Abstract
Objective:
To compare the efficacy and side effects of a cyclosporine microemulsion and tacrolimus in immunosuppressive therapy of renal transplantation.
Material and methods:
Between March 2003 and June 2005, the patients who had undergone kidney transplantation surgery and who were administered either basiliximab, a cyclosporine microemulsion, mycophenolate mofetil and prednisolone or basiliximab, tacrolimus, mycophenolate mofetil and prednisolone for baseline immunosuppressive therapy were recruited to our study. We evaluated the results of an 18-month follow-up period. The donors were called back weekly for a follow-up in the first month, fortnightly in the second month and then monthly for 18 months after discharge. A total of 41 patients were included in the study. The patients were evaluated as for demographic characteristics, acute rejection, cardiovascular and metabolic side effects, graft function, infections, hirsutism, gingival hyperplasia, cosmetic side effects, nephrotoxicity, drug changes and the survival rates.
Results:
There were no significant differences among the patients with regard to age, sex, donor type, dialysis periods, preoperative and postoperative systolic blood pressures, creatinine levels, hepatotoxicity, nephrotoxicity, occurrence of diabetes mellitus and the incidence of infection. The duration of hospitalization was prolonged in the cyclosporine A group. Acute rejection emerged in 5 patients (23.8%) in the tacrolimus group and in 4 patients (20%) in the cyclosporine A group. In the cyclosporin A group, the cholesterol and triglyceride levels were significantly higher than the tacrolimus group. The cosmetic side effects (gingival hyperplasia and hirsutism) as a reason for a change in medication were only observed in the cyclosporin A group, not in the tacrolimus group. A medication change was made in 8 patients in the cyclosporine A group and in 1 patient in the tacrolimus group. No death was observed in either group. Graft loss was observed in only 1 patient in the cyclosporine A group.
Conclusion:
Regarding the cosmetic side effects and hyperlipidemia, tacrolimus was found to be superior to cyclosporine A. Where hyperlipidemia is considered to be a risk factor for cardiovascular disease, tacrolimus use should be considered as a more acceptable treatment modality. However, the immunosuppressive regimen should be evaluated individually.
Keywords: Cyclosporine A, tacrolimus, transplantation
Introduction
Among factors determining the success rates in renal transplantation ABO blood group compatibility, HLA histocompatibility, pre-transplant blood transfusions, panel reactive antibody titer, live or cadaver donor, underlying primary disease, surgical technique, and immunosuppressive drug choice can be enumerated.[1]
Even though immunosuppressive treatments are successful in decreasing side effects developing in the long-term, they jeopardize patient, and graft survival. Even though various drugs are used, in standard schemas one of the two similar calcineurin inhibitors as cyclosporine A (CsA) or tacrolimus (Tc) is used as the basic immunosuppressive agent.
In our study, the efficacy, safety, and reliability of fundamental drugs, CsA, and Tc were analyzed in two separate treatment protocols used in renal transplantations realized in transplantation units.
Material and methods
This study included patients who had undergone renal transplantations in transplantation unit in Urology Clinics of Turkey High Specialization Training and Research Hospital Hospital and received baciliximab, CsA (microemulsion form), MMF (mycophenolate mofetil) and prednisolone or basiliximab, Tc, MMF, and prednisolone as initial immunosuppressive treatment. Outcomes of 18 months of follow-up of all patients were evaluated. Transplant recipients were controlled in the outpatient clinic weekly for the first, then every 15 days for the second month, and monthly thereafter. During the same period, transplant patients who didn’t want to comply with randomization protocol, those who lost to follow up after the first month or previously experienced toxic reactions while using CsA or Tc, and patients with graft failure for any reason were excluded from the study. A total of 46 patients had undergone renal transplantation, and 41 of them were included in the study.
Immunosuppressive protocols of the recipients were planned as follows:
CsA protocol: Basiliximab (on the day of the operation, and on the postoperative 4. days 20 mg IV infusion), MMF (2000 mg/d bid oral), CsA (4–6 mg/kg/d 2 bid oral). Prednol (500 mg on the day of the operation, and on the postoperative 1., and 2. days 250 mg, and 100 mg, respectively. Then the dose was tapered, and reduced to 10 mg from 12. days on.
Tc protocol: Basiliximab (on the day of the operation, and on postoperative 4. days 20 mg İV infusion), MMF (2000 mg/d bid oral), Tc (0.06–0.08 mg/kg/d bid oral). Prednisolone (500 mg on the day of the operation, and on the postoperative 1., and 2. days 250 mg, and 100 mg, respectively. Then the dose was tapered, and reduced to 10 mg from 12. days on.
For the measurement of CsA levels, blood samples were obtained 2 hours after the morning dose, and Tc levels were measured before the morning dose. Blood drug levels were measured in the immunology laboratories using FPIA (Axsym-Abbott)method for CsA, and MPEI (IMX-Abbott) for Tc measurements. Chart demonstrating whole blood levels of drugs is seen iN Table 1.
Table 1.
Chart demonstrating whole blood levels of the drugs used
| Cyclosporine A (µg/mL) | 1. month | 1.7 |
| 2. months | 1.5 | |
| 3. months | 1.3 | |
| 4–6. months | 1.1 | |
| 7–12. months | 0.9 | |
| >12 months | 0.8 | |
| Tacrolimus (ng/mL) | 2 weeks | 15–20 |
| 3–4 weeks | 10–15 | |
| 1–12 months | 5–10 | |
| >12 months | 5–7 |
Our protocol for prophylaxis of infection used for all recipients was as follows: Patients with PPD (+) received daily doses of 300 mg INH for 3 months. Cephazoline was used at a dose of 1 g bid IV for the first 3 days for three months. Trimethoprim + sulfa-methoxazole was used at daily oral doses of 160 mg for the first 6 months, and acyclovir 3200 mg/d oral for the first 4 months.
The transplant recipients were followed up for 18 months, and used two different calcineurin inhibitors.
Demographic characteristics (age, and gender of the recipient, type of donor, dialysis therapy, and its duration, hospital stay), immunological features (ABO groups of donors, and recipients, HLA types, crossmatches, and PSA titers), acute rejection, cardiovascular, and metabolic side effects (hypertension, hyperlipidemia, diabetes mellitus), graft functions (monitored by serum creatinine values), infection, hirsutismus, and gingival hyperplasia, cosmetic adverse effects, nephrotoxicity, hepatotoxicicty, drug change, and survival rates were evaluated.
Statistical analysis
For statistical evaluation, Statistical Package for the Social Sciences (SPSS Inc, Chicago, USA) 11.0.0 program was used. Continuous variables were calculated using Mann-Whitney Rank Sum or Student’s t test, categorical variables with chi-square, and ANOVA tests. For correlation analysis Pearson’s correlation test, and for the estimation of survival rates Kaplan-Meier survey analysis were used.
Results
Demographic data of the cases are shown in Table 2.
Table 2.
Demographic characteristics of the patients
| Group Tc (n:21) | Group CsA (n:20) | P value | ||
|---|---|---|---|---|
| Mean age (years) | 28.381±9.859 | 30.350±11.032 | 0.545 | |
| Male/Female | 19/2 | 14/6 | 0.130 | |
| Donor | Consanguineous Live | 17 | 14 | |
| Cadaver | 2 | 2 | 0.627 | |
| Non-consanguineous Live | 2 | 4 | ||
| Mean duration of dialysis therapy (month) | 22.714±23.277 | 23.300±11.522 | 0.920 | |
| Peritoneal dialysis/hemodialysis | 4/17 | 0/20 | 0.017 | |
| Duration of hospital stay (days) | 9.810±3.530 | 15.650±8.015 | 0.004 | |
Hospital stays were detected to be statistically significantly longer in the CsA group when compared with the Tc group.
Immunological features:
Renal transplantations were most frequently performed with 3 HLA mismatches in the Tc, and 2 HLA mismatches in the CsA groups. HLA incompatible grafts were also transplanted to the patients from their spouses in the CsA (n=1), and Tc (n=2) Tc groups. Only one patient in the Tc group developed acute rejection during the early postoperative period. Following the first episode, the patient is still being monitored with normal graft functions.
Acute rejection:
Acute rejection was also seen in the Tc (n=5; 23.8%), and CsA (n=4; 20.0%) (p=1.000) groups. In the Tc, and CsA groups biopsy-proven acute rejection episodes were resistant to prednisolone in 40, and 75% of the cases, respectively (p=0.764). Median time intervals from the time of transplantation to the onset of the first acute rejection episode were 51, and 87 days for the Tc, and CsA groups respectively (p=0.730).
Cardiovascular, and metabolic side effects:
Comparisons of pre-, and postoperative systolic blood pressures, and change in lipid profiles in both groups are seen in Table 3, and 4, respectively.
Table 3.
Comparison of systolic blood pressures (SBPs)
| Preoperative SBP (mmHg) | Postoperative SBP (mmHg) | P value | Frequency of hypertension | P value | |
|---|---|---|---|---|---|
| Tc | 119.286±22.0.80 | 126.905±25.173 | 0.108 | 6/21 | 0.433 |
| CsA | 110.300±18.114 | 119.000±21.250 | 0.139 | 4/20 |
Table 4.
Lipid profiles of CsA, and Tc groups, and their antihyperlipidemic treatment rates
| Tc (median) | P value | CsA (median) | P value | P value | ||
|---|---|---|---|---|---|---|
| Cholesterol | Preoperative | 187 mg/dL | 0.139 | 145 mg/dL | <0.001 | - |
| Postoperative | 190 mg/dL | 230 mg/dL | - | |||
| Triglyceride | Preoperative | 156 mg/dL | 0.546 | 109 mg/dL | <0.001 | - |
| Postoperative | 190 mg/dL | 208 mg/dL | - | |||
| Duration of initial treatment for hyperlipidemia | 60 days | - | 30 days | - | 0.432 | |
| Number of patients receiving treatment for hyperlipidemia | 5/21 | - | 13/20 | - | 0.012 |
In both groups, in only one case diabetes mellitus developed which required insulin treatment (p=1).
Graft function:
Median values of serum creatinine detected at, and following discharge from the hospital which we used for monitoring graft function are seen in Figure 1 (p>0.05).
Figure 1.
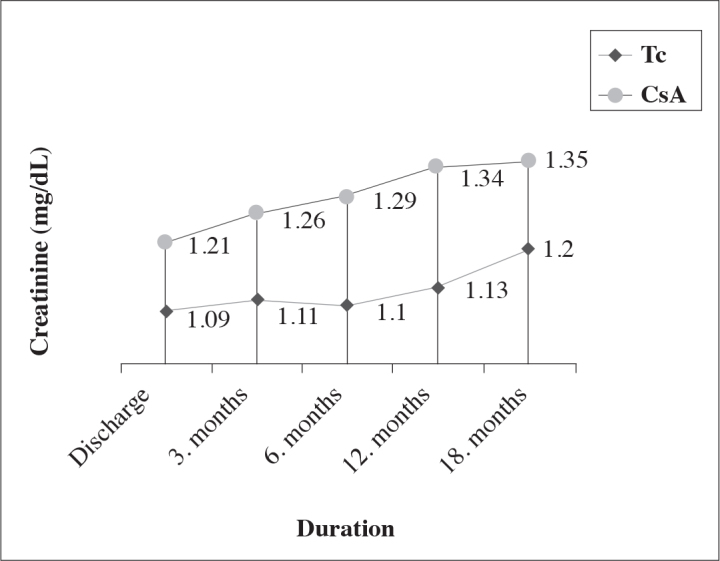
Graft function
Cosmetic side effects:
In the CsA group, as the most frequently encountered cosmetic adverse effects ie. hirsutismus, and gingival hyperplasia which led to modification of the drug therapy were seen in 10.0 (p:0.232), and 15.0% (p:0.107) of the cases in the CsA group, while it wasn’t observed at all in the Tc group.
Infection:
Infections requiring hospitalization developed in 25, and 30.0% of the patients in the Tc, and CsA groups, respectively without any statistically significant intergroup difference (p:0.130). One patient with CMV infection in the CsA group presented with gastrointestinal symptoms which led to decrease in graft functions during the period without the coverage of any prophylactic antiviral regimen. Hospitalized, and isolated patients responded to the treatment, and they were discharged with normal serum creatinine values.
Hepatotoxicity, Nephrotoxicity:
During the follow-up period excluding one patient in the CsA group, any case suggestive of nephrotoxicity was not encountered. A statistically significant difference was not found between two drug groups as for hepatotoxicity (p:0.232).
Drug change:
In the CsA group, drug therapy was changed in a total of 8 (40%) cases because of hirsutismus (n=2), gingival hyperplasia (n=3), hepatotoxicity (n=2), and nephrotoxicity (n=1). However in the Tc group drug therapy of one patient was changed because of the development of hepatotoxicity (p:0.001).
Survival:
None of the patients died in both groups. Overall patient survival rate was determined as 100 percent. Only in one patient in the CsA group (intragroup 5.0%; overall 2.43%) acute rejection refractory to prednisolone developed which did not respond to ATG (anti-thymocyte globulin) therapy. This patient was included in the hemodialysis program at the end of 2 months of follow-up, and accepted as a graft failure (p:0.488). Graft survival rates were 95.0% in the CsA, and 97.57% in the study group, respectively.
Discussion
Thanks to significant improvements in the field of renal transplantation, and innovations in the immunosuppressive therapy, important improvements have been realized in the graft survival rates. Immunosuppressive treatment with cyclosporine A decreased acute rejection rates markedly with a resultant rise in the improvement in 1-year graft survival rates from 55–60% to more than 80 percent.[2,3] Later on, another calcineurin inhibitor tacrolimus was approved by FDA with the emphasis on more tolerable side effect profile.[4] Because of significant impact of gastrointestinal system on CsA pharmacokinetics, microemulsion form of CsA has been developed, and this innovative form has increased efficacy, and safety of cyclosporine.[5,6]
Even though a statistically significant (p:0.017) difference was observed between Tc, and CsA groups as for demographic data, in correlation analyses any impact of this difference on graft survival has not been detected. This level of significance (p:0.004) could not be associated with graft functions in the long-run, while essentially the impact of surgical complications created such a level of difference.
An intergroup difference influential on graft survival was not observed when HLA compatibilites were compared. Intensive doses of immunosuppressive treatment regimen including basiliximab applied on all recipients of cadaver or live donor grafts as induction therapy, considerably block the development of acute rejection due to potent immune suppression in the early postoperative period.[7,8] This approach contributes specially to favourable early-term results even in cases of complete HLA incompatibility in recipients of live donor renal grafts.
Acute rejection attacks were seen in 5 patients in the Tc group, and with treatment any graft failure did not develop. However in the CsA group, acute rejection attacks developed in the CsA group, and one patient with a graft loss was included in the hemodialysis group. In a multicentered study, cadaver kidney recipients in both groups were compared, and acute rejection attacks were reported as 46.4, and 30.7%, in the CsA, and Tc groups, respectively.[9] In a series of 560 patients included in The European Tacrolimus vs. Cyclosporine Microemulsion in Renal Transplantation Study, biopsy-proven acute rejection rates in the first 6 months were 37.3, and 19.6% in the CsA, and Tc groups, respectively. In the same study, prednisolone-resistant rejection was seen less frequently in the Tc group (9.4 vs. 21%).[10]
Similarly, in a meta-analysis evaluating comparative studies performed between Tc, and both formulations of cyclosporines, it was indicated that treatment of 100 patients with cyclosporine instead of tacrolimus, prevented 12 acute rejection episodes in the first year.[11]
Contrary to studies which found similar rates of hypertension between Tc, and cyclosporins, with lower number of additional antihypertensive drugs in the Tc group, in our study, any significant intergroup difference was not found as for the number of antihypertensive drugs.[12]
The results of two separate multicentered studies conducted in Europe, and the USA, Tc was demonstrated to be more advantageous than CsA with lesser number of hypertensive complications, and some publications asserted that these two calcineurin inhibitors were not different as for risk of HT development.[4,10,13,14] In our study a significant difference could not be demonstrated between these two drugs.
In a study where results of 10 years of CsA use were reported, hyperlipidemia was not considered as an infrequently seen complication.[15] In a European comparator study, hypercholesterolemia was found to be significantly lower in the Tc group, in the long-term any intergroup difference was not found with respect to complication rates requiring antihyperlipidemic treatment.[10,13] In our study cholesterol, and triglyceride levels, and incidence of starting on an antihyperlipidemic drug therapy were found to be significantly higher in the CsA group when compared with the Tc group. Even though in the CsA group, higher posttransplant drug levels were observed, in both groups comparable treatment responses, and complete response rates were obtained. Protective effect of statin use on graft survival has been demonstrated.[16]
In a study, increased cumulative incidence of diabetes mellitus was found with time, and as risk factors black race, obesity, and tacrolimus use were indicated.[17] On the other hand, in a study where non-diabetic CsA users were investigated, incidence of diabetes mellitus in the first year reportedly rised to 30% by the tenth year.[18] In line with this information both calcineurin inhibitors can be held responsible for the increased rate of diabetes mellitus. In previous publications, some data implicating that Tc induces diabetes mellitus at a relatively higher frequency, recent articles have reported similar rates for both drug groups.[4,13,19–21] In our study groups, increases in pre-, and postoperative blood glucose levels have not been detected. However scarce number of our cases, and shorter follow-up periods constitute limitations of our study.
Most of the comparator studies encountered in the literature based on serum creatinine levels in order to determine graft function, have indicated lack of any significant difference between these two calcineurin inhibitors, while Krämer et al.[14] at the end of their comparative analysis of both drugs, detected serum creatinine levels in the Tc, and CsA groups as 1.36 mg/dL, and 1.61 mg/dL, respectively. In our study, serum creatinine values in the Tc group were maintained at a relatively lower level from the start, while intergroup difference was not found to be statistically significant.[4,10,12]
In our study, the incidence of infection has been in compliance with the results of comparator studies. Recently, infection is losing its place among causes of death thanks to prophylactic, and therapeutic antibiotherapy regimens.[22,23]
Cosmetic side effects were encountered in the CsA group in a higher frequency which caused drug change. Even though hirsutismus, and gingival hyperplasia are not life-threatening, they can lead to psychological problems especially in female recipients.[24] Organ toxicities those predominantly involving kidneys are restrictive factors for use of therapeutic doses of calcineurin inhibitors.[25] Renal toxicity of CsA is a well-known, and histopathologically defined adverse effect of the drug.[26] Tc with a similar histological profile has been introduced into market with a claim of a better side effect profile.[27] In our study, nephrotoxic effect detected in one patient led to exclusion of calcineurin inhibitors from the immunosuppressive protocol of this patient.
In this study, hepatotoxicity developed in both groups which was resolved with drug change without any statistically significant intergroup difference. As a main approach resorted to in the development of hepatotoxicity is either making a decrease in the drug dosage or switching to another drug.[28]
Change of drug therapy is a frequently applied method with the intention of individualization of the therapy. In comparative studies, the rationale of switching to another drug is frequently encountered episode(s) of rejection refractory to therapy. However, generally side effects of Tc requires crossing over to CsA therapy.[9,22] In patients converted from CsA to Tc therapy, a significant drop in serum creatinine levels was observed, and these levels were maintained during a 5-year surveillance period.[29] Even though drug change detected in our study was statistically significant, essentially these nonvital cosmetic causes lead the way. Relatively younger mean age of this patient group increased rates of drug change.
In short-, and long-term studies, Tc appears to be more advantageous than CsA, however, superiority of Tc over CsA has not been proved as for graft, and patient survival.[4,10,13,30] Overall, and graft survival rates of the patient group included in the study were detected as 100, and 97.5%, respectively.
Immunosuppressive regimen to be selected following renal transplantation should favourably effect both graft survival, and morbidity, and mortality of the patient. In our study both drugs of the calcineurin group were evaluated as for their impact on acute rejection, graft function, and their adverse effects as infectious episodes, hypertension, diabetes, hyperlipidemia, and cosmetic side effects. For cosmetic side effects, and hyperlipidemia, tacrolimus was found to be more favorable relative to cyclosporine A. When, risk of cardiovascular disease induced by hyperlipidemia was taken into consideration, tacrolimus appears to be more preferable. Nevertheless, each patient who will receive a renal transplant should be evaluated individually, monitored closely to detect the effects of immunosuppressive therapy, and its adverse effects in order to perform necessary interventions.
Footnotes
Conflict of Interest: No conflict of interest was declared by the authors.
References
- 1.Titiz Mİ. General approach to the renal transplantation. A practical approach to renal transplantation. Eczacıbaşı; İstanbul: 2004. [Google Scholar]
- 2.A randomized clinical trial of cyclosporine in cadaveric renal transplantation. Analysis at three years. The Canadian Multicentre Transplant Study Group. N Engl J Med. 1986;314:1219–25. doi: 10.1056/NEJM198605083141904. [DOI] [PubMed] [Google Scholar]
- 3.Kahan BD. Cyclosporine. N Eng J Med. 1989;321:1725–38. doi: 10.1056/NEJM198912213212507. [DOI] [PubMed] [Google Scholar]
- 4.Jensik SC. Tacrolimus (FK506) in kidney transplantation:tree-year survival results of the US multicenter, randomized, comperative trial. Transplant Proc. 1998;30:1216–8. doi: 10.1016/s0041-1345(98)00216-4. [DOI] [PubMed] [Google Scholar]
- 5.Meier-Kriesche HU, Kaplan B. Cyclosporine microemulsion and tacrolimus are associated with decreased chronic allograft failure and improved long-term graft survival as compared with sandimmune. Am J Transplant. 2002;2:100–4. doi: 10.1034/j.1600-6143.2002.020116.x. [DOI] [PubMed] [Google Scholar]
- 6.Pollard SG, Lear PA, Ready AR, Moore RH, Johnson RW. Comparison of microemulsion and conventional formulations of cyclosporine A in preventing acute rejection in de novo kidney transplant patients. The U.K. Neoral Renal Study Group. Transplantation. 1999;68:1325–31. doi: 10.1097/00007890-199911150-00018. [DOI] [PubMed] [Google Scholar]
- 7.Leonardi G, Messina M, Giraudi R, Pellu V, Fop F, Segoloni GP. Basiliximap in association with tacrolimus and steroids in caucasian cadaveric renal transplanted patients:significant decrease in early acute rejection rate and hospitalization time. Clin Transplant. 2004;18:113–8. doi: 10.1046/j.1399-0012.2003.00150.x. [DOI] [PubMed] [Google Scholar]
- 8.Hoshinaga K, Aikawa A, Murai M, Yamamoto H, Hirayama N, Shishido S, et al. Regimen of tacrolimus based immunosuppression with basiliximap, mycophenolate mofetil, and low-dose steroid reduces acute rejection in kidney transplants. Transplant Proc. 2005;37:1762–3. doi: 10.1016/j.transproceed.2005.02.066. [DOI] [PubMed] [Google Scholar]
- 9.Pirsch JD, Miller J, Deierhoi MH, Vincenti F, Filo RS. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. FK506 Kidney Transplant Study Group. Transplantation. 1997;63:977–83. doi: 10.1097/00007890-199704150-00013. [DOI] [PubMed] [Google Scholar]
- 10.Margreiter R, European Tacrolimus vs Ciclosporin Microemulsion Renal Transplantation Study Group Efficacy and safety of tacrolimus compared with ciclosporin microemulsion in renal transplantation: a randomised multicentre study. Lancet. 2002;359:741–6. doi: 10.1016/S0140-6736(02)07875-3. [DOI] [PubMed] [Google Scholar]
- 11.Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. BMJ. 2005;331:810. doi: 10.1136/bmj.38569.471007.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boots JM, van Duijnhoven EM, Christiaans MH, Nieman FH, van Suylen RJ, van Hooff JP. Single center experience with tacrolimus versus cyclosporine-Neoral in renal transplant recipients. Tranpl Int. 2001;14:370–83. doi: 10.1007/s001470100002. [DOI] [PubMed] [Google Scholar]
- 13.Morales JM, Andrés A, Dominguez-Gil B, Arriola M, Gutiérrez MJ, Hernández E, et al. Ten years of treatment with tacrolimus is related to an excellent renal function, allowing monoteraphy in a large proportion of cases: unicentric results of the tacrolimu versus cyclosporine A European multicentric study in kidney transplant patients. Transplant Proc. 2005;37:3738–42. doi: 10.1016/j.transproceed.2005.09.178. [DOI] [PubMed] [Google Scholar]
- 14.Krämer BK, Montagnino G, Del Castillo D, Margreiter R, Sperschneider H, Olbricht CJ, et al. Efficacy and safety of tacrolimus compared with cyclosporin A microemulsion in renal transplantation:2 year follow-up results. Nephrol Dial Transplant. 2005;20:968–73. doi: 10.1093/ndt/gfh739. [DOI] [PubMed] [Google Scholar]
- 15.Ota K, Takahashi K, Uchida K, Takahara S, Yagisawa T, Tanabe K. A 10 year follow up study of renal transplant recipients treated with cyclosporine. Clin Nephrology. 2000;53:182–7. [PubMed] [Google Scholar]
- 16.Del Castillo D, Cruzado JM, Manel Díaz J, Beneyto Castelló I, Lauzurica Valdemoros R, Gómez Huertas E, et al. The effects of hyperlipidemia on graft and patient outcome in renal transplantation. Nephrol Dial Transplant. 2004;19:67–71. doi: 10.1093/ndt/gfh1019. [DOI] [PubMed] [Google Scholar]
- 17.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transpl. 2003;3:178–85. doi: 10.1034/j.1600-6143.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 18.Cosio FG, Pesavento TE, Osei K, Henry ML, Ferguson RM. Posttransplant diabetes mellitus: increasing incidence in renal allograft recipientes transplanted in recent years. Kidney Int. 2001;59:732–7. doi: 10.1046/j.1523-1755.2001.059002732.x. [DOI] [PubMed] [Google Scholar]
- 19.Heisel O, Heisel R, Balshaw R, Keown P. New onset diabetes mellitus in patients receiving calcineurin inhibitors: a systematic review and meta-analysis. Am J Transplant. 2004;4:583–95. doi: 10.1046/j.1600-6143.2003.00372.x. [DOI] [PubMed] [Google Scholar]
- 20.First MR, Gerber DA, Hariharan S, Kaufman DB, Shapiro R. Posttransplant diabetes mellitus in kidney allograft recipients: incidence, risk factors and management. Transplantation. 2002;73:379–86. doi: 10.1097/00007890-200202150-00011. [DOI] [PubMed] [Google Scholar]
- 21.Irish W, Sherrill B, Brennan DC, Lowell J, Schnitzler M. Three year posttransplant graft survival in renal tranplant patients with graft function at 6 months receiving tacrolimus or cyclosporine microemulsion within a triple-drug regimen. Transplantation. 2003;76:1686–90. doi: 10.1097/01.TP.0000090865.20886.B7. [DOI] [PubMed] [Google Scholar]
- 22.Vincenti F, Jensik SC, Filo RS, Miller J, Pirsch J. A long term comparision of tacrolimus(FK506) and cyclosporine in kidney transplantation: evidence for improved allograft survival at five years. Transplantation. 2002;73:775–82. doi: 10.1097/00007890-200203150-00021. [DOI] [PubMed] [Google Scholar]
- 23.Mayer AD, Dmitrewski J, Squifflet JP, Besse T, Grabensee B, Klein B, et al. Multicenter randomized trial comparing tacrolimus(FK506) and cyclosporine in the prevention of renal allograft rejection. Transplantation. 1997;63:977–83. doi: 10.1097/00007890-199708150-00012. [DOI] [PubMed] [Google Scholar]
- 24.Erek E, Süleymanlar G, Serdengeçti K. Nephrology-dialysis, and transplantation in Turkey. Turkish Society of Nephrology Registry; 2000. [DOI] [PubMed] [Google Scholar]
- 25.Johnson RW. The clinical impact of nephrotoxicity in renal transplantation. Transplantation. 2000;69:14–7. doi: 10.1097/00007890-200006271-00004. [DOI] [PubMed] [Google Scholar]
- 26.d’Ardenne AJ, Dunnill MS, Thompson JF, McWhinnie D, Wood RF, Morris PJ. Cyclosporin and renal graft histology. J Clin Pathol. 1986;39:145–51. doi: 10.1136/jcp.39.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mihatsch MJ, Kyo M, Morozumi K, Yamaguchi Y, Nickeleit V, Ryffel B. The side-effects of ciclosporine-A and tacrolimus. Clin Nephrol. 1998;49:356–63. [PubMed] [Google Scholar]
- 28.Staatz CE, Willis C, Taylor PJ, Tett SE. Population pharmacokinetics of tacrolimus in adult kidney transplant recipients. Clin Pharmacol Ther. 2002;72:660–9. doi: 10.1067/mcp.2002.129304. [DOI] [PubMed] [Google Scholar]
- 29.Jordan ML, Shapiro R, Vivas C, Scantlebury V, McCauley J, McMichael J, et al. Outcome of tacrolimus conversion therapy for renal allograft rejection:5 year follow-up. Transplant Proc. 1999;31:81–3. doi: 10.1016/s0041-1345(99)00802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abou-Jaoude MM, Najm R, Shaheen J, Nawfal N, Abboud S, Alhabash M, et al. Tacrolimus (FK506) versus cyclosporine micro-emulsion (Neoral) as maintenance immunosuppression therapy in kidney transplant recipients. Transplant Proc. 2005;37:3025–8. doi: 10.1016/j.transproceed.2005.08.040. [DOI] [PubMed] [Google Scholar]


