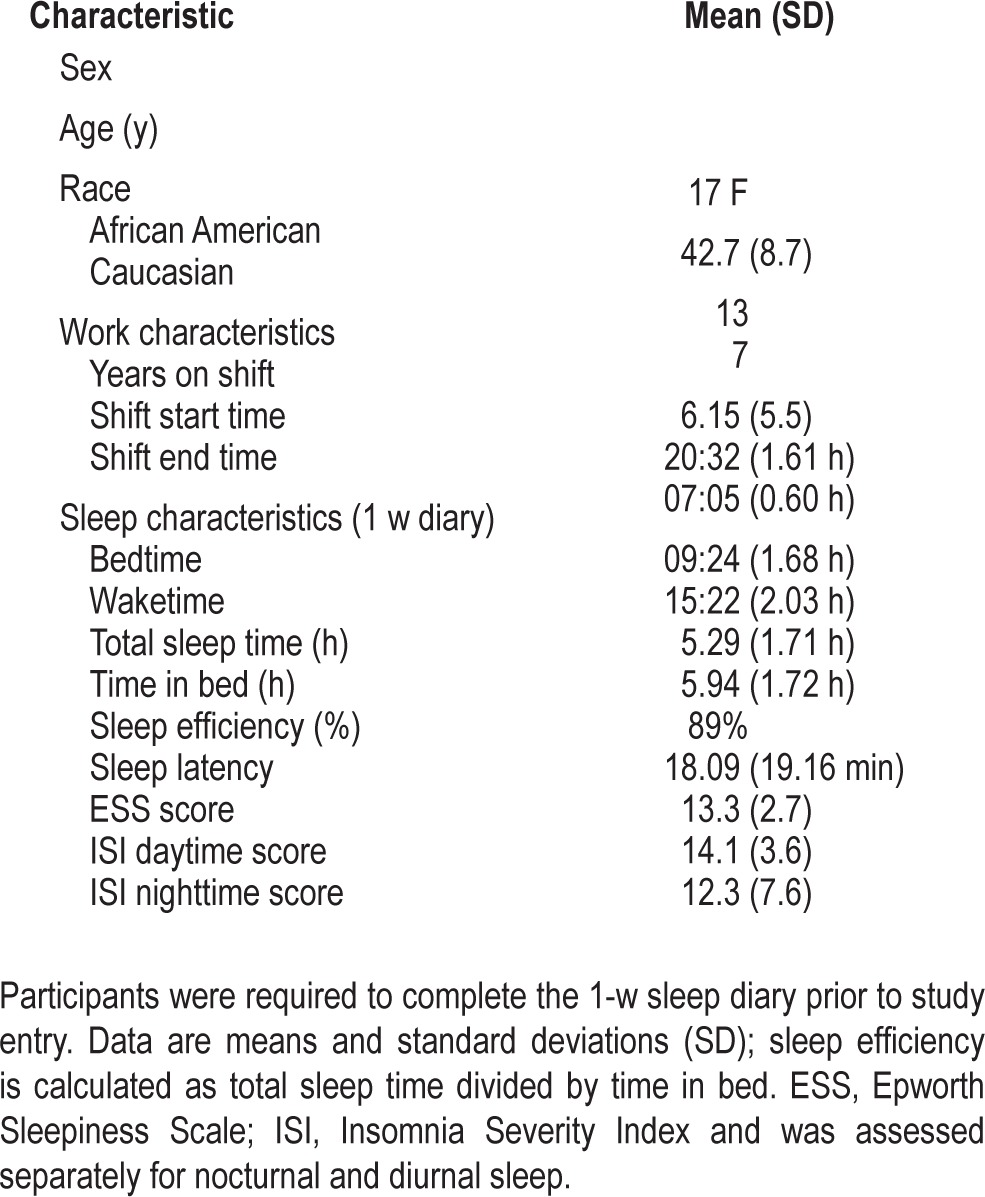
Twenty subjects between the ages of 27 and 55 y (mean = 42.7 ± 8.7 y, 17 F) were recruited from a large, urban medical center using flyers and online newsletters. Participants met inclusion criteria if they worked at least 5 night shifts each month, with 6 h or more worked between 22:00 and 08:00. Subjects were also required to meet International Classification of Sleep Disorders, Second Edition (ICSD-2) criteria for SWD, report symptoms of excessive sleepiness as well as Epworth Sleepiness Scale (ESS) ≥ 10, and be free of any other sleep medical or psychiatric disorders. A medical/sleep history and routine physical examination was performed by a physician to diagnose SWD, as well as to verify the absence of any other sleep, medical, or psychiatric disorders. In addition, the STOP questionnaire was used to screen out participants with a likelihood of sleep apnea.41 Prior to screening, all subjects also completed a 2-week sleep diary.
Exclusion criteria included the presence of any sleep and/ or health disorders other than SWD, as well as current pregnancy, history of substance abuse, use of any medication that acts on the central nervous system, contraindications to taking armodafinil, caffeine consumption > 500 mg/day, and smoking > 10 cigarettes/day.
Procedure
All subjects completed a randomized, double-blind, placebo-controlled, crossover protocol consisting of two counter-balanced overnight laboratory visits to the Henry Ford Sleep Disorders and Research Center. The study protocol was approved by the Henry Ford Hospital Institutional Review Board and subjects signed an informed consent before any procedures were performed and were compensated for participation. Each overnight visit lasted 12 h from 21:30 to 09:30, and took place in a private, sound-proof, and light-proof circadian suite within the sleep center, and had comfortable chairs, a bed, desk, and bathroom. For the entirety of both visits, subjects were kept in indoor light (∼500 lux at the angle of gaze) as measured using a calibrated light meter (Fisher Scientific, Pittsburgh, PA). Subjects were required to work for at least 3 consecutive nights and maintain their regular daytime sleep schedule prior to each study night, as well as refrain from any caffeine or alcohol use for 24 h prior to study nights. This was verified using sleep diary entries, which subjects were required to complete for a week prior to each study night.
Upon arriving to the sleep center at 21:30, subjects had electrodes attached for electroencephalography (EEG), electro-oculography (EOG), and electromyography (EMG), and these were monitored for the entirety of each study night to ensure wakefulness. In addition, they were also visually monitored by sleep center staff to ensure wakefulness throughout the study. When not completing study tasks or questionnaires, subjects were kept out of bed, and allowed to sit comfortably and watch TV, use a personal computer, or talk on the phone. Armodafinil 150 mg or placebo was administered at 23:45. Figure 1 presents a study timeline of study drug administration, and sleepiness and performance assessments throughout the protocol.
Figure 1.

Timeline of study protocol. Subjects arrived at 21:30. Armodafinil or placebo was administered at 23:45. Driving simulator sessions took place four times, at 03:00, 05:00, 07:00, and 09:00. Multiple sleep latency test (MSLT) naps were performed four times, at 01:30, 03:30, 05:30, and 07:30. The Karolinska Sleepiness Scale (KSS) was administered six times, at 22:00, 00:30, 02:30, 04:40, 06:30, and 08:30. The Digit Symbol Substitution Test (DSST) was administered six times, at 22:30, 23:00, 01:00, 04:40, 06:00, and 08:30. The Remote Associates Test (RAT) was administered at 04:00.
Driving Simulator Performance
Driving simulator performance was evaluated using the York Driving Simulator (York Computer Technologies, Ontario, Canada). This low-fidelity simulator was a driving task that presented a driver's-seat view of a typical highway scene, and subjects interacted with the simulator using a steering wheel and two pedals (brake and accelerator) connected to a 14” screen laptop running the simulator. The simulated environment consisted of a completely straight, two-lane highway, with posted speed limit signs of 60 mph, grass and trees beyond the road, and a speedometer in the lower middle section of the screen. The driving simulator has been shown to be sensitive to the impairing effects of sleep restriction and alcohol.14 At the screening appointment, subjects were given an in-depth description of the controls and operation of the driving simulator, and familiarized with the simulator during a 2-min practice session. Subjects were then given an additional 5-min practice session 15 min prior to their first driving task at each study night in order to re-familiarize them with the simulator.
At 3.25, 5.25, 7.25, and 9.25 h after drug or placebo administration (03:00, 05:00, 07:00, and 09:00), subjects completed a 30-min driving task on the simulator. These times were chosen because of our interest in driving performance near the circadian nadir, as well as during early-mid morning hours when many shift workers are commuting home from work. During each trial, if subjects drove onto the shoulder of the road, the desktop driving task stopped, and an on-screen message instructed the subject to press a button, which would reposition the car in the middle of the right-hand lane.
Endpoints were the standard deviation of lateral position (SDLP), or “weaving,” from the center of the right-hand lane, and off-road deviations, which occurred if the subject drove onto the shoulder on either side of the road. All of these measures are commonly used outcomes in driving research and have been found sensitive to various pharmacological manipulations.14,42
Subjects were also asked to evaluate their own driving ability on a two-item questionnaire immediately before and after each 30-min driving session. Question 1 asked, “How safely could you drive a car right now?” and allowed subjects to rate their perception from 1 (“Least Safe”) to 7 (“Most Safe”). Question 2 asked, “Would you get on the road now to drive a 30 minute commute?” and allowed subjects to answer, “Yes” or “No.”
Sleepiness Measures
Objective sleepiness was evaluated across the night using the multiple sleep latency test (MSLT). Nap sessions took place 1.75, 3.75, 5.75, and 7.75 h after drug administration (01:30, 03:30, 05:30, and 07:30), which is in accordance with standard MSLT protocol. As per standard protocol, lights were turned off at the beginning of each nap session, and subjects were instructed to lie comfortably in bed and given up to 20 min to fall asleep, at which point they were immediately awakened. Sleep latency was defined as the time from “lights out” to the first epoch of any stage of sleep on polysomnography (PSG). The primary measure was the mean overall MSLT for all nap trials. Each separate nap trial for the MSLT was also tested for significance as secondary measures.
Subjective sleepiness was evaluated using the Karolinska Sleepiness Scale (KSS), which was administered at 22:00, and 0.75, 2.75, 4.9, 6.75, and 8.75 h following drug administration (00:30, 02:30, 04:40, 06:30, and 08:30). This allowed for evaluation of sleepiness at both pre-drug and post-drug administrations.
Cognitive Performance
Cognitive performance was evaluated using the Digit Symbol Substitution Task (DSST), which was administered twice at baseline prior to drug or placebo administration (22:30 and 23:00), and 1.25, 4.9, 6.25, and 8.75 h following drug administration (01:00, 04:40, 06:00, and 08:30). The multiple assays were taken across the night, allowing for evaluation of both the effect of time since drug ingestion, as well as time of night. After being read instructions, subjects were given 90 sec to complete as many substitutions as possible. Endpoint was number completed.
Creativity as a measure of cognitive performance was evaluated using the Remote Associates Test (RAT), which was given 4.25 h after drug administration (04:00). This is a validated, 30-item, 40-min paper and pencil task which tests an individual's ability to form seemingly unrelated words or ideas into a meaningful or useful association. Each item presents three words, such as “speak,” “money,” and “street,” and the subject is instructed to come up with a fourth word which will meaningfully relate the three words. In this case, for example, the correct fourth word is “easy,” as in “speakeasy,” “easy money,” and “easy street.” The primary endpoint of this task is number of correct answers. The nature of this test allows for only one administration and therefore two different validated forms were used, with one form administered at each overnight session. Assessment occurred 4.25 h after drug administration, which coincided with the estimated circadian nadir in alertness. Previous controlled laboratory and field studies suggest that the RAT is a well-established and valid measure of creativity.43–45
Statistical Analysis
For the outcome variables—sleepiness, driving performance, and cognitive performance—repeated-measures analyses of variance (ANOVAs) were conducted to test for a main effect of drug, a main effect of time, and potential interactions of drug and time. Additional post hoc analyses were also performed where interactions were found, as well as for the final driving simulator session (9.25 h post-administration) regardless of interaction, because the stated aim of this study was to examine the effect of armodafinil on driving performance at the critical time at which many night workers are commuting home from work.
All analyses were performed on actual values, except when baseline measures were available, in which case change-from-baseline scores were calculated. Thus, for both the KSS and DSST, change scores were calculated for each time point in relation to baseline scores taken prior to drug administration.
RESULTS
Table 1 shows the demographic and pre-study sleep and shift work schedule data for the sample.
Table 1.
Participant demographic and sleep characteristics (n = 20).
Driving Simulator Performance
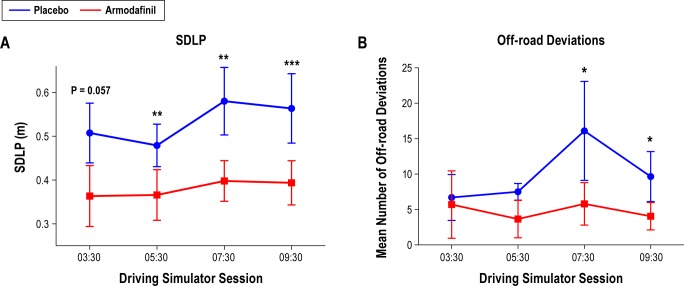
Compared to placebo, administration of armodafinil 150 mg improved driving performance on the desktop simulator across the night with respect to SDLP (F(1,19) = 18.02, P < 0.001) and off-road deviations (F(1,19) = 8.18, P = 0.01), with no effect of time (F(3,57) = 2.42, P = 0.08) and no significant interaction (F(3,57) = 0.46, P = 0.71) for either measure (Figure 2).
Figure 2.
Effects of armodafinil 150 mg administered at 23:45 on (A) standard deviation of lateral position (SDLP) (main effect of drug F(1,19) = 18.02, P < 0.001; no effect of time and no interaction) and (B) off-road deviations (main effect of drug F(1,19) = 8.18, P = 0.01; no effect of time and no interaction). * P < 0.05; ** P < 0.01; *** P < 0.001.
The a priori primary comparison of driving simulator performance 9.75 h following drug administration (at 09:30) revealed that armodafinil 150 mg improved driving simulator performance on this final session for both SDLP (Figure 2A; P = 0.001) and off-road deviations (Figure 2B; P = 0.03). Additional comparisons showed that armodafinil also improved SDLP 5.75 and 7.75 h following administration (05:30 and 07:30 [P < 0.01]), approached significance following administration (03:30 [P = 0.057]), and improved off-road deviations at 7.75 h post-administration (07:30 [P = 0.05]).
Estimates of Ability to Drive
A 2 (drug) × 2 (pre/post) × 4 (time) repeated-measures ANOVA for question 1 revealed a significant main effect of drug (F(1,13) = 9.02, P = 0.01), indicating that armodafinil increased subjects' ratings of ability to drive safely. A significant main effect of time (F(3,39) = 3.09, P = 0.038), showed that ratings of “safe driving” declined across the night. A main effect of pre/post (F(1,13) = 25.37, P < 0.001) showed decreased ratings of safe driving following completion of the driving task. Finally, an interaction of time × pre/post (F(3,39) = 4.61, P = 0.007) demonstrated that post-driving task ratings of safe driving were significantly decreased following the first exposure to the task regardless of drug condition. Within-subjects chi-square analyses using the McNemar test did not reveal any significant differences in terms of the percentage of subjects who responded “Yes” to the statement, “Would you get on the road right now to drive a 30 min commute?” (P > 0.05).
Sleepiness Measures
Objective Sleepiness
Mean overall MSLT sleep latency was calculated by averaging the four MSLT nap trials on each night (Figure 3A). Administration of armodafinil 150 mg produced an increase in MSLT of 5.97 ± 5.0 min relative to placebo, from 3.7 ± 0.6 min to 9.7 ± 5.2 min (P < 0.001). The repeated-measures ANOVA revealed a main effect of drug (F(1,19) = 28.28, P < 0.001), a main effect of time (F(3,57) = 19.76, P < 0.001), and no interaction. Subsequent post hoc comparisons revealed that armodafinil produced a significant improvement in MSLT nap trials at each of the four time points (P < 0.01).
Figure 3.
Effects of armodafinil 150 mg administered at 23:45 on (A) MSLT score (main effect of drug F(1,19) = 28.28, P < 0.001; main effect of time F(1,3) = 19.76, P < 0.001; no interaction), (B) KSS score (main effect of drug F(1,19) = 18.67, P < 0.001; main effect of time F(1,5) = 6.35, P < 0.001; drug × time interaction F(2,5) = 4.49, P = 0.001), (C) DSST score (main effect), and (D) RAT score (main effect). * P < 0.05; ** P < 0.01; *** P < 0.001. DSST, Digit Symbol Substitution Test; KSS, Karolinska Sleepiness Scale; MSLT, multiple sleep latency test; RAT, Remote Associates Test.
Subjective Sleepiness
The KSS was administered six times throughout the night, with the first KSS given 1.75 h before drug administration to serve as a baseline measure. Change scores were calculated from this baseline measurement for the five measurements that took place after drug administration, and a 2 (drug) × 5 (time) repeated-measures ANOVA was performed. This revealed a main effect of drug (F(1,19) = 8.77, P = 0.008), a main effect of time (F(4,76) = 4.42, P = 0.003), and a drug × time interaction (F(4,76) = 3.14, P = 0.019). Simple effects revealed that there was no significant difference in KSS score 45 min following armodafinil administration compared to placebo (3.6 ± 1.5 versus 3.8 ± 2.2, P = 0.69), but significant drug effects were found 2.75, 4.9, 6.75, and 8.75 h following drug administration (Figure 3B).
Cognitive Performance
Performance on the DSST improved after administration of armodafinil compared to placebo (Figure 3C). Subjects completed the DSST six times throughout the night, with two trials taking place before drug administration. No differences in baseline were observed between drug and placebo (57.7 ± 12.3 versus 57.6 ± 13.2, P = 0.97). A mean baseline score was calculated using the two pre-drug trials, and change scores were calculated for DSST trials 1.25, 4.92, 6.25, and 8.75 h following drug administration (Figure 3C). A 2 (drug) × 4 (time) repeated-measures ANOVA was conducted for these change scores and revealed no main effect of drug (F(1,19) = 3.05, P = 0.097), and no main effect of session (F(3,57) = 1.04, P = 0.38). Importantly, there was a significant drug × time interaction (F(1,3) = 5.45, P = 0.002). Follow-up analysis revealed that there was no significant improvement at 1.25 h (57.6 ± 10.7 versus 58.8 ± 12.5, P = 0.54), 4.92 h (59.7 ± 12.8 versus 56.1 ± 14.2, P = 0.068), and 8.75 h post-administration (61.3 ± 12.2 versus 58.1 ± 11.4, P = 0.068). There was a significant improvement in DSST score 6.25 h post-administration (62.4 ± 11.0 versus 54.8 ± 10.1, P = 0.001).
Armodafinil 150 mg significantly improved performance on the RAT from a mean score of 8.75 ± 4.9 to 11.25 ± 6.0 (P = 0.001), ∼5 h following drug administration (Figure 3D). After covarying for change in MSLT score between drug and placebo conditions, improvement on the RAT (i.e., drug effect) remained significant (F(1,19) = 16.2, P = 0.001).
DISCUSSION
The primary results of the current study are that armodafinil 150 mg significantly improved driving simulator performance concomitantly with levels of objective and subjective alertness compared with placebo during the night shift in subjects with SWD. Improvements in measures of simulated driving performance remained significant through the final assessment time point at 09:15. These findings have important implications for driving safety in shift workers and show that in the absence of circadian phase adjustments, symptomatic management with appropriate medications can attenuate negative consequences at critical points in the circadian cycle including common commute times in this population.
Although this is the first study to assess the effects of armodafinil on performance on a driving simulator in shift workers, the results are consistent with previous findings that 150 mg of armodafinil improved both clinician rated and patient-reported functioning of night shift workers between 04:00 and 08:00.46,47 In terms of objective measures of performance, the present results are consistent with a parallel group multicenter study by Czeisler and colleagues that showed armodafinil 150 mg for the treatment of SWD produced reductions in sleepiness and improved memory and attention performance throughout the night up to 08:00.48 However, in the current study as well as that of Czeisler et al.,48 MSLT trials between 05:00 and 08:00 remained shorter than 8 min. The current results are also consistent with studies demonstrating the effects of modafinil, the racemate of armodafinil, on improving psychomotor performance and verbal flexibility and originality during simulated night shifts27 and improved alertness in patients with SWD.49,50
An important and unique aspect of this study is in the requirement that the subjects must meet diagnostic criteria for SWD and yet there was no exclusion based on a minimal level of objective sleepiness to qualify for participation. Previous studies evaluating wake-enhancing drugs/stimulants required individuals with SWD to demonstrate a clinically significant level of sleepiness as determined by either the MSLT or the maintenance of wakefulness test.38,51 Clearly this strategy has a rationale in that a minimum level of alertness maximizes the ability to test the alerting effects of these drugs. However, this approach limits the generalizability to the larger SWD population, and therefore the clinical utility of subsequent results. Because objective demonstration of pathological sleepiness was not a requirement of the current study, this sample may be more representative of the broader SWD population. This difference may account for the larger effects of armodafinil on MSLT seen in the current study and in a previous study using an unselected sample of patients with SWD.52
Other studies have used normal volunteers exposed to models of shift work, including acute sleep deprivation and shifting sleep-wake schedule, to measure the effects of armodafinil on performance.27 Although these approaches provide important information as to the drug's pharmacodynamic activity, they are not generalizable to the clinical setting. This is evidenced by the fact that, at a regulatory level, there are no indications for the use of wake-promoting agents/stimulants for any acute cause of sleepiness. At a therapeutic level, there are changes in sleepiness and sleep efficiency as a function of the duration for which the patient has had SWD or been exposed to the night shift schedule. For example, among shift workers, the level of incident risk can increase across multiple night shifts (+36% for fourth consecutive night shift) as partial sleep loss accumulates and adds to the sleepiness produced by working in the trough of the circadian rhythm.23 Using light or other circadian interventions, some shift workers may adjust their circadian phase to the shift work schedule53 and may be less impaired with increasingly chronic shift work.54–57 For this reason, the current study not only evaluated actual shift workers, but required them to meet diagnostic criteria for SWD and to currently be working night shifts.
Although previous studies have shown that wake-promoting agents improve simulated driving performance following sleep deprivation33 or in patients with obstructive sleep apnea,37 one cannot rely on such data for generalizability to chronic shift workers. The current data clearly demonstrate that armodafinil 150 mg improves driving simulator performance in patients with SWD who usually work a night shift schedule. Importantly, because the commute home is the time of greatest risk of a car accident, the positive effect on driving performance persists through the 09:15 h of testing, as was the case for the MSLT results. This is evidenced by the absence of a drug × time interaction and, more importantly, by a significant difference in the primary driving measure (i.e. SDLP) at the 09:15 time point. In trying to dissect the mediators of improved driving performance, MSLT results were evaluated as a covariate. The results showed that although the level of alertness was a significant covariate in the driving performance results, the effect of armodafinil remained significant and robust even after correcting for improvement in alertness. Thus, variables other than sleepiness mediated armodafinil's effect on improved driving performance and need to be investigated. Finally, as was the case for sleepiness, the driving performance effects were paralleled by patient reports. Specifically, both their predictions of their ability to drive as well as their judgment as to how they performed were significantly elevated with treatment.
Finally, in the current study, armodafinil improved a measure of creativity. Although Walsh et al. have previously shown an improvement on creativity with modafinil, the study was done in normal volunteers under an acute sleep-work schedule reversal, thereby limiting generalizability.27 Identification of the potential mechanism(s) of armodafinil's effects on creativity are beyond the scope of the present paper and requires further study.
The current study has several limitations, including that only a single night of drug administration was evaluated. Because the subjects for this study had chronic SWD and the indications for these medications is chronic use, the chronic effects of these medications on driving performance need to be evaluated. It is important to point out that armodafinil and modafinil were previously shown to be effective with nightly use in SWD samples limited by restrictive MSLT entry criteria. In addition, only one dose of armodafinil was studied and it is possible that higher doses may have had more consistent effects on cognitive functioning. Future studies should examine the relative effects of different doses on driving following the night shift. In the current study, sleep was not measured during the daytime following the night shift. There is the possibility that sleep disruption may have been present following armodafinil administration, which could have the potential to negatively affect subsequent night shifts. However, in previous studies of armodafinil in SWD with similar dose and drug administration times, no differences in sleep were observed relative to placebo.48 Nonetheless, careful attention should be paid to the potential sleep disruptive effects of armodafinil, particularly in the clinical setting where time of administration is inherently more variable and where individual differences in metabolism may be more heterogeneous. Although standardized screening questionnaires and clinical evaluation were used to exclude participants with comorbid sleep disorders, PSG was not performed. Thus, it is possible that some patients with comorbid sleep disorders (e.g., sleep apnea) were included in the current study. However, the within-subjects design used is likely to have minimized the effect of comorbidity on the current findings. Although most did, not all subjects had vision corrected to 20/20. Finally, there was no objective screening for illicit drug use.
In summary, the results of this study demonstrate that armodafinil 150 mg significantly improves alertness, driving performance, and creativity in patients in whom SWD was diagnosed.
DISCLOSURE STATEMENT
This study is supported by an investigator initiated grant awarded by Teva Pharmaceutical Industries Ltd. Dr. Drake has received research support from Merck and Teva; participated in speaking engagements for Teva, Purdue, and Jazz; and consults for Teva. Dr. Roth has served as a consultant for Abbott, Accadia, AstraZeneca, Aventis, AVER, Bayer, BMS, Cypress, Ferrer, GlaxoSmithKline, Impax, Intec, Jazz, Johnson and Johnson, Merck, Neurocrine, Novartis, Proctor and Gamble, Pfizer, Purdue, Shire, Somaxon and Transcept. He has received research support from Cephalon, Merck, Transcept, Speakers Bureau and Purdue, and Proctor and Gamble. The other authors have indicated no financial conflicts of interest.
Footnotes
A commentary on this article appears in this issue on page 1887.
REFERENCES
- 1.McMenamin TM, Holden RJ, Bahls D, Real C. A time to work: recent trends in shift work and flexible schedules. Monthly Labor Review. 2007;130:3. [Google Scholar]
- 2.Kamdar BB, Tergas AI, Mateen FJ, Bhayani NH, Oh J. Night-shift work and risk of breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;138:291–301. doi: 10.1007/s10549-013-2433-1. [DOI] [PubMed] [Google Scholar]
- 3.Vyas MV, Garg AX, Iansavichus AV, et al. Shift work and vascular events: systematic review and meta-analysis. BMJ. 2012;345:e4800. doi: 10.1136/bmj.e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driscoll TR, Grunstein RR, Rogers NL. A systematic review of the neurobehavioural and physiological effects of shiftwork systems. Sleep Med Rev. 2007;11:179–94. doi: 10.1016/j.smrv.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Zucconi M. Article reviewed: Distribution of road accidents in policemen on shift-work on Italian highways: the contributing role of sleepiness. Sleep Med. 2001;2:561–3. doi: 10.1016/s1389-9457(01)00146-0. [DOI] [PubMed] [Google Scholar]
- 6.Barger LK, Lockley SW, Rajaratnam SM, Landrigan CP. Neurobehavioral, health, and safety consequences associated with shift work in safety-sensitive professions. Curr Neurol Neurosci Rep. 2009;9:155–64. doi: 10.1007/s11910-009-0024-7. [DOI] [PubMed] [Google Scholar]
- 7.Akerstedt T, Czeisler CA, Dinges DF, Horne JA. Accidents and sleepiness: a consensus statement from the International Conference on Work Hours, Sleepiness and Accidents, Stockholm, 8-10 September 1994. J Sleep Res. 1994;3:195. [Google Scholar]
- 8.Gumenyuk V, Roth T, Drake CL. Circadian phase, sleepiness, and light exposure assessment in night workers with and without shift work disorder. Chronobiol Int. 2012;29:928–36. doi: 10.3109/07420528.2012.699356. [DOI] [PubMed] [Google Scholar]
- 9.Rajaratnam SM, Barger LK, Lockley SW, et al. Sleep disorders, health, and safety in police officers. JAMA. 2011;306:2567–78. doi: 10.1001/jama.2011.1851. [DOI] [PubMed] [Google Scholar]
- 10.Swanson LM, Arnedt JT, Rosekind MR, Belenky G, Balkin TJ, Drake C. Sleep disorders and work performance: findings from the 2008 National Sleep Foundation Sleep in America poll. J Sleep Res. 2011;20:487–94. doi: 10.1111/j.1365-2869.2010.00890.x. [DOI] [PubMed] [Google Scholar]
- 11.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–62. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 12.Gold DR, Rogacz S, Bock N, et al. Rotating shift work, sleep, and accidents related to sleepiness in hospital nurses. Am J Public Health. 1992;82:1011–4. doi: 10.2105/ajph.82.7.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akerstedt T, Peters B, Anund A, Kecklund G. Impaired alertness and performance driving home from the night shift: a driving simulator study. J Sleep Res. 2005;14:17–20. doi: 10.1111/j.1365-2869.2004.00437.x. [DOI] [PubMed] [Google Scholar]
- 14.Arnedt JT, Owens J, Crouch M, Stahl J, Carskadon MA. Neurobehavioral performance of residents after heavy night call vs after alcohol ingestion. JAMA. 2005;294:1025–33. doi: 10.1001/jama.294.9.1025. [DOI] [PubMed] [Google Scholar]
- 15.Waggoner LB, Grant DA, Van Dongen HP, Belenky G, Vila B. A combined field and laboratory design for assessing the impact of night shift work on police officer operational performance. Sleep. 2012;35:1575–7. doi: 10.5665/sleep.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ware JC, Risser MR, Manser T, Karlson KH., Jr Medical resident driving simulator performance following a night on call. Behav Sleep Med. 2006;4:1–12. doi: 10.1207/s15402010bsm0401_1. [DOI] [PubMed] [Google Scholar]
- 17.Ftouni S, Sletten TL, Howard M, et al. Objective and subjective measures of sleepiness, and their associations with on-road driving events in shift workers. J Sleep Res. 2013;22:58–69. doi: 10.1111/j.1365-2869.2012.01038.x. [DOI] [PubMed] [Google Scholar]
- 18.Sandberg D, Anund A, Fors C, et al. The characteristics of sleepiness during real driving at night--a study of driving performance, physiology and subjective experience. Sleep. 2011;34:1317–25. doi: 10.5665/SLEEP.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews RW, Ferguson SA, Zhou X, Kosmadopoulos A, Kennaway DJ, Roach GD. Simulated driving under the influence of extended wake, time of day and sleep restriction. Accid Anal Prev. 2012;45:55–61. doi: 10.1016/j.aap.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 20.Marcus CL, Loughlin GM. Effect of sleep deprivation on driving safety in housestaff. Sleep. 1996;19:763–6. doi: 10.1093/sleep/19.10.763. [DOI] [PubMed] [Google Scholar]
- 21.Barger LK, Cade BE, Ayas NT, et al. Extended work shifts and the risk of motor vehicle crashes among interns. N Engl J Med. 2005;352:125–34. doi: 10.1056/NEJMoa041401. [DOI] [PubMed] [Google Scholar]
- 22.Smith L, Folkard S, Poole CJ. Increased injuries on night shift. Lancet. 1994;344:1137–9. doi: 10.1016/s0140-6736(94)90636-x. [DOI] [PubMed] [Google Scholar]
- 23.Folkard S, Tucker P. Shift work, safety and productivity. Occup Med (Lond) 2003;53:95–101. doi: 10.1093/occmed/kqg047. [DOI] [PubMed] [Google Scholar]
- 24.Wright KP, Jr, Hull JT, Hughes RJ, Ronda JM, Czeisler CA. Sleep and wakefulness out of phase with internal biological time impairs learning in humans. J Cogn Neurosci. 2006;18:508–21. doi: 10.1162/jocn.2006.18.4.508. [DOI] [PubMed] [Google Scholar]
- 25.Santhi N, Horowitz TS, Duffy JF, Czeisler CA. Acute sleep deprivation and circadian misalignment associated with transition onto the first night of work impairs visual selective attention. PLoS One. 2007;2(11):e1233. doi: 10.1371/journal.pone.0001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gumenyuk V, Roth T, Korzyukov O, et al. Shift work sleep disorder is associated with an attenuated brain response of sensory memory and an increased brain response to novelty: an ERP study. Sleep. 2010;33:703–13. doi: 10.1093/sleep/33.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh JK, Randazzo AC, Stone KL, Schweitzer PK. Modafinil improves alertness, vigilance, and executive function during simulated night shifts. Sleep. 2004;27:434–9. doi: 10.1093/sleep/27.3.434. [DOI] [PubMed] [Google Scholar]
- 28.Wright KP, Jr., Bogan RK, Wyatt JK. Shift work and the assessment and management of shift work disorder (SWD) Sleep Med Rev. 2013;17:41–54. doi: 10.1016/j.smrv.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Smith MR, Eastman CI. Shift work: health, performance and safety problems, traditional countermeasures, and innovative management strategies to reduce circadian misalignment. Nat Sci Sleep. 2012;4:111–32. doi: 10.2147/NSS.S10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mets M, Baas D, van Boven I, Olivier B, Verster J. Effects of coffee on driving performance during prolonged simulated highway driving. Psychopharmacology (Berl) 2012;222:337–42. doi: 10.1007/s00213-012-2647-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reyner LA, Horne JA. Early morning driver sleepiness: effectiveness of 200 mg caffeine. Psychophysiology. 2000;37:251–6. [PubMed] [Google Scholar]
- 32.Horne JA, Reyner LA. Counteracting driver sleepiness: effects of napping, caffeine, and placebo. Psychophysiology. 1996;33:306–9. doi: 10.1111/j.1469-8986.1996.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 33.Gurtman CG, Broadbear JH, Redman JR. Effects of modafinil on simulator driving and self-assessment of driving following sleep deprivation. Hum Psychopharmacol. 2008;23:681–92. doi: 10.1002/hup.983. [DOI] [PubMed] [Google Scholar]
- 34.Verster JC, Bekker EM, de Roos M, et al. Methylphenidate significantly improves driving performance of adults with attention-deficit hyperactivity disorder: a randomized crossover trial. J Psychopharmacol. 2008;22:230–7. doi: 10.1177/0269881107082946. [DOI] [PubMed] [Google Scholar]
- 35.Williams SC, Marshall NS, Kennerson M, Rogers NL, Liu PY, Grunstein RR. Modafinil effects during acute continuous positive airway pressure withdrawal: a randomized crossover double-blind placebo-controlled trial. Am J Respir Crit Care Med. 2010;181:825–31. doi: 10.1164/rccm.200908-1307OC. [DOI] [PubMed] [Google Scholar]
- 36.Williams SC, Rogers NL, Marshall NS, Leung S, Starmer GA, Grunstein RR. The effect of modafinil following acute CPAP withdrawal: a preliminary study. Sleep Breath. 2008;12:359–64. doi: 10.1007/s11325-008-0175-9. [DOI] [PubMed] [Google Scholar]
- 37.Kay G, Feldman N. Effects of armodafinil on simulated driving and self-report measures in obstructive sleep apnea patients prior to treatment with continuous positive airway pressure. J Clin Sleep Med. 2012;9:445–54. doi: 10.5664/jcsm.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Czeisler CA. Medical and genetic differences in the adverse impact of sleep loss on performance: ethical considerations for the medical profession. Trans Am Clin Climatological Assoc. 2009;120:249–85. [PMC free article] [PubMed] [Google Scholar]
- 39.Dinges DF, Arora S, Darwish M, Niebler GE. Pharmacodynamic effects on alertness of single doses of armodafinil in healthy subjects during a nocturnal period of acute sleep loss. Current Med Res Opinion. 2006;22:159–67. doi: 10.1185/030079906X80378. [DOI] [PubMed] [Google Scholar]
- 40.Swanson LM, Drake C, Arnedt JT. Employment and drowsy driving: a survey of American workers. Behav Sleep Med. 2012;10:250–7. doi: 10.1080/15402002.2011.624231. [DOI] [PubMed] [Google Scholar]
- 41.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812–21. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 42.Verster JC, Roth T. Thirty years of Dutch drugs and driving research: strengths and limitations of the on-the-road highway driving test and future challenges. Curr Psychopharmacology. 2012;1:97–102. [Google Scholar]
- 43.Gupta N, Jang Y, Mednick SC, Huber DE. The road not taken: creative solutions require avoidance of high-frequency responses. Psychological Sci. 2012;23:288–94. doi: 10.1177/0956797611429710. [DOI] [PubMed] [Google Scholar]
- 44.Cai DJ, Mednick SA, Harrison EM, Kanady JC, Mednick SC. REM, not incubation, improves creativity by priming associative networks. Proc Natl Acad Sci U S A. 2009;106:10130–4. doi: 10.1073/pnas.0900271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mednick MT. Research creativity in psychology graduate students. J Consult Psychol. 1963;27:265. doi: 10.1037/h0042429. [DOI] [PubMed] [Google Scholar]
- 46.Erman MK, Seiden DJ, Yang R, Dammerman R. Efficacy and tolerability of armodafinil: effect on clinical condition late in the shift and overall functioning of patients with excessive sleepiness associated with shift work disorder. J Occup Environ Med. 2011;53:1460–5. doi: 10.1097/JOM.0b013e318237a17e. [DOI] [PubMed] [Google Scholar]
- 47.Erman MK, Yang R, Seiden DJ. The effect of armodafinil on patient-reported functioning and quality of life in patients with excessive sleepiness associated with shift work disorder: a randomized, double-blind, placebo-controlled trial. Prim Care Companion CNS Disord. 2012;14(4) doi: 10.4088/PCC.12m01345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Czeisler CA, Walsh JK, Wesnes KA, Arora S, Roth T. Armodafinil for treatment of excessive sleepiness associated with shift work disorder: a randomized controlled study. Mayo Clin Proc. 2009;84:958–72. doi: 10.1016/S0025-6196(11)60666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Czeisler CA, Walsh JK, Roth T, et al. Modafinil for excessive sleepiness associated with shift-work sleep disorder. N Engl J Med. 2005;353:476–86. doi: 10.1056/NEJMoa041292. [DOI] [PubMed] [Google Scholar]
- 50.Erman MK, Rosenberg R For The USMSWSDSG. Modafinil for excessive sleepiness associated with chronic shift work sleep disorder: effects on patient functioning and health-related quality of life. Prim Care Companion J Clin Psychiatry. 2007;9:188–94. doi: 10.4088/pcc.v09n0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Czeisler CA, Walsh JK, Roth T, et al. Modafinil for excessive sleepiness associated with shift-work sleep disorder. N Engl J Med. 2005;353:476–86. doi: 10.1056/NEJMoa041292. [DOI] [PubMed] [Google Scholar]
- 52.Howard R, Roth T, Drake CL. The effects of armodafinil on objective sleepiness and performance in a shift work disorder sample unselected for objective sleepiness. J Clin Psychopharmacol. 2014;34:369–73. doi: 10.1097/JCP.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 53.Smith MR, Fogg LF, Eastman CI. Practical interventions to promote circadian adaptation to permanent night shift work: study 4. J Biol Rhythms. 2009;24:161–72. doi: 10.1177/0748730409332068. [DOI] [PubMed] [Google Scholar]
- 54.Boivin DB, James FO. Light treatment and circadian adaptation to shift work. Ind Health. 2005;43:34–48. doi: 10.2486/indhealth.43.34. [DOI] [PubMed] [Google Scholar]
- 55.James FO, Walker CD, Boivin DB. Controlled exposure to light and darkness realigns the salivary cortisol rhythm in night shift workers. Chronobiol Int. 2004;21:961–72. doi: 10.1081/cbi-200035944. [DOI] [PubMed] [Google Scholar]
- 56.Boivin DB, James FO. Circadian adaptation to night-shift work by judicious light and darkness exposure. J Biol Rhythms. 2002;17:556–67. doi: 10.1177/0748730402238238. [DOI] [PubMed] [Google Scholar]
- 57.Folkard S. Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm. Chronobiol Int. 2008;25:215–24. doi: 10.1080/07420520802106835. [DOI] [PubMed] [Google Scholar]