Abstract
The causes of male erectile dysfunction (ED) are quite variable and are now commonly divided into etiologies such as ischemia, smooth muscle damage, or altered blood flow. Although varying rates of ED have been reported in literature, the number of men with ED is projected to increase worldwide by 2025 to approximately 322 million. Since the introduction of phosphodiesterase 5 (PDE5) inhibitors, there has been a paradigm shift in the treatment of ED because PDE5 inhibitors address a broad spectrum of etiologies for ED. Today, the American Urological Association recommends the use of three PDE5 inhibitors (sildenafil, tadalafil, and vardenafil) as a first-line therapy for the treatment of ED. This review evaluates the pharmacological mechanism of PDE5 inhibitors along with the impact and use of sildenafil, vardenafil, tadalafil, and avanafil. By increasing intracellular cGMP levels, PDE5 inhibitors have been shown to be effective in the treatment of ED. Through their effects on other cellular signaling pathways, PDE5 inhibitors have the potential for treating other urologic conditions as well. The use of PDE5 inhibitors can also be combined to produce a synergistic effect in conditions such as male hypogonadism and benign prostatic hyperplasia in addition to ED.
Keywords: Erectile dysfunction, PDE5 inhibitors, sildenafil
Introduction
Erectile dysfunction (ED) is defined by the National Institute of Health as the incapacity to achieve and sustain a satisfactory erection for sexual intercourse.[1,2] This was first described over 2000 years ago by the ancient Egyptians; from that time, our knowledge of penile anatomy and physiology has changed remarkably.[1,2] Today, it is recognized that ED has organic causes such as ischemia, smooth muscle damage, or altered blood flow.[3] Other causes of ED are androgen deficiency, performance anxiety, Alzheimer’s disease, Parkinson’s disease, and in many cases, prescription drugs.[1–3] A common tool to evaluate ED in clinical trials is the 15-item self-administered questionnaire, the International Index of Erectile Function (IIEF); however, is has a limited use in routine clinical practice.[1,3]
Varying rates of ED have been reported throughout literature. It is estimated that up to 49 million men in the United States have some degree of ED and that the number is currently over 152 million worldwide. The Massachusetts Male Aging Study, which was the first epidemiological study of ED, suggests that ED is commonplace affecting up to 52% of men.[3–9] Projections for 2025 indicate approximately 322 million men worldwide will have ED.[2,4,5]
In the past, pharmacological treatment of ED was limited to cavernosal or arterial injections or intraurethral agents. However, the oral treatment of ED has changed since the chemist Leopold Spiegel described the aphrodisiac effects of yohimbine in 1896.[3] In 1998, oral pharmacotherapy for ED underwent a drastic advancement with the availability of phosphodiesterase 5 (PDE5) inhibitors as sildenafil was introduced into the market.
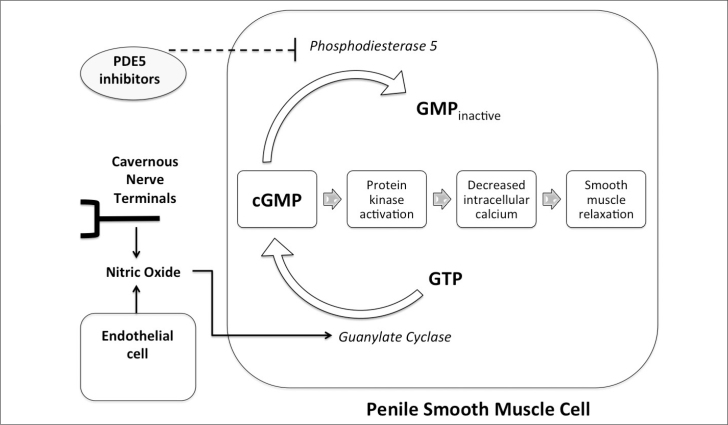
The phosphodiesterase inhibitors can be categorized into eleven different subtypes depending on the isozyme.[1,8] This enzyme is responsible for the degradation of 3′5′-guanosine monophosphate (cGMP), which in turn causes the deactivation of cGMP. Most human tissue expresses several types of phosphodiesterase isozymes. However, the human corpus cavernosum smooth muscle cells predominantly express the phosphodiesterase 5-isozyme subtype.[1,8] The smooth muscle dilation and vasodilation of blood vessels in the penis that cause an erection are regulated by nitric oxide and guanylate cyclase. The PDE5 inhibitors potentiate penile erections by increasing the levels of cGMP, as seen in Figure 1.[1,7,8] Although the currently available PDE5 inhibitors work in the same manner, they work at different plasma concentrations and more importantly vary in their degree of selectivity of the PDE5 isozyme. Because of this reason, patients complain of lumbago and myalgia with the inhibition of PDE11 and have visual color changes with the inhibition of PDE6.[1,3,8] The use of PDE5 inhibitors raises the concern that their interaction with organic nitrates can lead to an unsafe drop in blood pressure.[1,8] The American College of Cardiology recommends waiting 24 h prior to administering nitrates to patients who have taken sildenafil or vardenafil.[1] Patients’ who have taken tadalafil are recommended to wait 48 h for nitrate administration to avoid life-threatening cardiovascular problems.[1,3,6–8]
Figure 1.
Penile smooth muscle cell
With the increasing rates of ED worldwide and improved social networking, healthcare providers are increasingly prescribing PDE5 inhibitors and therapy for ED. Today, the American Urological Association (AUA) recommends the use of three PDE5 inhibitors (sildenafil, tadalafil, and vardenafil) as a first-line therapy for the treatment of ED. This review evaluates the urological impact and use of sildenafil, vardenafil, tadalafil, and avanafil.
Sildenafil
Sildenafil was the first oral PDE5 inhibitor used for the treatment of ED and has the largest clinical evidence to support of its use. It has a bioavailability of 40% and a peak plasma concentration of 1h.[1,2] After absorption, sildenafil is converted into an active metabolite N-desmethyl, which accounts for 20% of its pharmacological activity.[1,2] The half-life of both sildenafil and N-desmethyl metabolite is 4 h.[1] Absorption is slowed with the ingestion of food, especially a high fat meal.[9] Sildenafil is metabolized by the CYP3A4 and CYP2C9 pathways and has reduced clearance rates in volunteers older than 65 years.[1,2,9] Additionally, patients with hepatic cirrhosis were found to have elevated maximum concentrations. It is for this reason that the starting dose for men who are older than 65 and who have ED or severe cirrhosis is 25 mg 1 h prior to sexual activity.[1,9]
In clinical studies, sildenafil has been shown to improve IIEF scores especially in regard to questions on penetration and sexual intercourse.[1,8,9] Goldstein et al demonstrated a mean increase in achieving and maintaining erections from 60% to 130% above baseline on IIEF scores in a 24-week double-blind control study of 532 men.[10] Sildenafil has been shown to improve erections in 45–80% of all men with broad ED.[1,2,8] Specifically, studies have demonstrated a 90% response rate in men with depression and a 45% response rate after radical prostatectomy.[1,2] The efficacy of sildenafil in the management of ED in type I and II diabetes in a multicenter double-blind control study with dosing escalation demonstrated a 78% improvement in penetration frequency and a 93% increase in erection maintenance rates when compared with those using a placebo.[9]
Clinically, the chief adverse side effects in sildenafil trials are headache, dyspepsia, flushing, and abnormal vision.[9] Flushing and headache are attributed to the vasodilatory effect by sildenafil peripherally.[9] The 9% of men who have sensitivity to light and visual color changes are because in part of sildenafil’s weak affinity for PDE6 isozyme that is predominantly found in the photoreceptors of the eye.[1,9,10] Additionally, transient changes are seen in the electroretinogram, and patients with retinitis pigmentosa are advised to not take this medication.[9]
Vardenafil
Vardenafil is a potent PDE5 inhibitor that was Food and Drug Administration (FDA) approved to treat ED in 2003. Its chemical structure is very similar to that of sildenafil; however, it boasts a superior potency and selectivity (IC50 is 0.6 compared with 3.9 of sildenafil).[8] Like sildenafil, vardenafil has inhibitory effects on the PDE6 isoform (in the retina), which is responsible for the rare visual disturbance side effects. With respect to PDE6, the selectivity ratio of vardenafil is 15 compared with a selectivity ratio of approximately 7 for sildenafil. This translates to 15 times higher concentrations of vardenafil and seven times higher concentrations of sildenafil that are necessary to inhibit PDE6 in comparison to PDE5.[11,12] The half-life of vardenafil is 4 h, similar to that of sildenafil. It has an approximate duration of action of 6–8 h.
In general, vardenafil is well tolerated in healthy patients and patients with ED. In clinical trials, 22–61% of subjects reported adverse effects, increasing in a dose-dependent fashion. Most side effects were mild in severity, with headache and flushing being the most common.[5] In the largest single safety analysis trial of vardenafil, a trial of 762 subjects showed the most common side effects were headache (10–21%), rhinitis (9–17%), flushing (5–13%), and dyspepsia (1–6%).[13] Vardenafil, unlike other PDE5 inhibitors, causes lengthening of the QT interval. Therefore, it should not be taken by men taking other medications that prolong the QT interval or by men with a congenital prolonged QT interval.[14]
The efficacy and safety of vardenafil was previously demonstrated in a large population of patients and gave significantly better results than placebo in terms of successful intercourse attempts.[8] In one of the largest published studies to date, 805 men with ED were treated with 5 mg, 10 mg, or 20 mg of vardenafil or placebo. Significant increases in erectile function domain scores were seen in the 10 mg and 20 mg dosages (p<0.0001) [higher scores indicated greater erectile function].[13]
Tadalafil
Tadalafil is another selective PDE5 inhibitor that was approved by the FDA in 2003 and is recommended by the AUA as first-line treatment of ED. Its chemical structure is significantly different from that of sildenafil and vardenafil. In addition, it does not have inhibitory effects on the PDE6 isozyme. Therefore, there have been no significant visual side effects observed with tadalafil use, as seen with sildenafil and vardenafil.[5,14,15] Tadalafil does have affinity for the PDE11 isoform, which is expressed in the skeletal muscle, prostate, liver, kidney, pituitary gland, and testes. Some researchers believe that this may explain the back pain and myalgias that some patients experience while using this drug.[1,3,8,15,16]
The potency of tadalafil is similar to that of vardenafil (IC50 0.94 nM), and the plasma peak concentration is achieved approximately 2 h after oral dosing. The rate and extent of absorption is not reduced by fatty foods or alcohol differentiating it from sildenafil and vardenafil.[5] Tadalafil is predominantly metabolized via CYP3A4. Based on studies with drugs such as midazolam, lovastatin, theophylline, and warfarin, tadalafil does significantly inhibit or induce the clearance of drugs metabolized by CYP isoforms.[17] Tadalafil has a terminal half-life of 17.5 h and an approximate duration of action of 24–36 h. Unlike sildenafil and vardenafil, tadalafil is FDA approved for daily dosage (in addition to as needed), usually between 2.5–5 mg daily. The most frequent adverse effects noted with tadalafil use is headache, dyspepsia, and back pain.[5]
A recent large clinical trial with a total of 1112 men supported the efficacy of tadalafil. Men with mild to severe ED with numerous etiologies were evaluated at fixed doses of tadalafil and placebos. In this 12-week trial, Brock et al.[18] showed that tadalafil significantly enhances all efficacy outcomes. In total, 81% of men reported improved erections compared with 35% in the control group, and 75% of sexual intercourse attempts were successful.
Avanafil
In 2012, avanafil became the fifth and newest PDE5 inhibitor approved by the FDA for the treatment of ED. Avanafil (4-[(3-chloro-4-methoxybenzyl)amino]-2-[2-(hydro-xymethyl)-1-pyrrolidinyl]-N-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide;(S)-2-(2-hydroxymethyl-1-pyrrolidinyl)-4-(3-chloro-4-methoxybenzylamino)-5-[(2-pyrimidinylmethyl) carbamoyl]pyrimidine) is a pyrimidine derivative with a molecular weight of 483.95 Da.[6,7] Avanafil has been found in Phase III clinical trials to be a fast-acting and highly selective PDE5 inhibitor. Its oral bioavailability is 69%, and it has a peak plasma concentration of 35 min with a half-life of 1.5 h.[6] Unlike sildenafil, it has a diminished inhibition of PDE6 isozyme, and studies on anesthetized dogs demonstrate that it is less likely to affect retinal function.[6,7] Studies on both sildenafil and avanafil demonstrate decreases in mean arterial pressure with lessened effects in the avanafil group. Phase III clinical trials were recently completed evaluating avanafil in men with ED and a mean IIEF score of 12.7. This study was performed at three dosing ranges (50 mg, 100 mg, and 150 mg). The data exhibit dose-dependent increases in successful vaginal intercourse from 45% to 64%, 46% to 74%, and 48% to 77%, respectively, with increases in IIEF scores of 12.7–22.2.[6,7] Common side effects include headaches, flushing, nasal congestion, muscle cramps, and postural hypotension, which were seen at higher doses.[6,7] Clinical studies demonstrate that avanafil is a fast-acting PDE5 inhibitor with activity 15 min after administration.[6]
Synergistic Effects
PDE5 inhibitors have been shown to have a beneficial impact in patients with lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH).[19] While LUTS is ultimately a multifactorial and multidimensional problem, similarities between the pathophysiological mechanisms of LUTS and ED include the nitric oxide–cyclic guanosine monophosphate (NO/cGMP) pathway, RhoA/Rho-kinase signaling, pelvic ischemia, and autonomic adrenergic over activity.[19,20] These similarities may explain why PDE5 inhibitors such as tadalafil and sildenafil are successfully utilized in the treatment of BPH-related LUTS. Taken alone, PDE5 inhibitors reduce the International Prostate Score Symptoms scores by nearly three points compared with the scores using a placebo and demonstrate moderate synergy when taken in combination with α-blockers.[19,20] There is currently a lack of objectivity, however, pertaining to the effects of PDE5 inhibitors (i.e., tadalafil) on other quantifiable variables such as Qmax.[19,21] The AUA does not include PDE5 inhibitors in its clinical guidelines for LUTS, but the European Association of Urology lists the class of medications under “new emerging drugs” for the treatment of male LUTS.[22,23]
Another synergistic effect of PDE5 inhibitors has been demonstrated by their relationship with testosterone repletion therapy. It is known that testosterone deficiency leads to a reduction in nitric oxide synthetase expression and activity, which is one of the driving factors in erectile function.[24,25] Testosterone deficiency is most commonly defined at <300 ng/dL. This threshold is controversial because there is a variable response in patients to this value.[24,25] This reduction can be restored with testosterone therapy. Therapy can in fact enhance the response to PDE5 inhibitors in hypogonadal men with ED who failed previous monotherapy treatment with either testosterone or a PDE5 inhibitor alone.[26] In a systematic review, Alhathal et al.[27] found that in 14 studies, combination therapy of testosterone plus a PDE5 inhibitor (12 testosterone + sildenafil, 2 testosterone + tadalafil) demonstrated an overall efficacy of 34–100% in patients with ED who failed on either drug alone or in patients who had previously experienced suboptimal improvement on monotherapy. Recently, a multicenter, double-blind, placebo-controlled TADEST study examined 173 patients with low testosterone levels and ED.[26,27] Men who received 1% testosterone along with tadalafil had a significant increase in IIEF scores.[26,27] In addition to restoring sexual function, PDE5 inhibitors have also been shown to enhance the Leydig cell secretory function, increasing sperm function and aiding sperm motility.[28] The emerging studies point to the efficacy of combination therapy (testosterone supplementation and PDE5 inhibitor use) over monotherapy in the management of ED, especially in men who have a low testosterone level.
In conclusion, the causes of ED are variable, and since the introduction of PDE5 inhibitors, there has been a paradigm shift in the treatment of ED. With the increasing rates of ED worldwide PDE5 inhibitors will also play an important role in the management of ED across a broad spectrum of etiologies. Additionally, the emergence of newer more selective PDE5 inhibitors, which may cause patients with ED may prefer these drugs. Furthermore, with a growing body of knowledge revealing the potentiating effects of PDE5 inhibitors in the management of hypogonadism and BPH that the role of PDE5 inhibitors in urology has yet to be revealed.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - T.S.H., L.J.; Design - T.S.H., L.J.; Supervision - T.S.H., L.J.; Funding - T.S.H., L.J.; Materials - T.S.H., L.J.; Data Collection and/or Processing - T.S.H., L.J.; Analysis and/or Interpretation - T.S.H., L.J.; Literature Review - T.S.H., L.J.; Writer -T.S.H., L.J.; Critical Review - T.S.H., L.J.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Setter SM, Iltz JL, Fincham JE, Campbell RK, Baker DE. Phosphodiesterase 5 inhibitors for erectile dysfunction. Ann Pharmacother. 2005;39:1286–95. doi: 10.1345/aph.1E487. http://dx.doi.org/10.1345/aph.1E487. [DOI] [PubMed] [Google Scholar]
- 2.Lue TF, Hon S. Pathophysiology of erectile dysfunction. Campbell-Walsh Urology. 2000:688–70. [Google Scholar]
- 3.Houry SK, Harlip IS. Sexual medicine sexual dysfunction in men and women. 2010:19–22. [Google Scholar]
- 4.Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84:50–6. doi: 10.1046/j.1464-410x.1999.00142.x. http://dx.doi.org/10.1046/j.1464-410x.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- 5.Crowe SM, Streetman DS. Vardenafil treatment for erectile dysfunction. Ann Pharmacother. 2004;38:77–85. doi: 10.1345/aph.1D019. http://dx.doi.org/10.1345/aph.1D019. [DOI] [PubMed] [Google Scholar]
- 6.Limin M, Johnsen N, Hellstrom WJ. Avanafil, a new rapid-onset phosphodiesterase 5 inhibitor for the treatment of erectile dysfunction. Expert Opin Investig Drugs. 2010;19:1427–37. doi: 10.1517/13543784.2010.518955. http://dx.doi.org/10.1517/13543784.2010.518955. [DOI] [PubMed] [Google Scholar]
- 7.Alwaal A, Al-Mannie R, Carrier S. Future prospects in the treatment of erectile dysfunction: focus on avanafil. Drug Des Devel Ther. 2011;5:435–43. doi: 10.2147/DDDT.S15852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuthe A. Phosphodiesterase 5 inhibitors in male sexual dysfunction. Curr Opin Urol. 2003;13:405–10. doi: 10.1097/00042307-200309000-00008. http://dx.doi.org/10.1097/00042307-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Basu A, Ryder RE. New treatment options for erectile dysfunction in patients with diabetes mellitus. Drugs. 2004;64:2667–88. doi: 10.2165/00003495-200464230-00004. http://dx.doi.org/10.2165/00003495-200464230-00004. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med. 1998;338:1397–404. doi: 10.1056/NEJM199805143382001. http://dx.doi.org/10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- 11.Saenz de Tejada I, Frutos JA, Gaudo M, Florio V. Comparative selectivity: profiles of tadalafil, sildenafil and vardenafil using an in vitro phosphodiesterase activity assay. Int J Impot Res. 2002;14:20–32. [Google Scholar]
- 12.Porst H. IC351 (tadalafil, Cialis): update on clinical experience. Int J Impot Re. 2002;14:57–64. doi: 10.1038/sj.ijir.3900807. http://dx.doi.org/10.1038/sj.ijir.3900807. [DOI] [PubMed] [Google Scholar]
- 13.Hellstrom WJ, Gittelman M, Karlin G, Segerson T, Thibonnier M, Taylor T, et al. Vardenafil for treatment of men with erectile dysfunction: efficacy and safety in a randomized, double-blind, placebo-controlled trial. J Androl. 2002;23:763–71. [PubMed] [Google Scholar]
- 14.Carson CC., 3rd Cardiac safety in clinical trials of phosphodiesterase 5 inhibitors. Am J Cardiol. 2005;96:37–41. doi: 10.1016/j.amjcard.2005.07.010. http://dx.doi.org/10.1016/j.amjcard.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Eardley I, Cartledge J. Tadalafil (Cialis) for me with erectile dysfunction. Int J Clin Pract. 2002;56:300–4. [PubMed] [Google Scholar]
- 16.Brock GB. Tadalafil: a new agent for erectile dysfunction. Can J Urol. 2003;10:17–22. [PubMed] [Google Scholar]
- 17.Briganti A, Salonia A, Deho’ F, Zanni G, Barbieri L, Rigatti P, et al. Clinical update on phosphodiesterase type-5 inhibitors for erectile dysfunction. World J Urol. 2005;23:374–84. doi: 10.1007/s00345-005-0022-6. http://dx.doi.org/10.1007/s00345-005-0022-6. [DOI] [PubMed] [Google Scholar]
- 18.Brock GB, McMahon CG, Chen KK, Costigan T, Shen W, Watkins V, et al. Efficacy and safety of tadalafil for the treatment of erectile dysfunction: results of integrated analyses. J Urol. 2002;168:1332–6. doi: 10.1016/S0022-5347(05)64442-4. http://dx.doi.org/10.1016/S0022-5347(05)64442-4. [DOI] [PubMed] [Google Scholar]
- 19.Gacci M, Corona G, Salvi M, Vignozzi L, McVary KT, Kaplan SA, et al. A systematic review and meta-analysis on the use of phosphodiesterase 5 inhibitors alone or in combination with α-blockers for lower urinary tract symptoms due to benign prostatic hyperplasia. Eur Urol. 2012;61:994–1003. doi: 10.1016/j.eururo.2012.02.033. http://dx.doi.org/10.1016/j.eururo.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 20.Andersson KE, de Groat WC, McVary KT, Lue TF, Maggi M, Roehrborn CG, et al. Tadalafil for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: pathophysiology and mechanism(s) of action. Neurourol Urodyn. 2011;30:292–301. doi: 10.1002/nau.20999. http://dx.doi.org/10.1002/nau.20999. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan SA. Tadalafil for the treatment of benign prostatic hyperplasia: When the moment does not add up. Eur Urol. 2013;63:517–8. doi: 10.1016/j.eururo.2012.11.004. http://dx.doi.org/10.1016/j.eururo.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan S. Tadalafil for the treatment of benign prostatic hyperplasia: When the moment does not add up. Eur Urol. 2012:11–12. doi: 10.1016/j.eururo.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Clinical practice guidelines. Management of BPH. American Urological Association Web site 2010, revised. http://www.auanet.org/content/clinical-practice-guidelines/clinical-guidelines.cfm?sub=bph.
- 24.Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, et al. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2013;64:118–40. doi: 10.1016/j.eururo.2013.03.004. http://dx.doi.org/10.1016/j.eururo.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Mills TM, Stopper VS, Wiedmeier VT. Effects of castration and androgen replacement on the hemodynamics of penile erection in the rat. Biol Reprod. 1994;51:234–8. doi: 10.1095/biolreprod51.2.234. http://dx.doi.org/10.1095/biolreprod51.2.234. [DOI] [PubMed] [Google Scholar]
- 26.Penson DF, Ng C, Rajfer J, Gonzalez-Cadavid NF. Adrenal control of erectile function and nitric oxide synthase in the rat penis. Endocrinology. 1997;138:3925–32. doi: 10.1210/endo.138.9.5402. http://dx.doi.org/10.1210/endo.138.9.5402. [DOI] [PubMed] [Google Scholar]
- 27.Alhathal N, Elshal AM, Carrier S. Synergetic effect of testosterone and phophodiesterase-5 inhibitors in hypogonadal men with erectile dysfunction: A systematic review. Can Urol Assoc J. 2012;6:269–74. doi: 10.5489/cuaj.11291. http://dx.doi.org/10.5489/cuaj.11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dimitriadis F, Tsambalas S, Tsounapi P, Kawamura H, Vlachopoulou E, Haliasos N, et al. Effects of phosphodiesterase-5 inhibitors on Leydig cell secretory function in oligoasthenospermic infertile men: a randomized trial. BJU Int. 2010;106:1181–5. doi: 10.1111/j.1464-410X.2010.09243.x. http://dx.doi.org/10.1111/j.1464-410X.2010.09243.x. [DOI] [PubMed] [Google Scholar]