Abstract
Objective:
The aim of this study was to determine the frequencies of Y chromosome microdeletions in infertile azoospermic and oligozoospermic Turkish men and in healthy control subjects.
Material and methods:
Sixty-four azoospermic and 51 oligozoospermic patients infertile patients, and 70 healthy men who had a child without the aid of assisted reproductive technologies were included in this study. DNA was extracted from peripheral blood samples collected from the patients. Following multiplex PCR performed with 15 different primer sequences, Y chromosome AZFa, AZFb, AZFc and AZFd region microdeletions were determined by agarose gel electrophoresis.
Results:
Y chromosome microdeletions were detected in 8 (12.5%) patients in the azoospermia group and 3 (5.9%) patients in the oligozoospermia group. The overall frequency of Y chromosome microdeletions in all infertile cases was 9.6%. Y chromosome microdeletions were not found in the healthy control group. Among the infertile cases, there were 4 (3.48%) AZFa, 2 (1.74%) AZFb, 3 (2.61%) AZFc and 7 (6.09%) AZFd region microdeletions. Y chromosome microdeletions were not found among healthy men in the control group.
Conclusion:
The presence of Y chromosome microdeletions among azoospermic and oligozoospermic infertile males suggests that routine genetic testing and genetic counseling prior to the use of assisted reproduction techniques are necessary.
Keywords: AZFa, AZFb, AZFc, AZFd, male infertility, Y chromosome microdeletion
Introduction
Infertility which is defined as inability to produce offspring despite regular and unprotected sexual intercourse after a period of one year, is detected in nearly 15% of the married couples.[1] Approximately 50% of the cases are secondary to male-factor infertility. Genetic factors of male infertility include i) chromosomal aneupolidies, ii) microdeletions, and iii) single gene defects.[2]
Originally in the year 1976, Tiepolo and Zuffardi defined azoospermia factor region (AZF) in infertile men using a large -scale deletion mapping.[3] Subsequent studies have demonstrated that AZF is the region where genes which have important functions in the proliferation, and differentiation are coded. AZF region are divided into four non-overlapping subregions as AZFa (proximal), AZFb (intermediate), AZFc (distal), and AZFd (between AZFb, and AZFc).[4,5] Dependent on microdeletions occurring on these regions different phenotypic characteristics can emerge.[6]
AZFa microdeletions have been associated with germinal aplasia (Sertoli cell only syndrome, SCOS).[7] In cases of large AZFb deletion regions, azoospermia was observed, while in cases of partial AZFb deletion, mild and severe degrees of oligospermia can occur.[8] Deletions which occur in the AZFc region can cause type II SCOS or hypospermatogenesis.[9] However AZFd deletions can manifest themselves as abnormal sperm morphology despite mild degrees of oligospermia or normal sperm counts.[10]
In infertile cases, Y chromosomal microdeletions have been reported at incidence rates ranging between 1–55 percent.[8,11] In normal male population, its rate ranges between 0.7–1 percent.[12] In this study we aimed to analyze, and compare Y chromosome deletions in infertile azoospermic, oligozoospermic men, and normal healthy control group.
Material and methods
This study enrolled a total of 185 infertile cases with diagnosis of azoospermia (n=64), oligozoospermia (n=51), and also 70 healthy men who had known to have children without any aid from assisted reproductive techniques. From each patient 3 mL blood sample was drawn into tubes containing EDTA, and responses to inquiries about their medical history were recorded. Our study protocol was approved by Ege Univeristy, Faculty of Medicine, Research Ethics Committee with the decree 08-3/9, dated 06.04.2008. DNA was isolated from peripheral blood samples drawn from control, and study groups using High Pure PCR Template Preparation Kit (Roche Applied Science). For the analysis of Y microdeletion, AZOpleks IV AZF (Diagen) detection kit containing primers specific to the regions (sY84, sY82, sY255, sY134, sY127, sY142, sY153, sY254, sY141, sY158, sY152, sY86, QX7, sY233) which are required for the identification of 14 microdeletions on all AZF regions, and SRY region on Y chromosome was used.
For each case, 4 different PCR mixtures were prepared. PCR protocol was realized in the following steps: denaturation (94°C for 15 min), amplification (10 cycles): denaturation (94°C for 15 secs), conjugation (61°C for 30 secs), polimerization (72°C for 45 secs), 25 cycles: denaturation (94°C for 15 secs), conjugation (59°C for 30 secs), polimerization (72°C for 45 secs), polimerization (72°C for 4 min). Then PCR products were made visible using 3% agarose gel electrophoresis, and sizes of PCR products specific to STS regions contained in PCR mixtures were evaluated.
Statistical analysis
Y microdeletion findings detected in azoospermic, oligozoospermic, and control groups were compared using Fisher’s Exact Test, and chi-square test.
Results
This study enrolled 70 healthy controls, 64 azoospermic, and 51 oligozoospermic men, and their Y chromosome microdeletions were analyzed. Mean ages of the control, azoospermic, and azoospermic groups were detected as 40.48±8.38, 35.46±4.82, and 36.80±5.39 years, respectively.
Y chromosome microdeletion data were determined in azoospermic, and oligospermic groups as shown in Table 1. In the azoospermic group 8 of 64 (12.5%, 95% CI: 1.35–14.65%), and in the oligospermic group 3 of 51 (5.9%, 95% CI: 1.68–7.68%) infertile cases had Y chromosome microdeletiones. (Table 1, Figure 1). When all infertile cases were evaluated, microdeletions were detected in 9.6% of the patients. When azoospermic, oligo zoospermic, and control groups were compared, a significantly increased rate of Y microdeletion carriership was found in the azoospermic, and oligospermic groups (p=0.004, Table 2). In fact its rate was significantly higher in the infertile group relative to the control group (p=0.007, Table 3). In comparison with the control group, the rate of Y microdeletion carriership significantly increased in cases with azoospermia. (p=0.002), while any significant difference was not observed between the oligozoospermic cases, and the control group (p=0.072).
Table 1.
AZF region deletions determined in azoospermic, and olgigozoospermic patients
| AZFa | AZFb | AZFc | AZFd | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sY(82) | sY(84) | sY(86) | sY127 | sY134 | sY142 | sY141 | sY158 | sY233 | sY254 | sY255 | QX7 | sY152 | sY153 | ||
| Azoospermia | Case 1 | x | |||||||||||||
| Case 2 | x | ||||||||||||||
| Case 3 | x | ||||||||||||||
| Case 4 | x | x | |||||||||||||
| Case 5 | x | x | x | ||||||||||||
| Case 6 | x | x | x | x | x | ||||||||||
| Case 7 | x | ||||||||||||||
| Case 8 | x | x | x | x | x | x | x | x | x | x | |||||
| Case 9 | x | ||||||||||||||
| Oligozoospermia | Case 10 | x | |||||||||||||
| Case 11 | x | ||||||||||||||
AZF: azoospermia factor region
Figure 1.
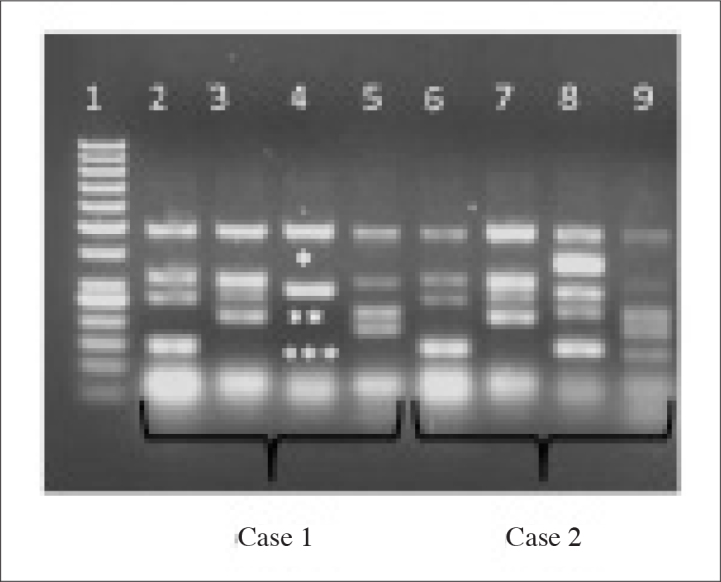
Results of mulplex PCR analysis 1. row: 50 base double molecular weight marker; 2.–5. rows:Case 1; 6.–9. rows:Case 2; In Case 1, deletions were found in sY254*, sY158**, and sY152*** In Case 2,any deletion was not found Case 1 Case 2
Table 2.
Comparison of microdeletion rates in azoospermic, oligozoospermic, and control groups
| Group | Microdeletion (+) | Microdeletion (−) | p |
|---|---|---|---|
| Azoospermic (n=64) | 8 | 56 | 0.004 |
| 12.5% | 87.5% | ||
| Oligozoospermic (n=51) | 3 | 48 | |
| 5.9% | 94.1% | ||
| Control (n=70) | 0 | 70 | |
| 0% | 100% |
Table 3.
Comparison of microdeletion rates in infertile (azoospermic, oligozoospermic), and control groups
| Group | Microdeletion (+) | Microdeletion (−) | p |
|---|---|---|---|
| Infertil (n=115) | 11 | 104 | 0.007 |
| 9.6% | 90.4% | ||
| Control (n=70) | 0 | 70 | |
| 0% | 100% |
In cases with infertility, deletions in the AZFa (n=4; 3.48%), AZFb (n=2; 1.74%), AZFc (n=3; 2.61%), and AZFd (n=7; 6.09%) regions were detected. In the azoospermic group in all AZF regions of one case, and AZFc, and AZFd regions of 2 cases, microdeletions were found. However in 7 cases with infertility, microdeletion was determined in only one AZF region.
Y chromosome microdeletion was not found in 70 healthy controls included in our study population.
Discussion
In our study, we aimed to investigate the incidence of AZF microdeletions in Turkish men, and Y chromosome micro-deletion carriership was detected in 9.6% of infertile cases. However, chromosome aneuploidies in these cases included in our study were not evaluated.
Y chromosome microdeletions lead to impairment of the spermatogenetic process, and effect normal spermatogenesis adversely. Incidence rates of deletions can differ between ethnic groups, and geographic distribution of the population studied.[13] Different incidence rates have been reported for Y chromosome microdeletions encountered in the Turkish population Balkan et al.[12] 1.3%; Sargın et al.[14] 3.3%, Akın et al.[15] 3.93%, Tağa (thesis study)[16] 6.3, Çetinkaya[17] (thesis study) 9.09%, Müslümanoğlu et al.[18] 22.64%. In other populations, incidence rates of Y chromosome microdeletion have been reported to range between 1, and 55 percent.[11,19]
In most of the studies which investigated Y chromosome micro-deletions, AZFd region has not been analyzed.[14–17] However in our study, AZFd region was analyzed, and AZFd microdeletions were found in 6.09% of the infertile men. AZFd region which is localized between AZFb, and AZFc regions was described by Ken-First et al.[4]. In infertile cases the rate of AZFd region deletions have been reported as 15.1 percent.[18] In our study, its rate was found to be 6.08 percent. In our study, AZFc microdeletion was detected in 3 (5.88%) azoospermic cases. AZFc deletions effect spermatogenetic process, but as reports have indicated they do not always cause infertility. In azoospermic cases with AZFc microdeletions, the chance of sperm retrieval to achieve fertilization is quite high in testicular sperm extraction (TESE) method. However it should not be forgotten that male offsprings of these cases will also carry AZFc microdeletions.[20] Complete deletion of AZFa or AZFb regions usually results in total loss of germ cells. Therefore, TESE, and intracytoplasmic sperm injection (ICSI) are not successful in these cases. Chance of spermatozoa retrieval in cases with partial AZFb deletion using TESE has been reportedly 50 percent.[5]
Functions of 14 of 31 Y chromosome genes have been defined.[21] AZFb proximal region of Y chromosome contains CDY2, RPS4Y2, and XKRY, while distal-AZFb/proximal-AZFc region has BPY2.1, CDY1.1, DAZ1, DAZ2 genes. In the AZFc region, only two genes (CSPG4LY, GOLGA2LY) are encoded. From this aspect, AZFb microdeletions are thought to induce testicular pathologies more frequently than AZFc microdeletions. DAZ (Deleted in Azoospermia) gene family is expressed in gonads, and has important functions in the development of germ cells. DAZ genes in the AZFc region have been reportedly subjected to deletion in 12–15% of azoospermic men.[9] However in our study DAZ deletion was detected in 3 (5.88%) azoospermic cases.
In recent years, thanks to TESE, and ICSI procedures, spermatozoa can be retrieved from oligozoospermic, and azoospermic cases to achieve fertilization. However, reports indicate that in Y chromosome microdeletion carriers, success rate in sperm retrieval using TESE method is only 10 percent.[17] Since TESE bypasses natural selection process of spermatozoa, it carries the risk of transfer of genetic abnormalities to the coming generation. Cram et al.[22] reported transmission of Y microdeletions from father to son during ICSI procedure. Therefore, determination of chromosome abnormalities, and AZF region microdeletions before ICSI procedures has a crucial importance in genetic counseling.
Urology guidelines indicate that in azoospermic and/or severe oligozoospermic infertile cases investigation of Y chromosome microdeletions should be carried out routinely.[20] European Academy of Andrology (EAA), and European Molecular Genetics Quality Network (EMQN) released a guideline in order to ensure standardization, and quality control in the diagnosis of microdeletions.[20] With the introduction of this guideline in practice, further information can be gathered about sequence of Y chromosomes, and pathogenesis of microdeletions. In the guidelines search for the deletions in the regions of AZFa (sY84, sY86), AZFb (sY127, sY134), and AZFc (sY254, sY255) was recommended in the infertile men. In our study, in addition to the recommended STS regions, AZFa (sY82), AZFb (sY142), AZFc (sY141, sY158, sY233, QX7), and AZFd (sY152, sY153) regions were also analyzed, and excluding QX7, in all of these regions microdeletions were observed. Rate of all types of deletions in the regions not recommended in the guidelines was 59.26%, and majority of these deletions constituted AZFd deletions. Müslümanoğlu et al.[18] reported deletion in the AZFd region as 15.1%, and also maturation arrest, and Sertoli cell-only syndrome were reported in these cases. With this respect we think that this guideline fails to represent Turkish population, and especially AZFd region should be included in the analyses.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Ege University School of Medicine (04.06.2008, 08-3/9).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.U., B.S., Z.E.; Design - M.U., B.S., Z.E.; Supervision - Z.E.; Funding - Z.E.; Materials - R.A., M.U., B.S.; Data Collection and/or Processing - V.B.C.; Analysis and/ or Interpretation - A.Ş.K., A.T.V., Z.M.; Literature Review - A.Ş.K., V.B.Ç.; Writer - A.Ş.K., V.B.Ç.; Critical Review - Z.E.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Iammarrone E, Balet R, Lower AM, Gillott C, Grudzinskas JG. Male infertility. Best Pract Res Clin Obstet Gynaecol. 2003;17:211–29. doi: 10.1016/s1521-6934(02)00147-5. [DOI] [PubMed] [Google Scholar]
- 2.Vogt PH. Molecular genetics of human male infertility: from genes to new therapeutic perspectives. Curr Pharm Des. 2004;10:471–500. doi: 10.2174/1381612043453261. [DOI] [PubMed] [Google Scholar]
- 3.Tiepolo L, Zuffardi O. Localization of factors controlling spermatogenesis in the nonfluorescent portion of the human Y chromosome long arm. Hum Genet. 1976;34:119–24. doi: 10.1007/BF00278879. [DOI] [PubMed] [Google Scholar]
- 4.Kent-First M, Muallem A, Shultz J, Pryor J, Roberts K, Nolten W, et al. Defining regions of the Y-chromosome responsible for male infertility and identification of a fourth AZF region (AZFd) by Y-chromosome microdeletion detection. Mol Reprod Dev. 1999;53:27–41. doi: 10.1002/(SICI)1098-2795(199905)53:1<27::AID-MRD4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 5.Vogt PH. Azoospermia factor (AZF) in Yq11: towards a molecular understanding of its function for human male fertility and spermatogenesis. Reprod Biomed Online. 2005;10:81–93. doi: 10.1016/s1472-6483(10)60807-3. [DOI] [PubMed] [Google Scholar]
- 6.Vogt PH, Edelmann A, Kirsch S, Henegariu O, Hirschmann P, Kiesewetter F, et al. Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11. Hum Mol Genet. 1996;5:933–43. doi: 10.1093/hmg/5.7.933. [DOI] [PubMed] [Google Scholar]
- 7.Poongothai J, Gopenath TS, Manonayaki S. Genetics of human male infertility. Singapore Med J. 2009;50:336–47. [PubMed] [Google Scholar]
- 8.Foresta C, Moro E, Ferlin A. Y chromosome microdeletions and alterations of spermatogenesis. Endocr Rev. 2001;22:226–39. doi: 10.1210/edrv.22.2.0425. [DOI] [PubMed] [Google Scholar]
- 9.Reijo R, Lee TY, Salo P, Alagappan R, Brown LG, Rosenberg M, et al. Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene. Nat Genet. 1995;10:383–93. doi: 10.1038/ng0895-383. [DOI] [PubMed] [Google Scholar]
- 10.Ceylan GG, Ceylan C, Elyas H. Genetic anomalies in patients with severe oligozoospermia and azoospermia in eastern Turkey: a prospective study. Genet Mol Res. 2009;8:915–22. doi: 10.4238/vol8-3gmr616. [DOI] [PubMed] [Google Scholar]
- 11.van der Ven K, Montag M, Peschka B, Leygraaf J, Schwanitz G, Haidl G, et al. Combined cytogenetic and Y chromosome microdeletion screening in males undergoing intracytoplasmic sperm injection. Mol Hum Reprod. 1997;3:699–704. doi: 10.1093/molehr/3.8.699. [DOI] [PubMed] [Google Scholar]
- 12.Balkan M, Tekes S, Gedik A. Cytogenetic and Y chromosome microdeletion screening studies in infertile males with Oligozoospermia and Azoospermia in Southeast Turkey. J Assist Reprod Genet. 2008;25:559–65. doi: 10.1007/s10815-008-9272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krausz C, McElreavey K. Y chromosome microdeletions in ‘fertile’ males. Hum Reprod. 2001;16:1306–7. doi: 10.1093/humrep/16.6.1306. [DOI] [PubMed] [Google Scholar]
- 14.Sargin CF, Berker-Karauzum S, Manguoglu E, Erdogru T, Karaveli S, Gulkesen KH, et al. AZF microdeletions on the Y chromosome of infertile men from Turkey. Ann Genet. 2004;47:61–8. doi: 10.1016/j.anngen.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Akin H, Onay H, Turker E, Ozkinay F. Primary male infertility in Izmir/Turkey: a cytogenetic and molecular study of 187 infertile Turkish patients. J Assist Reprod Genet. 2011;28:419–23. doi: 10.1007/s10815-011-9542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tağa S, Danışman, Dikmen N. Detection of Y chromosome microdeletions (AZF genes) in infertile men in the Çukurova region. 2008 Çukurova Üniversitesi Sağ. Bil Ens Doktora Tezi. [Google Scholar]
- 17.Çetinkaya M, Danışman, Önder AU. Investigation, and interpreattion of the incidence of chromosome microdeletions in infertile Turkish Men. 2008 İstanbul Üniversitesi Cerrahpaşa Tıp Fakültesi Uzmanlık Tezi. [Google Scholar]
- 18.Muslumanoglu MH, Turgut M, Cilingir O, Can C, Ozyurek Y, Artan S. Role of the AZFd locus in spermatogenesis. Fertil Steril. 2005;84:519–22. doi: 10.1016/j.fertnstert.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Foresta C, Ferlin A, Garolla A, Moro E, Pistorello M, Barbaux S, et al. High frequency of well-defined Y-chromosome deletions in idiopathic Sertoli cell-only syndrome. Hum Reprod. 1998;13:302–7. doi: 10.1093/humrep/13.2.302. [DOI] [PubMed] [Google Scholar]
- 20.Simoni M, Bakker E, Krausz C. EAA/EMQN best practice guidelines for molecular diagnosis of y-chromosomal microdeletions. State of the art 2004. Int J Androl. 2004;27:240–9. doi: 10.1111/j.1365-2605.2004.00495.x. [DOI] [PubMed] [Google Scholar]
- 21.Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, Cordum HS, Hillier L, Brown LG, et al. Y chromosome analysis of infertile men and their sons conceived through intracytoplasmic sperm injection: vertical transmission of deletions and rarity of de novo deletions. Fertil Steril. 2000;74:909–15. doi: 10.1016/s0015-0282(00)01568-5. [DOI] [PubMed] [Google Scholar]
- 22.Cram DS, Ma K, Bhasin S, Arias J, Pandjaitan M, Chu B, et al. Y chromosome analysis of infertile men and their sons conceived through intracytoplasmic sperm injection: vertical transmission of deletions and rarity of de novo deletions. Fertil Steril. 2000;74:909–15. doi: 10.1016/s0015-0282(00)01568-5. [DOI] [PubMed] [Google Scholar]


