Abstract
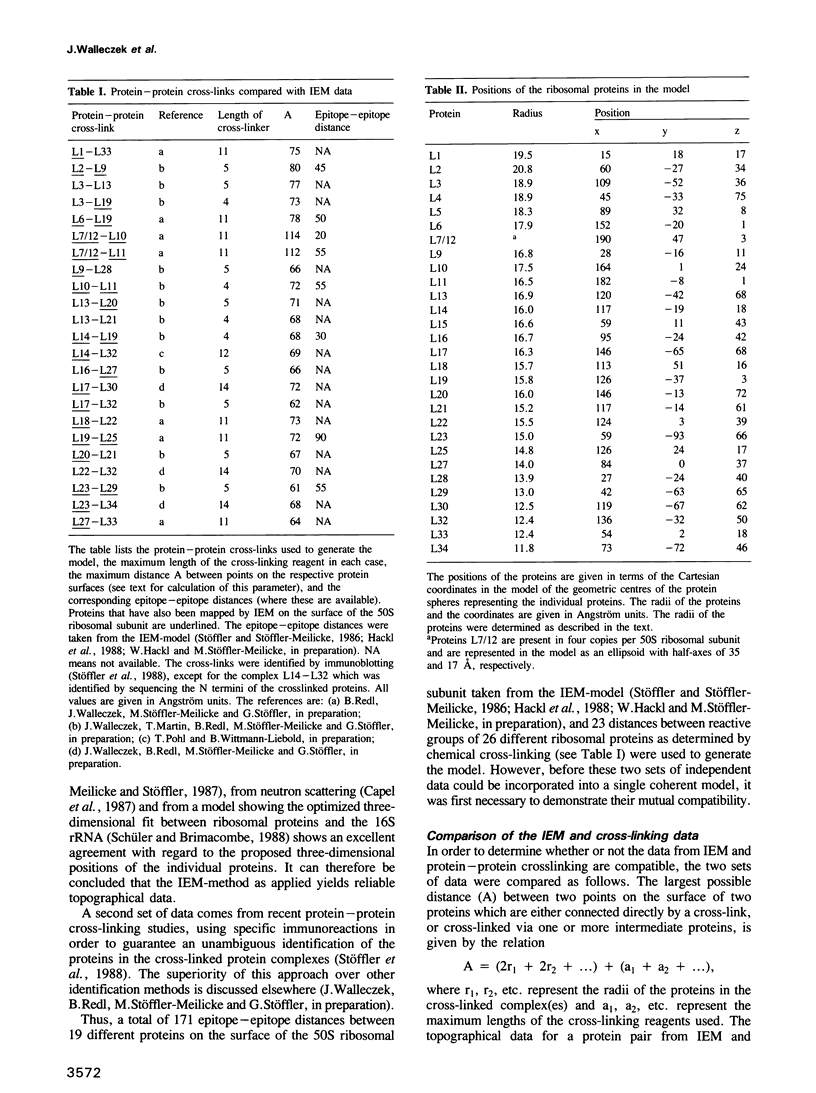
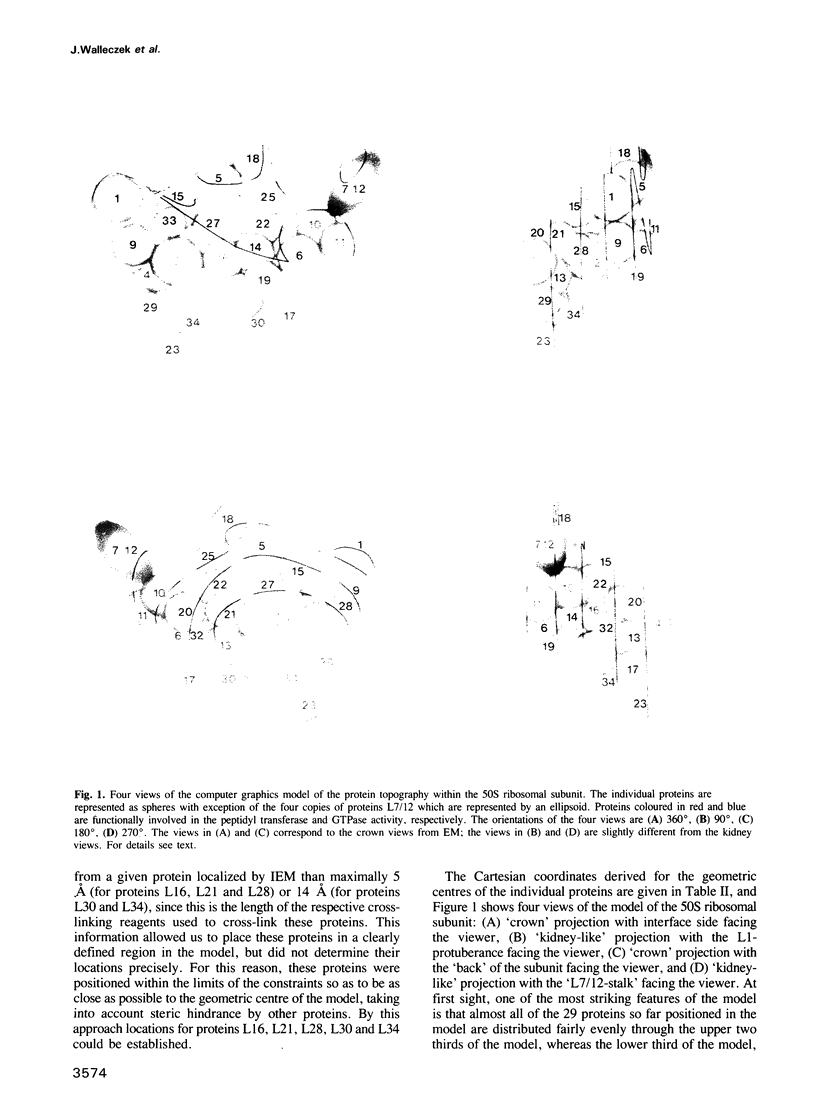
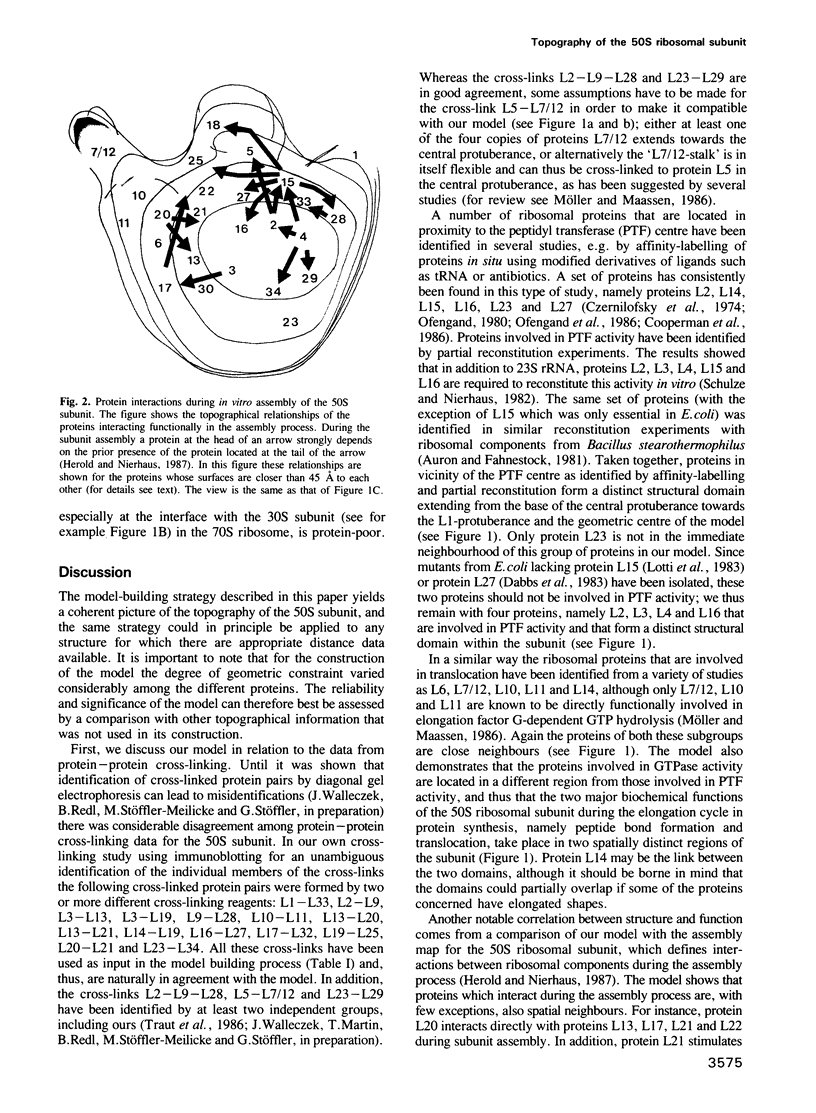
A three-dimensional model for the arrangement of 29 of the 33 proteins from the Escherichia coli large ribosomal subunit has been generated by interactive computer graphics. The topographical information that served as input in the model building process was obtained by combining the immunoelectron microscopically determined network of epitope-epitope distances on the surface of the large ribosomal subunit with in situ protein-protein cross-linking data. These two independent sets of data were shown to be compatible by geometric analysis, thus allowing the construction of an inherently consistent model. The model shows (i) that the lower third of the large subunit is protein-poor, (ii) that proteins known to be functionally involved in peptide bond formation and translocation are clustered in two separate regions, (iii) that proteins functionally interdependent during the self-assembly of the large subunit are close neighbours in the mature subunit and (iv) that proteins forming the early assembly nucleus are grouped together in a distinct region at the 'back' of the subunit.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auron P. E., Fahnestock S. R. Functional organization of the large ribosomal subunit of Bacillus stearothermophilus. J Biol Chem. 1981 Oct 10;256(19):10105–10110. [PubMed] [Google Scholar]
- Brimacombe R., Atmadja J., Stiege W., Schüler D. A detailed model of the three-dimensional structure of Escherichia coli 16 S ribosomal RNA in situ in the 30 S subunit. J Mol Biol. 1988 Jan 5;199(1):115–136. doi: 10.1016/0022-2836(88)90383-x. [DOI] [PubMed] [Google Scholar]
- Capel M. S., Engelman D. M., Freeborn B. R., Kjeldgaard M., Langer J. A., Ramakrishnan V., Schindler D. G., Schneider D. K., Schoenborn B. P., Sillers I. Y. A complete mapping of the proteins in the small ribosomal subunit of Escherichia coli. Science. 1987 Dec 4;238(4832):1403–1406. doi: 10.1126/science.3317832. [DOI] [PubMed] [Google Scholar]
- Czernilofsky A. P., Collatz E. E., Stöffler G., Kuechler E. Proteins at the tRNA binding sites of Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1974 Jan;71(1):230–234. doi: 10.1073/pnas.71.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackl W., Stöffler-Meilicke M., Stöffler G. Three-dimensional location of ribosomal protein BL2 from Bacillus stearothermophilus, a key component of the peptidyl transferase center. FEBS Lett. 1988 Jun 6;233(1):119–123. doi: 10.1016/0014-5793(88)81367-x. [DOI] [PubMed] [Google Scholar]
- Herold M., Nierhaus K. H. Incorporation of six additional proteins to complete the assembly map of the 50 S subunit from Escherichia coli ribosomes. J Biol Chem. 1987 Jun 25;262(18):8826–8833. [PubMed] [Google Scholar]
- Lotti M., Dabbs E. R., Hasenbank R., Stöffler-Meilicke M., Stöffler G. Characterisation of a mutant from Escherichia coli lacking protein L15 and localisation of protein L15 by immuno-electron microscopy. Mol Gen Genet. 1983;192(3):295–300. doi: 10.1007/BF00392165. [DOI] [PubMed] [Google Scholar]
- Meisenberger O., Pilz I., Stöffler-Meilicke M., Stöffler G. Small-angle X-ray study of the 50 S ribosomal subunit of Escherichia coli. A comparison of different models. Biochim Biophys Acta. 1984 Apr 5;781(3):225–233. doi: 10.1016/0167-4781(84)90087-3. [DOI] [PubMed] [Google Scholar]
- Radermacher M., Wagenknecht T., Verschoor A., Frank J. Three-dimensional structure of the large ribosomal subunit from Escherichia coli. EMBO J. 1987 Apr;6(4):1107–1114. doi: 10.1002/j.1460-2075.1987.tb04865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards F. M. Areas, volumes, packing and protein structure. Annu Rev Biophys Bioeng. 1977;6:151–176. doi: 10.1146/annurev.bb.06.060177.001055. [DOI] [PubMed] [Google Scholar]
- Schulze H., Nierhaus K. H. Minimal set of ribosomal components for reconstitution of the peptidyltransferase activity. EMBO J. 1982;1(5):609–613. doi: 10.1002/j.1460-2075.1982.tb01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüler D., Brimacombe R. The Escherichia coli 30S ribosomal subunit; an optimized three-dimensional fit between the ribosomal proteins and the 16S RNA. EMBO J. 1988 May;7(5):1509–1513. doi: 10.1002/j.1460-2075.1988.tb02970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöffler-Meilicke M., Stöffler G. The topography of ribosomal proteins on the surface of the 30S subunit of Escherichia coli. Biochimie. 1987 Oct;69(10):1049–1064. doi: 10.1016/0300-9084(87)90005-8. [DOI] [PubMed] [Google Scholar]
- Stöffler G., Redl B., Walleczek J., Stöffler-Meilicke M. Identification of protein-protein cross-links within the Escherichia coli ribosome by immunoblotting techniques. Methods Enzymol. 1988;164:64–76. doi: 10.1016/s0076-6879(88)64035-3. [DOI] [PubMed] [Google Scholar]
- Stöffler G., Stöffler-Meilicke M. Immunoelectron microscopy of ribosomes. Annu Rev Biophys Bioeng. 1984;13:303–330. doi: 10.1146/annurev.bb.13.060184.001511. [DOI] [PubMed] [Google Scholar]
- Wada A., Sako T. Primary structures of and genes for new ribosomal proteins A and B in Escherichia coli. J Biochem. 1987 Mar;101(3):817–820. doi: 10.1093/jb/101.3.817. [DOI] [PubMed] [Google Scholar]