Abstract
Background
Simultaneous kidney and pancreas (SPK) transplantation is an attractive option for end-stage renal disease patients with type 1 diabetes. Although SPK transplantation is superior to remaining on dialysis, the survival advantage for SPK recipients compared to kidney transplantation alone (KTA) is controversial.
Methods
Using data obtained from the Scientific Registry of Transplant Recipients, we compared patient and graft survivals for 7308 SPK and 4653 KTA adult patients with type I diabetes transplanted in 1998 to 2009. Because SPK and KTA recipients are differently selected, comparison groups were chosen to maximize overlap in the case mixes. Most previous studies contrasted (unadjusted) Kaplan-Meier survival curves or, if covariate-adjusted, reported hazard ratios (HRs). Using newer statistical methods, we avoid relying on hazard ratios (which are seldom of inherent interest) and directly compare covariate-adjusted survival curves. Specifically, we compare average covariate-adjusted SPK- and KTA-specific survival curves (and 10-year area under the curve; ie, restricted mean survival time) to emulate a randomized clinical trial.
Results
Mean restricted mean kidney graft survival time was significantly greater by 0.18 years (P = 0.045) for SPK compared to KTA. Similarly, patient survival was 0.17 years greater (P = 0.033) for SPK than KTA. Increased graft survival was primarily observed in younger SPK recipients. Supplementary analysis revealed that the SPK hazards were nonproportional, meaning that it would be difficult to quantify the cumulative effect of SPK through a standard Cox regression analysis.
Conclusions
Using this novel methodology, we demonstrate that SPK is associated with statistically but not clinically significant increases in graft and patient survival.
Using a novel statistical approach with covariate-adjusted survival curves, Sung and colleagues show a statistically but not clinically significant graft and patient survival advantage to SPK compared to PTA.
Simultaneous kidney and pancreas (SPK) transplantation is an attractive option for patients with type 1 diabetes and end-stage renal disease. The incremental benefit of the pancreas transplant includes: freedom from insulin, normal glycemic control, freedom from hypoglycemic events, improvement or delay in secondary complications of diabetes, and potentially longer kidney graft survival. Because the immunosuppressive burden is similar to recipients of kidney transplantation alone (KTA), the incremental risks are largely secondary to the perioperative risks of the pancreas transplant procedure itself, which can be substantial.
Advising kidney and pancreas transplant candidates as to the best transplant option has proven to be challenging. This is due to the variety of available choices and, in part, to the multitude of factors that may influence outcomes: availability of a living donor, waiting time for deceased donor kidneys and pancreas, local allocation algorithms and, most importantly, differences in recipients and donor organ quality between SPK versus KTA. Although all kidney or kidney-pancreas transplants provide a survival advantage compared with remaining on dialysis, analyses examining the incremental survival benefit of the pancreas have yielded mixed results. In these registry-based, observational studies, a number of methods are used to address differences between SPK and KTA recipients, which reflect more restrictive SPK selection criteria for both donors and recipients. Regression modeling, restrictions on donor and recipient inclusion criteria, matched kidney analyses, and comparative benefit models have been used but fail to completely eliminate this selection bias.
The majority of existing studies either compared Kaplan-Meier survival curves or estimated hazard ratios (HRs) based on Cox regression. Kaplan-Meier curves are not risk-adjusted and, hence, may yield biased results. Hazard ratios based on Cox regression are suboptimal for guiding clinical decision making when the baseline risks are low (because the HR will overstate the actual effect on absolute survival) or if the hazards are not proportional.
We restricted the KTA group based on covariate patterns present in SPK recipients. We adjust for treatment group-specific imbalances using Cox regression. Survival probabilities for SPK and KTA are then compared by taking the difference between the 2 treatment-specific average survival curves over a 0- to 10-year follow-up period. The resulting contrast has factored out differences between SPK and KTA case mixes because the SPK and KTA average survival curves are computed across the same set of subjects (the entire study population, regardless of the transplant actually received). This novel analytic construct then generate results that would be obtained through a randomized clinical trial, in the sense that survival curves are compared in a manner which factors out case mix differences.
PATIENTS AND METHODS
Study Population and (Restricted) Comparison Groups
Data were obtained from the Scientific Registry of Transplant Recipients. Information is submitted by the transplant centers to the Organ Procurement and Transplantation Network.
A schematic description of the study cohort is depicted in Figure 1. We identified 9065 SPK and 11,288 KTA transplants between January, 1, 1998, and December 31, 2009, to type I diabetic patients aged 18 years or older. Presence and type of diabetes were identified through diagnosis codes obtained from the Scientific Registry of Transplant Recipients standard analysis files. After examining various descriptive statistics, we eliminated subgroups that appeared to be absent from, or greatly under-represented, in the SPK transplants. Specifically, after restricting recipient body mass index (BMI) to 32 or less and age at transplant to 55 years or younger, and donor BMI to less than 31 and age to 47 years, there remained 7308 SPK and 4653 KTA transplants. Additionally, because center effects can play a substantial role in posttransplant outcomes, we eliminated patients at centers that only performed KTA transplants (151 of 222 centers remained). After applying the center criterion, the resulting study cohort consisted of 7255 SPK and 3998 KTA transplants.
FIGURE 1.
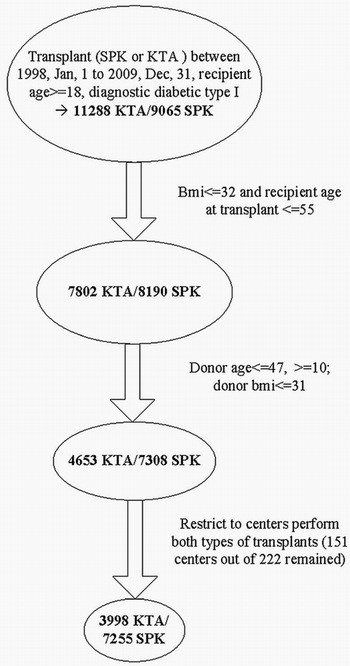
Description of study cohort.
Follow-Up Time and Events
The SPK and KTA recipients were compared with respect to (i) time until death and (ii) time until graft loss, with the latter defined as the earliest of death, reported date of graft loss, and repeat kidney transplantation. Graft loss is commonly defined this way in the existing literature. Under this definition, time until graft loss represents survival time with a functioning (primary) graft. The alternative, death-censored graft survival, is prone to dependent censoring and generally produces an uninterpretable survival curve due to competing risks issues.1 Follow-up began at the time of transplant and ended at the earliest of occurrence of the event of interest, loss to follow-up, or the end of the observation period December 31, 2009.
Covariate Adjustment
Because type of transplant (SPK or KTA) is not randomized, valid comparison of SPK and KTA transplants requires proper covariate adjustment. The cohort selection process described above only ensures overlap across the SPK- and KTA-risk subgroups, a necessary but not sufficient condition for an unbiased comparison between SPK and KTA. Covariate adjustment is still required and, as such, we adjusted for the following recipient factors: calendar year of transplant, sex, age at transplant, BMI, years at dialysis, angina, chronic obstructive pulmonary disease, cerebrovascular disease, peripheral vascular disease, race, ethnicity, blood type, panel-reactive antibodies and center; and donor factors: donor age and the log of the Kidney Donor Risk Index.2
SPK Versus KTA: Avoidance of the HR
Our interest is in comparing SPK and KTA survival curves (and area under the curves) for graft loss and patient survival because differences in survival probability are more clinically interpretable than ratios of hazard functions. Although covariate adjustment typically results in an analysis focusing on HRs, our primary analysis does not rely on the SPK/KTA HR. This is mainly for 3 reasons. First, the difference in survival is inherently of greater interest than the HR, as evidenced by the scarcity of published analyses where the authors report the HR in the absence of the need for covariate adjustment. Second, the HR does not provide information on the actual difference in survival; for example, it is possible to have HR of 2 and a difference in 5-year survival of 5%. In this example, the magnitude of the HR may serve to substantially overstate the difference in survival. Third, if the SPK and KTA hazards are not proportional and, even more so, if the SPK/KTA HR changes from greater than 1 to less than 1 over time (as we suspect), the cumulative effect of SPK is not discernable from the follow-up–specific HRs estimated through a time-dependent Cox model (the so-called Cox nonproportional hazards model).
Separate Cox Models by Transplant Type
We still use Cox regression, but not for the purposes of estimating the SPK versus KTA HR. Instead, we fit separate Cox models for SPK and KTA patients. From this exercise, no assumptions are made regarding the nature of the relationship between SPK and KTA outcomes; for example, no proportional hazards assumption, or anything analogous to it, is made. After fitting the SPK and KTA Cox models, we predict survival curves for each patient under each of 2 scenarios: (i) the patient receives an SPK transplant (ii) the patient receives KTA transplant. Survival functions are fitted under each of the 2 scenarios irrespective of which type of transplant the patient actually receives.
Comparison of Average SPK and KTA Survival Curves
The average SPK (or KTA) survival curve is then the average across all subjects when they receive SPK (or KTA). The area under the survival curve (out to 10 years after transplantation) is the mean number of years lived out of the first 10 years. As a subanalysis, we analyzed the 18 to 39 and 40 to 55 recipient age groups separately.
The afore-described method of contrasting survival curves was initially proposed by Chen and Tsaitis,3 with various extensions having since been developed.4-6 The method has been rigorously demonstrated to reproduce the Kaplan-Meier survival curves that would be obtained (e.g., in our case) if SPK/KTA transplants were randomized. Note that the average difference in SPK and KTA survival curves is covariate-adjusted because the same patients are inputted.
Time-Dependent Cox Model
For comparison purposes and to evaluate the degree to which the SPK/KTA HR changes over posttransplant follow-up time, we fitted a time-dependent Cox model. The HR for SPK/KTA can be interpreted as the death rate for an SPK patient, divided by the death rate for a KTA patient, covariate-adjusted. This model allows for time-varying HRs, such that the afore-described SPK/KTA contrast is allowed to differ by time since transplant: 0 to 3 months, 3 to 6 months, 6 months to 1 year, 1 to 2 years, 2 to 3.5 years, 3.5 to 5 years, and 5 to 10 years. The utility of such time-dependent HR models is restricted to description, as opposed to treatment decisions. For example, the 1- to 2-year HR has meaning only to a patient that has survived 1 year, and this HR “expires” at year 2 (at which time a different HR applies). Such issues have been previously described in detail.7
Sensitivity Analyses
The methods we use for contrasting SPK and KTA estimate what is known as the average causal effect (ACE). Because there are different ways to estimate the ACE, as a sensitivity analysis, we estimated the ACE using various well-established alternative methods: namely, propensity scoring,8 Inverse Probability of Treatment Weighting9 and the effect of treatment on the treated.10
All statistical analyses were carried out using SAS v9.3 (SAS Institute; Cary, NC).
RESULTS
Baseline recipient and donor characteristics for the SPK and KTA groups are described in Table 1. The discrepancy between SPK and KTA was significant for the following covariates: sex, angina, cerebrovascular disease, peripheral vascular disease, race, panel-reactive antibodies, Kidney Donor Risk Index, recipient age, donor age, calendar year of transplant, and years on dialysis before transplantation. Although some of the differences are slight, the number of differences (among factors known to be strong predictors of survival) implies that an unadjusted analysis would be confounded.
TABLE 1.
Baseline recipients and donor characteristics by SPK versus KTA groups
The time-varying HR for SPK relative to KTA is plotted in Figure 2 with respect to (a) graft loss and (b) death. Both plots follow a similar pattern. Early post-transplant, the death rate is considerably higher for SPK patients (HR > 1), but the HR then decreases rapidly. As an example of the limitations of this time-dependent HR analysis, it is difficult to ascertain from Figure 2B if 10-year survival probability is higher for SPK or KTA. The death hazard is lower for SPK for a longer time period, but the HR is much higher than 1 for the intervals where SPK has the higher death rate.
FIGURE 2.
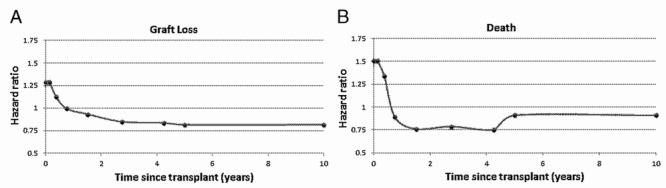
A, Relative risk (hazard ratio) of SPK kidney graft loss relative to KTA over time. B, Relative risk (hazard ratio) of SPK recipient death relative to KTA over time.
A patient attempting to use Figure 2B to assist in deciding between SPK and KTA should note, for example, that HR is equal to 0.89 applies only to a patient who survives the first 6 months after transplantation and, therefore, is not useful for a choice made at the time of transplantation. Similarly, an HR of 1.5 for months 0 to 3 is of limited value because this HR only applies during the 0- to 3-month posttransplant interval.
The estimated, covariate-adjusted survival curves for SPK and KTA are plotted in Figure 3 with respect to (a) graft loss and (b) death. For SPK, average 1-, 3-, 5- and 10-year graft survival probabilities are 93.1%, 86.6%, 80.1%, and 64.3%; with the corresponding graft survival for KTA being 93.9%, 86.6%, 78.5%, and 59.0%. Average 1-, 3-, 5-, and 10-year survival probabilities are estimated at 95.7%, 91.6%, 87.6%, and 73.7% for SPK; the corresponding probabilities being 96.5%, 91.3%, 85.7%, and 70.9% for KTA. Consider the solid line in Figure 3A pertaining to SPK graft survival. The HRs and SPK baseline hazard (estimated using Cox regression) were used to predict a graft survival curve for each patient in the study population. The solid line represents the average across these graft survival curves. If all patients in the study population received an SPK transplant, this line represents what would be the average graft survival.
FIGURE 3.
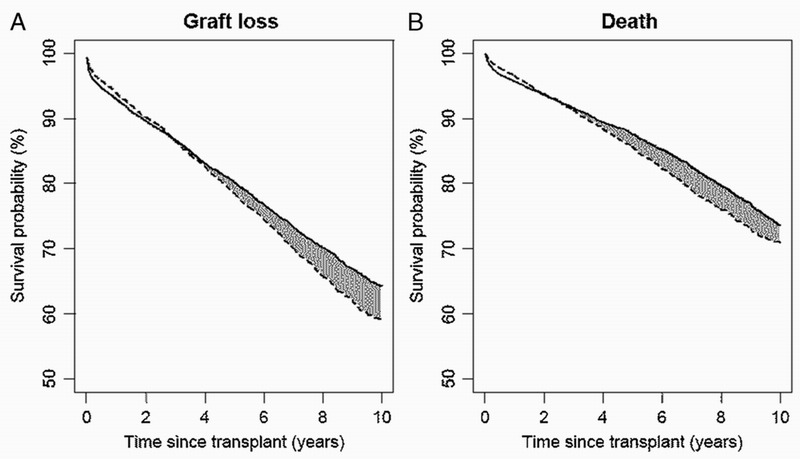
Estimated survival curves for (A) graft loss and (B) death adjusted for patients and donor characteristics using the method of Chen and Tsiatis.2 Solid line indicates SPK and dashed line indicates KTA.
From Figure 3A, kidney graft survival and patient survival are initially higher for KTA than SPK. Graft survival for SPK and KTA are equal at 2.9 years after transplantation, whereas patient survival is equal at 1.9 years. Thereafter, the comparison favors SPK.
Note that the crossing points are greater in Figure 3A and B than in Figure 2A and B, reflecting the cumulative nature of survival probability. Exploring this issue in greater detail, Figure 2 shows that the HR is initially increased for SPK versus KTA. However, the increased HR is short-lived, with the HR decreasing sharply and crossing 1, indicating equality of hazards (death rates), at about the 3-month mark. The survival function is by nature a cumulative measure, meaning that equality in the SPK and KTA survival functions will occur at a later time point than the time at which the HR drops below 1, as evidenced by Figure 3.
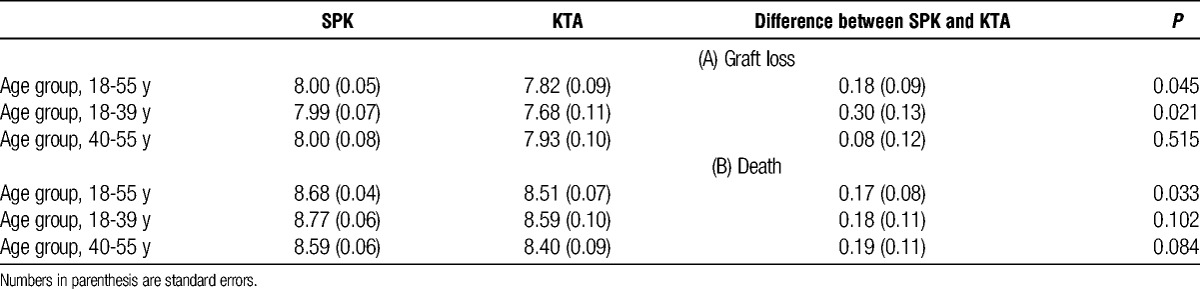
With respect to the time to kidney graft loss, the estimated mean lifetimes up to 10 years for SPK and KTA are 8.00 (with standard error (SE) of 0.05) years and 7.82 (SE = 0.09) years respectively. Compared with KTA, SPK has an added benefit of 0.18 (SE = 0.09) years of 10 years after the transplantation; the difference is statistically significant (P = 0.045). With respect to the time to death alone, the estimated mean lifetimes are 8.68 (SE = 0.04) and 8.51 (SE = 0.07) for SPK and KTA, respectively. Again, compared with KTA, SPK has the benefit of additional 0.17 years of 10 years (SE = 0.08) (P = 0.033).
It would be expected that both graft and patient survivals among SPK patients would be affected by pancreas graft loss. Among the SPK patients in our study, the 10-year cumulative incidence of pancreas graft loss was approximately 25%.
We further conducted analyses for subgroups of recipients with age at transplantation from 18 to 39 years (n = 5434) and from 40 to 55 years (n = 5824), respectively. The estimated, covariate-adjusted survival curves for SPK and KTA are plotted in Figure 4 and Figure 5, respectively. In addition, the estimated mean lifetimes up to 10 years and the difference between SPK and KTA for these subgroups as well as the whole study population are shown in Table 2. Younger SPK recipients had significantly higher difference in estimated mean graft lifetimes compared with KTA recipients (0.30 years, P = 0.021).
FIGURE 4.
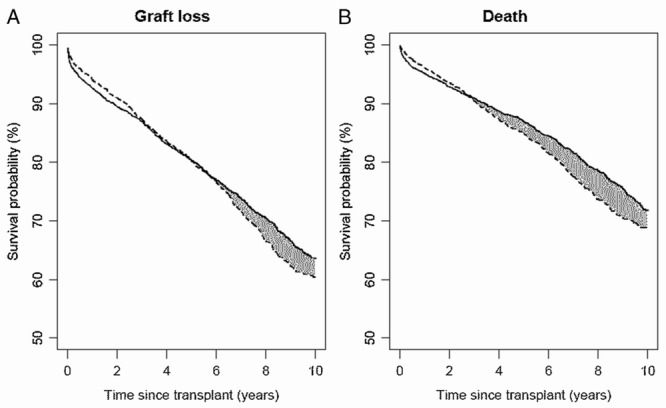
For recipients with age at transplant from 18 to 39 years: estimated survival curves for (A) graft loss and (B) death adjusted for patients and donor characteristics using the method of Chen and Tsiatis.2 Solid line indicates SPK and dashed line indicates KTA.
FIGURE 5.
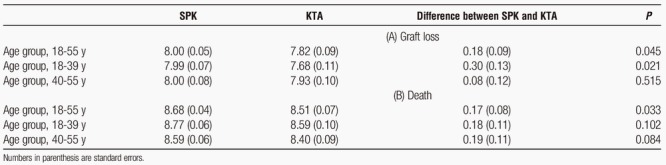
For recipients with age at transplant from 40–55: estimated survival curves for (A) graft loss and (B) death adjusted for patients and donor characteristics using the method of Chen and Tsiatis.2 Solid line indicates SPK and dashed line indicates KTA.
TABLE 2.
Mean lifetimes up to 10 years for SPK and KTA and their differences based on (a) graft loss and (b) death for recipients with age-at-transplants from 18 to 55, from 18 to 39, and from 40 to 55
Sensitivity Analysis
Propensity Scoring
The first alternative method we examined featured the propensity score (i.e., the probability of receiving SPK) based on a logistic regression model fitted to the SPK and KTA patients and using the previously listed set of covariates. The idea is that SPK receipt is approximately randomized among patients with the same propensity score. Each of the SPK and KTA cohorts are broken up into 10 groups, with cut point corresponding to propensity score deciles. Nonparametric survival curves are computed within decile-specific subgroup, and then averaged. The resulting patient and graft survival curves were very close to those in Figure 3A and B.
Inverse Weighting
The second alternative method we examined was Inverse Probability of Treatment Weighting. This is performed by first defining patient-specific weights. The weight for a SPK patient is 1 over the afore-described propensity score; the weight for a KTA patient is 1 over the probability of receiving KTA. Confounding is avoided in the sense that the weighted versions of the SPK and KTA cohorts have the same adjustment covariate distribution. Weighted nonparametric survival curves for SPK an KTA were estimated and, again, found to be very similar to those in Figure 3A and B.
Effect of Treatment on the Treated
We also estimated the effect of treatment on the treated, by essentially comparing SPK versus KTA using methods from our main line analysis, but this time (i) averaging over the SPK patients only and (ii) averaging over the KTA patients only. Meaningful discrepancies between either (i) or (ii) and those we report earlier would imply that there are regions of the covariate space that are markedly under-represented (or even absent) for 1 of the 2 transplant types. However, results were in fact very similar in the ACE and both (i) and (ii).
Based on these analyses, our results do not appear to be sensitive to the particular methods we chose to contrast survival curves.
DISCUSSION
Although it has been uniformly demonstrated that SPK transplantation improves survival compared with remaining on dialysis, the specific contribution of the pancreas transplant to this survival advantage is less certain and, similar to analyses of solitary pancreas transplant outcome,11 most analyses that compare SPK to KTA recipients have suggested that at most the incremental benefit of the pancreas transplant is modest.11-17 Overall survival outcomes are superior for SPK recipients compared with deceased donor but not living donor KTA recipients.
Comparisons between SPK and KTA transplants are complicated by differences in SPK and KTA recipients. The SPK recipients are younger, more robust, have fewer comorbidities and almost exclusively have type 1 diabetes. They are considered more likely to survive long term than the typical diabetic KTA recipient regardless of the transplant type. In addition, many ways in which they differ, such as cardiovascular disease, functional status, and diabetes type, are not well characterized in the Organ Procurement and Transplantation Network database and, thus, are not accurate adjustments in registry analyses. Therefore, it is difficult to conclude that superior outcomes for SPK recipients are due to the pancreas transplant or the type of patient that receives SPK.
Deceased donor kidney quality is also different for SPK and KTA transplants. Although it is rare for an SPK transplant to come from a donor older than 50 years, these kidneys are routinely used for KTA. Even for donors younger than 50 years, donor selection is much more rigorous for SPK than for KTA because selection criteria for pancreas donors are more rigorous. Although some criteria are well characterized, like donor hypertension and diabetes, others, such as vascular disease or hemodynamic criteria, are not. Thus, differences in donor selection may also contribute to differences in SPK versus KTA outcome in ways not amenable to covariate adjustment.
Most analyses in the literature have used Cox models to adjust for differences in donor and recipient characteristics, with the effect of SPK reported in terms of HRs.11-13,16,17 In some cases, survival probability for SPK and KTA has also been compared using Kaplan-Meier methods, such that adjustments were not made for confounding.11,12,16,17 Alternatively, Ojo used a time-dependent, Cox nonproportional hazards model to study the effect of SPK in terms of adjusted 10-year survival rates and expected remaining lifetimes.15 Marroquin compared SPK versus KTA using half-life times, where the estimated half-life times were stratified by diagnosis, adult versus pediatric transplant and transplant number but did not fully adjust for other possible confounding factors.14
Efforts have also made to control for confounding by restricting comparison to a much smaller subset of patients. For example, Israni et al13 restricted his study cohort to SPK waitlisted patients to control for recipient status. Bunnapradist et al12 when restricting donor age to younger than 35 years and recipient age to younger than 40 years, was unable to replicate the survival advantage for SPK recipients observed in the larger, more inclusive cohort. Weiss et al17 also controlled for donor factors by comparing SPK with KTA who received a kidney from a pancreas donor. This stringent selection of the comparison group resulted in a dramatically reduced sample size, with only 101 subjects in the KTA group. Moreover, differences in crude survival probabilities may not be a good basis for fair comparison because the comparison groups may still differ with respect to other factors.
We used a statistical method that is formally and rigorously developed for estimating average treatment-specific survival curves, adjusting for differences in case mix.2 This method allows us to estimate the treatment-specific survival curves and restricted mean lifetimes that would be estimated from a randomized clinical trial where suitable patients were randomly assigned to receive either SPK or KTA. In terms of modeling, our method does not use an HR to contrast SPK and KTA because the reporting of a single HR assumes that SPK and KTA hazards are proportional, an assumption which has been shown to be false in previous studies. One could expand the model to yield a time-dependent HR, but this produces a set of HRs which are only interpretable conditionally and instantaneously. In the presence of time-dependent differences, the cumulative effect is much more useful to patients and providers. We estimate differences between SPK and KTA survival, and area under the survival curve; both of which are inherently cumulative measures.
Using this method, we demonstrated a modest survival benefit for SPK recipients. The initial patient and kidney graft survival advantage for KTA (perhaps secondary to increased surgical morbidity and mortality for SPK recipients) is countered by a flatter survival curve thereafter, with overall survival equivalent at about 2 years. That the graft survival HR continues to fall for 4 years after transplant suggests that the initial relative risks of SPK may extend beyond the added surgical risks of the procedure and may perhaps be related to differences in immunosuppression. However, average 10-year mean graft and patient survival time were increased by only 0.18 and 0.17 years, respectively. Although statistically significant, such differences are much less than those suggested by the HRs in a Cox model. The specific increase in restricted mean patient survival observed in older recipients suggests that these recipients are not only acceptable SPK candidates, but maybe more like to experience a small survival benefit attributable to the pancreas.
Although time-dependent risks beyond 10 years may differ for SPK and KTA recipients, these would not be likely to diverge significantly given the relatively limited pancreas graft survival beyond 10 years in SPK recipients. However, the divergence of the survival curves (both kidney graft and patient survival) suggests that a more clinically meaningful survival benefit might be present if pancreas transplant survival can be improved. This concept is supported by observations that pancreas transplant recipients with a failed transplant have far inferior outcomes compared to those with long-term survival.18
This analysis does not and cannot address the well-documented beneficial impact on quality of life and secondary complications of diabetes that have been demonstrated in pancreas transplant recipients. These benefits are important to consider for provider and patients in clinical decision-making. Indeed, in light of the very modest impact of the pancreas transplant on graft and patient survivals, these may be considered to be the primary rationale for considering SPK in this population.
Restriction of the cohort to recipients and donors likely to be candidates for SPK had the effect of reducing differences between SPK and KTA survival. Nevertheless, important differences in both donor and recipient characteristics between the 2 groups persisted such that covariate adjustment remained an important characteristic of the methodology. Using novel methodology, this study adds further support to the literature suggesting that, for SPK, any survival advantage of the pancreas transplant itself is modest compared to the benefit of being a healthier recipient, receiving a kidney from a healthier donor, or being transplanted earlier.
Footnotes
The authors declare no funding or conflicts of interest.
R.S.S. participated in research design, writing of the paper, and data analysis. M.Z. participated in research design, writing of the paper, performance of the research, contributed new reagents or analytic tools, participated in data analysis. D.E.S. participated in research design, writing of the paper, performance of the research, contributed new reagents or analytic tools, participated in data analysis. X.S. participated in research design, writing of the paper, performance of the research, contributed new reagents or analytic tools, participated in data analysis. J.C.M. participated in research design, writing of the paper, and data analysis.
REFERENCES
- 1. Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: Wiley; 2002. [Google Scholar]
- 2. Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the Kidney Donor Risk Index. Transplantation. 2009; 88: 231– 236. [DOI] [PubMed] [Google Scholar]
- 3. Chen P, Tsiatis AA. Causal inference on the difference of the restricted mean life between two groups. Biometrics. 2001; 57: 1030– 1038. [DOI] [PubMed] [Google Scholar]
- 4. Zhang M, Schaubel DE. Estimating differences in restricted mean lifetime using observational data subject to dependent censoring. Biometrics. 2011; 67: 740– 749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang M, Schaubel DE. Contrasting treatment-specific survival using double-robust estimators. Stat Med. 2012; 31: 4255– 4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang M, Schaubel DE. Double-robust semiparametric estimator for differences in restricted mean lifetimes in observational studies. Biometrics. 2012; 6: 999– 1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wei G, Schaubel DE. Estimating cumulative treatment effects in the presence of nonproportional hazards. Biometrics. 2008; 64: 724– 732. [DOI] [PubMed] [Google Scholar]
- 8. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983; 70: 41– 55. [Google Scholar]
- 9. Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect on the survival of HIV-positive men. Epidemiology. 2000; 11: 561– 570. [DOI] [PubMed] [Google Scholar]
- 10. Pearl J. Causality: Models, Reasoning, and Inference. New York: Cambridge; 2009. [Google Scholar]
- 11. Gruessner RW, Sutherland DE, Gruessner AC. Mortality assessment for pancreas transplants. Am J Transplant. 2004; 4: 2018– 2016. [DOI] [PubMed] [Google Scholar]
- 12. Bunnapradist S, Cho YW, Cecka JM, Wilkinson A, Danovitch GM. Kidney allograft and patient survival in type I diabetic recipients of cadaveric kidney alone versus simultaneous pancreas/kidney transplants: a multivariate analysis of the UNOS database. J Am Soc Nephrol. 2003; 14: 208– 213. [DOI] [PubMed] [Google Scholar]
- 13. Israni AK, Feldman HI, Propert KJ, Leonard M, Mange KC. Impact of simultaneous kidney-pancreas transplant and timing of transplant on kidney allograft survival. Am J Transplant. 2005; 5: 374– 382. [DOI] [PubMed] [Google Scholar]
- 14. Marroquin CE, Edwards EB, Collins BH, Desai DM, Tuttle-Newhall JE, Kuo PC. Half-life analysis of pancreas and kidney transplants. Transplantation. 2005; 80: 272– 275. [DOI] [PubMed] [Google Scholar]
- 15. Ojo AO, Meier-Kriesche HU, Hanson JA, et al. The impact of simultaneous pancreas-kidney transplantation on long-term patient survival. Transplantation. 2001; 71: 82– 90. [DOI] [PubMed] [Google Scholar]
- 16. Reddy KS, Davies D, Ormond D, et al. Impact of acute rejection episodes on long term graft survival following simultaneous kidney pancreas transplantation. Am J Transplant. 2003; 3: 439– 444. [DOI] [PubMed] [Google Scholar]
- 17. Weiss AS, Smits G, Wiseman AC. Twelve-month pancreas graft function significantly influences survival following simultaneous pancreas-kidney transplantation. Clin J Am Soc Nephrol. 2009; 4: 988– 995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Norman SP, Kommareddi M, Ojo AO, Luan FL. Early pancreas graft failure is associated with inferior late clinical outcomes after simultaneous kidney-pancreas transplantation. Transplantation. 2011; 92: 796– 801. [DOI] [PMC free article] [PubMed] [Google Scholar]


