Abstract
Objective:
Renal ischemia-reperfusion injury may occur due to nephron-sparing surgery in patients with a solitary kidney or restricted renal parenchymas. Prophylactic agents do not always achieve their intended effects and may exhibit side effects. The present study was designed to investigate the possible protective effects of lycopene against hypoxia-induced renal damage.
Material and methods:
Twelve Wistar rats were used in the study. Female Wistar rats were divided into two groups of six rats each; the first group served as the control, and the second group was treated for two days with oral lycopene (4 mg/kg per day) before surgery. All Wistar rats were subjected to right nephrectomy and abdominal aorta clamping for 45 minutes to induce ischemia, followed by 24 hours of reperfusion. Blood samples were collected from all rats twice before surgery and 24-hours after surgery for analyses of serum urea, creatinine, sodium, and potassium levels. Left nephrectomies were performed following reperfusion. Then histopathological scores were estimated, and malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD), glutathione (GSH), and glutathione peroxidase (GSH-Px) levels in renal tissue samples were measured, and compared between groups.
Results:
There were no significant differences between the control, and the lycopene group with respect to postischemic urea, creatinine, or potassium levels. A significant difference between the groups was observed with respect to postischemic sodium levels (p=0.028). Pathological scores were higher in the control group than in the lycopene group (p<0.05). Mean tissue MDA levels in the control group were higher than in the lycopene group (p=0.055). The mean tissue GSH-Px levels were similar in the control, and lycopene groups. The mean GSH levels in the control group were higher than in the lycopene group (p>0.05). The mean tissue SOD levels were similar in the control, and lycopene groups. The mean CAT levels in the control group were higher than in the lycopene group (p>0.05).
Conclusion:
Lycopene may have a protective effect on the short-term biochemical and histopathological parameters following renal ischemia/perfusion injury.
Keywords: Ischemia-reperfusion injury, lycopene, renal ischemia
Introduction
Nowadays, nephron-sparing surgeries are most frequently performed surgical modalities for the early diagnosis, and treatment of renal tumours, and with technological advances, frequency of laparoscopic approaches applied to these tumours has increased. During these surgeries, renal artery is temporarily occluded which might cause ischemia/reperfusion damage because of warm ischemia.[1]
Formation of free oxygen radicals caused by reperfusion after an ischemic episode plays important roles in the pathogenesis of acute ischemic renal failure. Increase in the levels of malondialdehyde (MDA) which is a marker of lipid peroxidation has been more frequently detected during reperfusion following an ischemic episode, and anti-oxidant agents demonstrated an alleviating effect on the tissue damage caused by these reactive oxygen radicals.[2] After removal of the causative agent of hypoxia, reperfusion starts to take effect. Polymorphonuclear leukocytes (PMNLs) migrate, and settle in the affected tissue, and release free oxygen radicals which aggravate destructive changes in the tissue. Excessive formation of oxygen radicals results in necrosis, protein denaturation, and DNA damage caused by various mechanisms including tissue damage, and peroxidation of membrane lipids.[3]
The effects of vitamin E therapy on renal histological changes developed during renal tissue damage induced by ischemia/reperfusion have been analyzed, and vitamin E therapy alleviated tissue damage associated with reperfusion.[2] Other therapeutic agents (incl. aminosteroids) currently used to prevent ischemia/reperfusion damage have not gained importance in clinical practice. Neither pentoxyfilline nor mannitol has a satisfactory attenuating impact on tissue damage incurred.[4] Lycopene has a protective effect against oxidative damage caused by DNA.[5] In chronic alcoholism it protects liver against the effects of apoptosis.[6] Besides, it has a tumour suppressive activity.[7] In the surgical treatment of prostate cancer, lycopene has demonstrated positive contributory effect on surgical margin negativity.[8] Besides it has a protective effect against cutaneous apoptosis induced by ultraviolet lights.[9] However, currently, any optimal technique which ideally prevents renal damage, and shortens duration of warm ischemia or any optimal agent protecting renal tissue is not available. Antioxidant characteristics of lycopene effective in tissues have been demonstrated following exposure to various oxidative agents including ferric nitrilotriacetate, gentamicin, cisplatinum, and alcohol.[6,10–12] However protective effect of lycopene against postoperative oxidative damage induced in renal tissue has not been cited in any literature study. To this end, herein, we have investigated short-term prophylactic effect of lycopene (if any) on renal ischemia/reperfusion damage.
Material and methods
For our study we obtained the written approval of the Adnan Menderes University, Institutional Ethics Committee of Animal Experiments (ADÜ-HADYEK) granted in the 5th Session with registration #: B.30.2.ADÜ.0.06.00.00/124-HEK/2008/009.
Preparation of the rats:
In this study 12 Wistar-Albino strain female rats weighing 190–250 g which were procured from the animal laboratory of our Faculty of Veterinary Science were used. Blood samples from tails of all rats which were kept in separate cages in the Laboratory of Experimental Animals of Faculty of Veterinary Science were drawn under room temperature, and transferred at +4 degres of ambient temperature for analysis of serum urea, creatinine, potassium, and sodium levels.
Rats in the control group (n=6) received 0.5 mL maize oil, and study group of rats were fed with 0.5 mL maize oil and lycopene (4 mg/kg bwt) given as a gavage solution once daily for two days.[11,12] Then the rats were deprived of food, and water for 6 hours, and general anesthesia was induced with a mixture of xylazine hydrocloride (8 mg/kg) and ketamine HCl (35 mg/kg) given intraperitoneally.
Experimental Model:
Through midline abdominal incision, intraperitoneal access was achieved, and intraabdominal visceral organs were deviated to the left, and covered with a packing impregnated with 0.9% Na Cl. To compensate for the insensible losses, 1 cc 0.9% NaCl solution was injected subcutaneously, and thermal pads containing active ingredients of iron, active carbon, and salt were placed under the rats to keep their body temperature at a certain level. Right kidneys of the rats were identified, separated, and freed from adjacent structures with blunt, and sharp dissection. Renal pedicle was elevated with suspension sutures, and ligated with 4/0 silk sutures. Then intraabdominal organs were deviated to the right side to identify the left kidney. Upper pole of the left kidney was freed from its attachments. Afterwards, abdominal aorta was localized, and clamped immediately below the insertion site of the superior mesenteric artery (Figure 1). After an ischemic period of 45 minutes[13] aortic clamp was opened. After reperfusion was ensured, peritoneum was closed with 4/0 continuous vicryl, and skin with intermittent 4/0 silk sutures.
Figure 1.
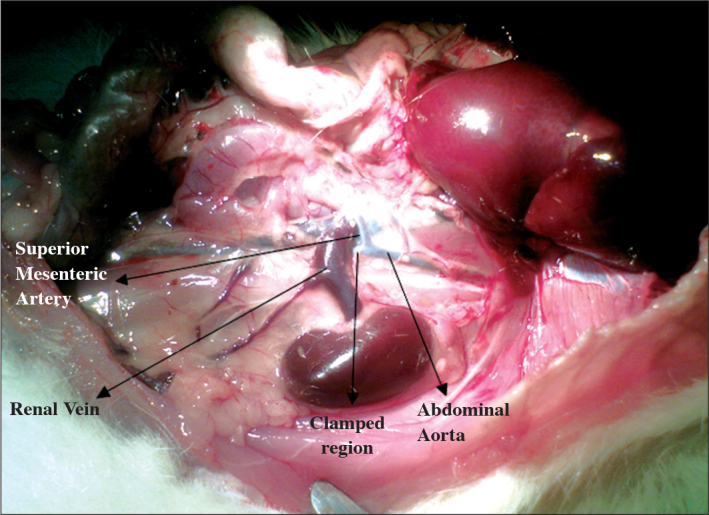
Renal vein. abdominal aorta. superior mesenteric artery. clamped region
Biochemical, and histopathological analysis:
After 24 hours of ischemia/reperfusion period, a mixture of xylazine hydrocloride (8 mg/kg bwt), and ketamine HCl (35 mg/kg bwt) was rein-stituted intraperitoneally to induce general anesthesia. Previous incision line was opened, and extended up to thorax. Then from all rats in both groups, 1.5 cc intracardiac blood samples were drawn to determine 24. hour serum urea, creatinine, Na+, and K+ values. Left nephrectomy was performed, and then the rats were sacrificed. Half of the renal tissue samples were immersed in 10% formaldehyde solution for histopathological examination. The remaining 50% of the samples were transferred under cold chain conditions, and kept at −85°C to be later analyzed using respective analytical methods for MDA (malondialdehyde; Ohkawa method), CAT (catalase; Aebi method), SOD (superoxide dismutase; Sun method), GSH (glutathion; Buetler method), and GSH-Px (glutathion peroxidase; Kakkar method). Cut surface of each renal tissue sample fixated in 10% formaldehyde in the pathology laboratory was sampled, and following routine tissue examination procedures, the specimens were embedded in paraffin blocks. From paraffin blocks 5-micron thick sections were cut. Cut sections were stained with hematoxylineosin (H&E) and PAS for histopathological evaluation, and Masson’s trichrome dye for the assessment the presence of fibrosis. Twenty fields of vision randomly selected from all H&E stained sections were examined under 20X magnification.
Tissue samples were examined as for the presence of glomerular changes, tubular dilation, cellular vacuolization, protein casts, necrosis, interstitial inflammation, and congestion, and percentage deviations from the normal histology were evaluated as follows:
0=Normal histology
1=<10%
2=10–50%
3=50–90%
4=>90% tissue damage.[13]
Histopathological scoring was based on morphologic features such as swelling of tubular cells, loss of brush-like edges of tubular cells, nuclear condensation, and loss of nuclei.
0=Normal morphology
1=<1/3
2=1/3–2/3
3=>2/3 morphologic loss in two thirds of the tissue.[14]
Tissue samples were homogenized at 4°C in 50 mM (1/10 g/mL) phosphate buffer (pH 7.4) containing a protease inhibitor phenylmethanesulphonyl fluoride (PMSF), (0.2 µM) and 1mM ethylenediamine tetra acetic acid (EDTA) Homogenates were centrifuged at 10.000 rpm for 5 minutes, and equal aliquots of supernatant were transferred into Ependorf tubes. The samples were frozen at −85°C for further analysis of other parametres (CAT, MDA, GSH, GSH-px, and SOD).
Statistical analysis
Mann-Whitney U, and Wilcoxon Tests were used. Wilcoxon test was employed for the statistical analysis of biochemical parameters (urea, creatinine, sodium, potassium), and intragroup comparisons of pre-, and post-ishemic values, and intergroup comparisons of parameters estimated after ischemia/reperfusion period.
Statistical, and comparative evaluation of the data retrieved from histopathological analysis of renal tissue specimens obtained from the rats in the control, and lycopene groups, and those related to enzymatic, and oxidative parameters (MDA, CAT, SOD, GSH, and GSH-Px) were performed using Mann-Whitney U Test.
P<0.05 was considered as the cut-off value for statistical significance. For statistical evaluation of the study outcomes SPSS 16 program was used.
Results
Biochemical findings:
A statistically significant difference was detected as for pre-, and post-ischemic mean urea, and creatinine values both in the control, and lycopene groups (Tables 1, 2).
Table 1.
Comparison of average pre-, and postischemic urea, creatinine (Cr), sodium, and potassium values of rats in the control group
| Control group | Urea (mg/dL) | Cr (mg/dL) | Sodium (mmol/L) | Potassium (mmol/L) |
|---|---|---|---|---|
| Preischemic period | 57.3 | 0.45 | 141.5 | 5.1 |
| Postischemic period | 148.8 | 1.17 | 133.6 | 4.85 |
| p | 0.046 | 0.027 | 0.028 | >0.05 |
Table 2.
Comparison of average pre-. and postischemic urea. creatinine (Cr). sodium. and potassium values of rats in the lycopene group
| Lycopene group | Urea (mg/dL) | Cr (mg/dL) | Sodium (mmol/L) | Potassium (mmol/L) |
|---|---|---|---|---|
| Preischemic period | 61.2 | 0.45 | 140.8 | 4.3 |
| Postischemic period | 159 | 1.37 | 137.6 | 5 |
| p | 0.046 | 0.046 | >0.05 | >0.05 |
A statistically significant difference was detected between pre-, and post-ischemic mean Na+ values measured in the control group (Table 1). Any difference between pre-, and post-ischemic mean Na+ values was not detected in the lycopene group (Table 2). Any difference between pre-, and postischemic mean potassium values of rats in the control, and the lycopene groups was not detected (Tables 1, 2).
Histopathological findings:
Histopathologically, renal tissue samples excised from the rats in the control, and the study groups were analyzed as for inflammation, congestion, tubular changes, dilatation of the Bowman’s capsule, proteinaceous casts, vacuolization, necrotic areas, and glomerular changes. Inflammation was not observed in the control, and study groups. Congestion was not seen in any of the rats in the lycopene group, while in all the (n=6; 100%) rats in the control group areas of congestion were detected with statistically significant difference between groups (p=0.001).
Various percentages of tubular changes were observed in the control, and lycopene groups (10–50%, in 83.3% of the rats, and <10% in 16.7% of the rats in the control; and 10–50% in 50%, and <10% in 50% of the rats in the lycopene groups, respectively) (p>0.05). Dilatation of the Bowman’s capsule was detected in all (100%) rats in the control group, while in 33.3% of the rats in the lycopene group any sign of dilatation was not found (p>0.05). Proteinaceous casts were found in the control, and lycopene groups (83.3, and 33.3%, respectively) (p>0.05). Tissue analysis of the rats in the control group revealed the presence of various percentages (50–90%) of areas of vacuolization in 16.7% of the rats in the control group. However in none of the rats in the lycopene group vacuolization was detected (p>0.05). Large areas of necrosis was observed in the control, and lycopene groups (33.3, and 16.7% of the rats, respectively (p>0.05).
Mean pathological scores of the rats inm the control, and lycopene groups were 2.17±0.41, and 1.5±0.55, respectively with a statistically significant difference between scores (p<0.05).
Histological findings:
Comparative mean values of MDA, CAT, SOD, GSH, and GSH-Px obtained from histological analysis of renal tissue of rats in the control, and the lycopene group following 45 minutes of ischemia, and 24 hours of reperfusion are presented in Table 3.
Table 3.
Intergroup comparison of average values of SOD. CAT. GSH. GSH-Px. and MDA as detected in histological analyses
| Histological analysis | SOD ng/g wet tissue | CAT U/g wet tissue | GSH mg/g wet tissue | GSH-Px mU/g wet tissue | MDA nmol/g wet tissue |
|---|---|---|---|---|---|
| Control group | 46.14 | 229.74 | 5.53 | 166.17 | 68.96 |
| Lycopene group | 45.65 | 231.28 | 3.43 | 177.57 | 28.97 |
| p | >0.05 | >0.05 | >0.05 | >0.05 | =0.055 |
As seen in Table 3, in our study, any statistically significant intergroup difference was not detected as for mean values of tissue enzyme levels. As an indicator of lipid peroxidation (ie. tissue damage) mean tissue MDA level which increases mostly following ischemia/reperfusion period was found to be nearly 2.4-fold higher in the control group relative to the lycopene group.
Discussion
Our study demonstrates that short-term lycopene administration before 45 minutes of renal ischemia, and 24 hours of reperfusion decreases renal damage.
Literature reviews have shown the development of renal failure after exposure of rats to various drugs (gentamicin, cisplatinum), and heavy metals. To alleviate renal damage incurred after exposure to these agents, attempts have been made to demonstrate the protective effect of a nonenzymatic, antioxidant agent lycopene on histopathologic changes, enzymatic changes in tissues, and serum renal function tests. It has been demonstrated that lycopene suppresses the impact of oxidative damage on liver in chronic alcoholism, and also tumoral activity, in addition to its ameliorating contribution to prostate cancer therapy, and favourable effect on apoptosis.[6–9] Various studies have shown that ischemia-reperfusion induced in LPN, and APN lead to renal oxidative damage. However, any study concerning the antioxidative effect of lycopene on oxidative damage induced following renal ischemia-reperfusion caused by surgical interventions has not been cited in the literature. In our study, protective effect of lycopene on oxidative renal damage occurred after induced renal hypoxia has been demonstrated, and since lycopene was the only variable in comparator groups, the group fed with maize oil was evaluated as a control group.
In our study, a statistically significant difference was detected between pre-, and post-ischemic urea, creatinine, and Na+ values in the control group of rats which we induced ischemia. This phenomenon demonstrates that our ischemia model can induce nephropathy with resultant abnormal serum urea, creatinine, and Na+ levels. We have observed that after induction of ischemia, preischemic urea, and creatinine levels of the rats fed with lycopene, could not be maintained with lycopene administration. However Na+ value in the lycopene group was not altered despite induction of ischemia. However, pre-, and postischemic K+ values did not differ statistically between both groups. Non-impairment of Na+ value after ischemia/reperfusion in the lycopene group has demonstrated protective effect of lycopene on tubular functions.
In recent years, free radicals which have been held responsible for the pathogenesis of many diseases, also gained importance as for complications after LPN, and APN. Some antioxidant enzymes (ie. SOD, GSH-Px, and CAT), GSH, thioles, anti-oxidant vitamins (ie. vitamin E, and C), trace elements (ie. selenium), and low-molecular weight compounds (ie. uric acid, bilirubin) are the most important components of the defense mechanisms. The critical role of free oxygen radicals formed after an ischemic episode in the pathogenesis of acute renal failure has been demonstrated. In addition, it has been indicated that levels of MDA which is an indicator of lipid peroxidation increases mostly during reperfusion following ischemia, and with antioxidant agents the severity of destructive changes might be alleviated.[2]
In our study, mean value of the antioxidant GSH-Px was higher in the lycopene group. Mean value of free oxygen radical, SOD was found to be higher than that of the control group. The difference between the control, and the lycopene groups as for SOD, CAT, GSH, and GSH-Px was at a significance level of p>0.05. Mean value of MDA which is an indicator of postischemic renal tissue damage was 68.96 nmol/g in the control group, while in the lycopene group where we applied lycopene during the postischemic period its mean value was relatively lower (ie. 28.97 nmol/g) (p=0.055), but without any statistical difference between groups. Extremely wider safety interval of lycopene, and increase in the antioxidant effect of lycopene with its gradual increase in its plasma concentration have been also demonstrated.[15,16] We think that our unique, pilot study which guides the way, and sheds light on this subject will demonstrate statistically significant difference between average tissue enzyme levels thanks to increase in the sampling size, duration of administration and/or dose of lycopene which is an antioxidant with wider safety margin. The most important justification of our assertion is that we have revealed the presence of a statistically significant difference between pathological scores of the groups.
Pathological scoring system has been used both to monitor renal tissue damage after ischemia-reperfusion period, and compare the control, and the study groups as for the severity of renal damage.[14]
Congestion was less frequently observed in rats fed with lycopene (p<0.001). Vacuolisation was not seen in rats fed with lycopene. Necrotic areas were less frequently encountered in the lycopene group. Formation of proteinaceous corpuscles was less often seen in the lycopene group without reaching statistical difference. Decreased incidence of tubular dilatation was observed in the lycopene group (p>0.05). As a consequence, pathological scoring which demonstrates cellular damage was detected to be lower in the lycopene group with a statistical difference between groups (p<0.05).
In conclusion, duration of cross-clamping was observed adequate to induce ischemia-reperfusion injury. Besides, among parameters of renal functions, levels of Na+ did not change, and preserved in the lycopene group. This phenomenon suggested certain protective role of lycopene on tubular functions. Accumulation of abnormal proteinaceous materials was more frequently detected in the renal tissue of rats of the control group (p<0.05). Mean value of MDA which exerts genotoxic, mutagenic, and carcinogenic effects at acellular level was found to be lower in the lycopene group (p=0.055). Similarly, mean value of an antioxidant enzyme, GSH-Px, was higher, while that of the oxidative enzyme, SOD was lower in the lycopene group. All of these findings have suggested lycopene as an effective molecule, however intergroup difference did not reach a statistically significant level. Lack of statistically significant difference between groups might be due to small sampling size of our study. When all these results were evaluated, we have concluded that lycopen might potentially protect renal tissues from ischemia/reperfusion injury in preoperatively selected cases among candidates for renal surgery.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Adnan Menderes University.
Informed Consent: This is a experimental studies so there was no patients permits.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.P.; Design - A.P., H.G.; Supervision - H.G.; Funding - A.P., H.G.; Materials - A.P., M.B.; Data Collection and/or Processing - A.P., H.G.; Analysis and/or Interpretation - A.P., H.G., M.B., C.Ü., Ç.Y., N.K., N.Ç.; Literature Review - A.P., H.G., M.B., Ç.Y., N.Ç.; Writer - A.P., H.G.; Critical Review - A.P., H.G., M.B., C.Ü., Ç.Y., N.K., N.Ç.; Other - A.P., C.Ü., Ç.Y., N.Ç.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Bilen CY. Laparoscopic radical, and nephron-sparing surgery. Üroonkoloji Kitabı. 2007;2:997–1010. [Google Scholar]
- 2.Selçuk NY, Yakan B, San A, Başoğlu M, Tonbul Z, Kızıltunç A, Gündoğdu C. The evaluation of lipid peroxidation and alpha-tocopherol treatment in experimental warm renal ischemia and reperfusion. Official Journal of the Turkish Nephrology Association. 1996;1:5–10. [Google Scholar]
- 3.Kehrer JP. Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol. 1993;23:21–48. doi: 10.3109/10408449309104073. [DOI] [PubMed] [Google Scholar]
- 4.Knochel JP, Carter NW. The role of muscle cell injury in the pathogenesis of acute renal failure after exercise. Kidney Int Suppl. 1976;6:58–64. [PubMed] [Google Scholar]
- 5.Porrini M, Riso P. Lymphocyte Lycopene concentration mad DNA protection from oxidative demage is increased in women after a short period of tomato consumption. J Nutr. 2000;130:189–92. doi: 10.1093/jn/130.2.189. [DOI] [PubMed] [Google Scholar]
- 6.Aşcıoğlu YT. The effect of lycopene on chronic alcoholic liver damage in rats. 2005. pp. 47–9. Sisli Etfal Training and Research Hospital, Department of Biochemistry.
- 7.Pool-Zobel BL, Bub A, Müller H, Wollowski I, Rechkemmer G. Consumption of vegetables reduces genetic damage in humans: first results of a human intervention trial with carotenoid-rich foods. Carcinogenesis. 1997;18:1847–50. doi: 10.1093/carcin/18.9.1847. [DOI] [PubMed] [Google Scholar]
- 8.Kucuk O, Sarkar FH, Sakr W, Djuric Z, Pollak MN, Khachik F, et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol Biomarkers Prev. 2001;10:861–8. [PubMed] [Google Scholar]
- 9.Rousseau EJ, Davison AJ, Dunn B. Protection by beta-carotene and related compounds against oxygen-mediated cytotoxicity and genotoxicity: Implications for carcinogenesis and anticarcinogenesis. Free Radic Biol Med Review. 1992;13:407–33. doi: 10.1016/0891-5849(92)90183-h. [DOI] [PubMed] [Google Scholar]
- 10.Matos HR, Capellozi VL, Gomes OF, Mascio PD, Medeiros MH. Lycopene inhibits DNA damage and liver necrosis in rats treadted with ferric nitrilotriacetate. Arch Biochem Biophys. 2001;396:171–7. doi: 10.1006/abbi.2001.2611. [DOI] [PubMed] [Google Scholar]
- 11.Karahan İ, Ateşşahin A, Yılmaz S. Protective effect of lycopene on gentamicin-induced oxidative stres and nephrotoxicity in rats. Toxicology. 2005;215:198–204. doi: 10.1016/j.tox.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Ateşşahin A, Yılmaz S, Karahan İ. Effects of lycopene against cisplatin-induced nephrotoxicity and oxidative stress in rats. Toxicology. 2005;212:116–23. doi: 10.1016/j.tox.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Williams P, Lopez H. Characterization of renal ıschemiareperfusion ınjury in rats. JPM. 1997;37:1–7. doi: 10.1016/s1056-8719(96)00141-4. [DOI] [PubMed] [Google Scholar]
- 14.Avlan D, Tamer L, Ayaz L, Polat A, Öztürk C, Özturhan H, et al. Effects of trapidil on renal ischemia-reperfusion injury. J Pediatr Surg. 2006;41:1686–93. doi: 10.1016/j.jpedsurg.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 15.Gerster H. The potential role of lycopene for human health. J Amer Coll Nutr. 1997;16:109–26. doi: 10.1080/07315724.1997.10718661. [DOI] [PubMed] [Google Scholar]
- 16.Jonker D, Kuper CF, Fraile N. Ninety day oral toxicity study of lycopene from Blakeslea trispora in rats Reg. Toxicology. 2003;37:396–406. doi: 10.1016/s0273-2300(03)00013-8. [DOI] [PubMed] [Google Scholar]


