Abstract
Brain-derived neurotrophic factor (BDNF) and its receptors tyrosine kinase B (trkB), and cyclic adenosine monophosphate response element binding protein (CREB) have been suggested as the neurobiological risk factors causing depressive disorder. Serotonin (5-hydroxytryptamine, 5-HT) plays an important role in the pathogenesis of depression. We in-vestigated the effect of treadmill exercise on social interaction in relation with BDNF and 5-HT expressions following stress in rats. Stress was induced by applying inescapable 0.2 mA electric foot shock to the rats for 7 days. The rats in the exercise groups were forced to run on a motorized treadmill for 30 min once a day for 4 weeks. Social interaction test and western blot for BDNF, TrkB, pCREB, and 5-HT1A in the hippocampus were performed. The results indicate that the spend time with unfamiliar partner was decreased by stress, in contrast, treadmill exercise increased the spending time in the stress-induced rats. Expressions of BDNF, TrkB, and pCREB were decreased by stress, in contrast, treadmill exercise enhanced expressions of BDNF, TrkB, and pCREB in the stress-induced rats. In addition, 5-HT1A receptor expression was de-creased by stress, in contrast, treadmill exercise enhanced 5-HT1A expression in the stress-induced rats. In the present study, treadmill exercise alleviated stress-induced social interaction impairment through enhancing hippocampal plasticity and serotonergic function in the hippocampus. These effects of treadmill exercise are achieved through 5-HT1A receptor activation.
Keywords: Social interaction, Brain-derived neurotrophic factor, Tyrosine kinase B, CREP, 5-hydroxytryptamine 1A receptor, Treadmill exercise
INTRODUCTION
Mood change in sadness, misery, and unhappiness frequently occurs after disappointment and stressful events, and this continuous mood change is the main feature of depression in children and adolescence. Violence and bullying in school are the major stressful events causing depression in the children and adolescence (Goldstein et al., 2007; Grant et al., 2004; Kaltiala-Heino et al., 2010).
Malfunction of hippocampus is strongly associated with depression (Castrén et al., 2007; Michelsen et al., 2008). Brain derived neurotrophic factor (BDNF) and its receptors tyrosine kinase B (trkB), cyclic adenosine monophosphate response element binding protein (CREB) have been suggested as the neurobiological risk factors causing depressive disorder (Sulser, 2002; Mattson et al., 2004). BDNF, a member of the neurotrophin family factor, and trkB control neuronal plasticity that a play important role in the depression (Castrén and Rantamäki, 2010). Transcription factor CREB, one of the various transcription factors, binds as dimer to the cyclic adenosine monophosphate response (CRE). Over-expression of CREB in the hippocampus exerts anti-depressive effect (Chen et al., 2001).
Serotonin (5-hydroxytryptamine, 5-HT) plays an important role in the pathogenesis of depression (Cowen, 2008). Tryptophan hydroxylase (TPH) is the rate-limiting enzyme of 5-HT synthesis and modulates 5-HT activity in the brain. TPH regulates presynaptic 5-HT release by 5-HT1A autoreceptor. BDNF and serotonin are known to be synergistically regulated. BDNF enhances the growth and survival of 5-HT and 5-HT stimulates the expression of BDNF (Mattson et al., 2004). Interaction of synaptic plasticity and serotonergic system play key role in the depression.
The beneficial effects of physical exercise on brain functions have been reported in many studies. Exercise protects against the progress of several neurological diseases including Alzheimer’s disease (Adlard et al., 2005), Parkinson’s disease (Sung et al., 2012) and ischemic stroke (Willey et al, 2009). Exercise improves symptoms of anxiety and depression (Kim et al., 2015; Wipfli et al., 2011). However, little information is available regarding the effect of exercise on depression-related social interaction abnormality caused by stress during adolescence stage. In the present study, we investigated the effect of treadmill exercise on social interaction in relation with BDNF and 5-HT expressions following stress using young rats.
MATERIALS AND METHODS
Animals and treatments
Sprague-Dawley rats, weighing 100±10 g (5 weeks), were obtained from commercial breeder (Orient Co., Seoul, Korea) for the experiment. The experimental procedures were performed in accordance with the animal care guidelines of the National Institutes of Health (NIH) and the Korean Academy of Medical Sciences. The rats were housed under controlled temperature (20± 2°C) and lighting (07:00 to 19:00 h) conditions with food and water available ad libitum. The rats were randomly divided into 4 groups (n=10 in each group): control group, control and exercise group, stress-induced group, and stress-induced and exercise group.
Induction of stress
Stress was induced by applying inescapable 0.2 mA electric foot shock to the rats, as the previously described method (Kim et al., 2015; Li et al., 2011). One set of electric foot shock composed of 6 sec duration and repeated 10 times with 30 sec interval. The rats in the stress-induced groups received three sets of electric foot shock per day. Exposure of rats to the electric foot shock continued for 7 days.
Exercise protocol
The rats in the exercise groups were forced to run on a motorized treadmill for 30 min once a day for 4 weeks. The exercise load consisted of running at a speed of 5 meters/min for the first 5 meters/min, 8 meters/min for the next 5 min, and 15 meters/min for the last 20 min, with a 0° inclination. The rats in the control groups were left on the treadmill without running for the same period as the exercise groups.
Social interaction test
The social interaction test was performed, as the previously described method (Kim et al., 2014). Two weight matched rats, drawn from different cages, were used for this test. The normal rat was used as the dummy partner and the index rat was marked with an oil pen. The index rat was randomly assigned to an unfamiliar partner in the same treatment group, and the rats were then placed in the center of an open arena (length 85 cm×width 75 cm×height 30 cm). The animals were closely observed and the interactions exhibited by the index rat were recorded over a period of 7 min. The social behaviors (genital investigation, sniffing, following, grooming) demonstrated by the index rat were visually assessed throughout the duration of the test period. Aggressive behavior and passive contact were not counted in the score of social interaction test.
Western blot for BDNF, TrkB, pCREB, and 5-HT1A
Western blot for were performed according to the previously described method (Han et al., 2014; Jeon et al., 2014; Kim et al., 2012). The animals were sacrificed immediately after determination of social interaction test. The animals were fully anesthetized with Zoletil 50® (10 mg/kg, i.p.; Vibac, Carros, France), and hippocampus and dorsal raphe were collected, then immediately frozen at −70°C. Protein from each hippocampus and dorsal raphe were extracted. The tissues were homogenized with lysis buffer containing 50 mM Tris–HCl (pH 8.0), 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2·6H2O, 1 mM EGTA, 1 mM PMSF, 1 mM Na2VO4, and 100 mM NaF, then ultra- centrifuged at 50,000 rpm for 1 hr. Protein content was measured using a Bio-Rad colorimetric protein assay kit (Bio-Rad, Hercules, CA, USA). Protein (30 μg) was separated on SDS-polyacrylamide gels and transferred onto a nitrocellulose membrane. Anti-GAPDH antibody (1:3,000; Abcam, Cambridge, UK), anti- BDNF (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-TrkB (1:1,000; Santa Cruz Biotechnology), anti-pCREB antibody (1:1,000; Santa Cruz Biotechnology), anti-CREB antibody (1:1,000; Merck Millipore), and anti-5-HT1A (1:1,000; Abcam) were used as the primary antibodies. Horseradish peroxidase-conjugated anti-mouse antibody for β-actin, anti-rabbit antibody for GAPDH, BDNF, TrkB, CREB, and 5-HT1A, and anti-goat antibody for pCREB were used as the secondary antibodies. Experiments were performed in normal laboratory conditions and at room temperature, except for the transferred membranes. Transferred membranes were performed at 4°C with the cold pack and pre-chilled buffer. Band detection was performed using the enhanced chemiluminescence (ECL) detection kit (Amersham Pharmacia Biothech GmbH, Freiburg, Germany).
Data analysis
For confirming the expressions of BDNF, TrkB, pCREB, and 5-HT1A, the detected bands were calculated densitometrically using Molecular Analyst™ (Bio-Rad). The data were analyzed with one-way ANOVA and then Duncan post-hoc tests. All values are expressed as the mean±standard error of the mean (SEM), and P value<0.05 was considered significant.
RESULTS
Effect of treadmill exercise on social interaction
To evaluate the effect of treadmill exercise on social interaction, modified social interaction test was performed. The results of social interaction test are presented in Fig. 1. An average time of social interaction was 162.72±8.58 sec in the control group, 182.33 ±10.76 sec in the control and exercise group, 39.83±10.07 sec in the stress-induced group, and 83.90±11.21 sec in the stress-induced and exercise group. The results indicate that the spend time with unfamiliar partner was decreased by induction of stress (P< 0.05). In contrast, treadmill exercise increased the spending time with unfamiliar partner in the stress-induced rats (P<0.05).
Fig. 1.
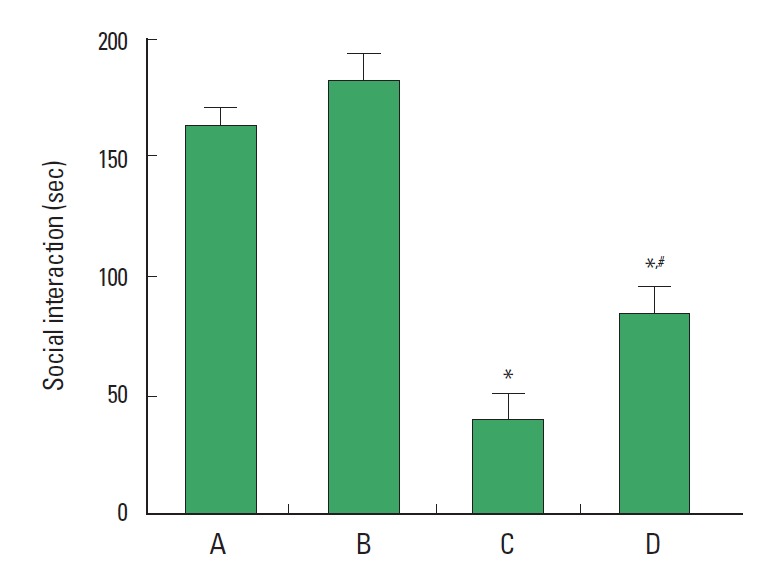
Effect of treadmill exercise on social interaction task. (A) Control group, (B) control and exercise group, (C) stress-induced group, (D) stress-induced and exercise group. The data are presented as the mean±standard error of the mean (SEM). *represents P<0.05 compared to the control group. #represents P<0.05 compared to the stress-induced group.
Effect of treadmill exercise on BDNF and TrkB expressions in hippocampus
BDNF and TrkB expressions are presented Fig. 2. When the level of BDNF in the control group was set at 1.00, the level of BDNF was 0.96±0.01 in the control and exercise group, 0.44± 0.02 for the stress-induced group, and 0.58±0.01 for the stress-induced and exercise group. When the level of TrkB in the control group was set at 1.00, the level of TrkB was 0.88±0.02 in the control and exercise group, 0.36±0.04 in the stress-induced group, and 0.73±0.02 in the stress-induced and exercise group. Expression of BDNF and TrkB in the hippocampus was decreased by induction of stress (P<0.05). In contrast, treadmill exercise enhanced expression of BDNF and TrkB in the stress-induced rats (P<0.05).
Fig. 2.
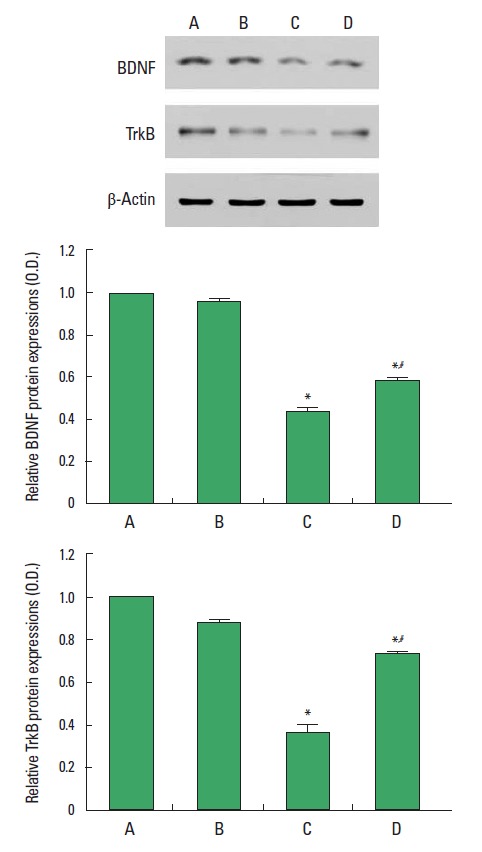
Effect of treadmill exercise on BDNF and TrkB expression in the hippocampus. (A) Control group, (B) control and exercise group, (C) stress-induced group, (D) stress-induced and exercise group. The data are presented as the mean±standard error of the mean (SEM). *represents P<0.05 compared to the control group. #represents P<0.05 compared to the stress-induced group.
Effect of treadmill exercise on pCREB expression in hippocampus
pCREB expression is presented Fig. 3. When the level of pCREB in the control group was set at 1.00, the level of pCREB was 0.96 ±0.04 in the control and exercise group, 0.66±0.03 in the stress-induced group, and 0.85±0.03 in the stress-induced and exercise group. Expression of pCREB in the hippocampus was decreased by induction of stress (P<0.05). In contrast, treadmill exercise enhanced pCREB expression in the stress-induced rats (P<0.05).
Fig. 3.
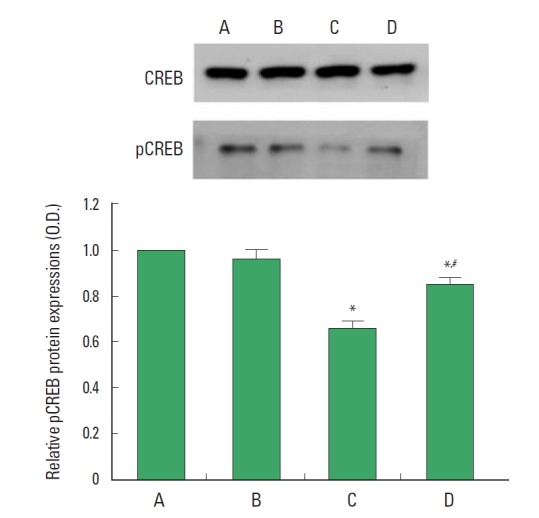
Effect of treadmill exercise on pCREB expression in the hippocampus. (A) Control group, (B) control exercise group, (C) stress-induced group, (D) stress- induced and exercise group. The data are presented as the mean±standard error of the mean (SEM). *represents P<0.05 compared to the control group. #represents P<0.05 compared to the stress-induced group.
Effect of treadmill exercise on 5-HT1A expression in hippocampus
5-HT1A expression is presented Fig. 4. When the level of 5-HT1A in the control group was set at 1.00, the level of 5-HT1A was 0.92 ±0.04 in the control and exercise group, 0.72±0.03 in the stress- induced group, and 0.86±0.02 for the stress-induced and exercise group. Expression of 5-HT1A in the hippocampus was decreased by induction of stress (P<0.05). In contrast, treadmill exercise enhanced 5-HT1A expression in the stress-induced rats (P<0.05).
Fig. 4.
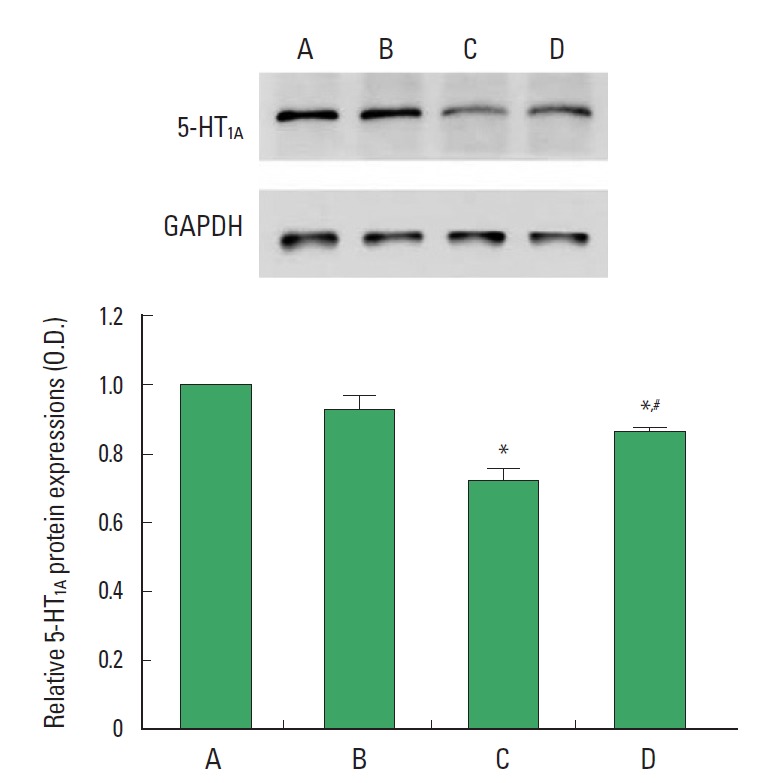
Effect of treadmill exercise on 5-HT1A expression in the hippocampus. (A) Control group, (B) control and exercise group, (C) stress-induced group, (D) stress-induced and exercise group. The data are presented as the mean±standard error of the mean (SEM). *represents P<0.05 compared to the control group. #represents P<0.05 compared to the stress-induced group.
DISCUSSION
Defect in the social interaction is the major symptom of depression, and it is related with impaired synaptic plasticity and serotonergic function (Mattson et al., 2004; Michelsen et al., 2008). In the preset study, stress-induced depressive rats showed less time with unfamiliar partner in the social interaction test.
Down-regulation of BDNF and trkB in the hippocampus is associated with depressive patients (Dwivedi et al., 2003; Lee et al., 2007). Alteration of synaptic plasticity causes depression (Duman, 2002), and increased CREB in the hippocampus produces anti-depressant like effects (Carlezon et al., 2005). Survival-promoting effect of hippocampal BDNF signaling by exercise is dependent on CREB, and activation of CREP is an important part of hippocampal survival-enhancing mechanism of anti-depressants (Chen and Russo-Neustadt, 2009). In the present study, expression BDNF, trkB, and pCREB were decreased in the hippocampus of stress- induced depressive rats.
5-HT from dorsal and median raphe ramifies to the most of the brain areas, and 5-HT is implicated in the cognitive and affective disorders, such as anxiety and depression (Cools et al., 2008). 5-HT receptors are also involved in the pathophysiology of depression, and 5-HT receptors are target for the mechanism of antidepressants. One of the 5-HT receptor subtypes, 5-HT1A receptor is mostly expressed in the mammalian brain (Albert and Lemonde, 2004). 5-HT1A receptor is especially expressed in the hippocampus, and suppressed 5-HT1A mRNA was observed in the patient with depression (López-Figueroa et al., 2004; Meltzer et al., 2004). Decrement in hippocampal 5-HT1A receptor function induced by mild stress in BDNF-deficient mice was prevented by administration of the selective serotonin reuptake inhibitor fluoxetine, which increased activation of TrkB (Burke et al., 2013). They suggested that decreased BDNF expression is implicated in the stress-related mental illness (Burke et al., 2013). In the present study, 5-HT1A expression in the hippocampus was decreased in the stress-induced depressive rats.
Exercise improved symptoms of depressive disorder patient (Dimeo et al., 2001), and regular exercise suppressed neuroticism, anxiety, and depression (DeMoor et al., 2006). In the present study, treadmill exercise increased the time with unfamiliar partner in the depressive rats, suggesting that treadmill exercise exerted anti-depressive effect.
Exercise influences brain plasticity by promoting neurogenerative, neuroadaptive and neuroprotective processes, and some of the process are regulated by neurotrophic factors (Ding et al., 2011; Huang et al., 2006; Kim et al., 2014). In addition, serotonin function is also enhanced by exercise (Kim et al., 2015; Korb et al., 2010). In the present study, treadmill exercise enhanced expressions of BDNF and pCREB in the hippocampus of the depressive rats. Treadmill exercise also enhanced 5-HT1A receptor expression in the hippocampus of the depressive rats.
The present results show that stress during induces impairment of social interaction through reduction of synaptic plasticity and serotonergic function in the hippocampus. Treadmill exercise alleviate s impairment of social interaction by enhancing hippocampal plasticity and serotonergic function in the hippocampus. These effects of treadmill exercise are achieved through 5-HT1A receptor.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2010-327-G00099).
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PR, Lemonde S. 5-HT1A receptors, gene repression, and depression: guilt by association. Neuroscientist. 2004;10:575–593. doi: 10.1177/1073858404267382. [DOI] [PubMed] [Google Scholar]
- Burke TF, Advani T, Adachi M, Monteggia LM, Hensler JG. Sensitivity of hippocampal 5-HT1A receptors to mild stress in BDNF-deficient mice. Int J Neuropsychopharmacol. 2013;16:631–645. doi: 10.1017/S1461145712000466. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev Neurobiol. 2010;70:289–297. doi: 10.1002/dneu.20758. [DOI] [PubMed] [Google Scholar]
- Castrén E, Võikar V, Rantamäki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7:18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Russo-Neustadt AA. Running exercise-induced up-regulation of hippocampal brain-derived neurotrophic factor is CREB-dependent. Hippocampus. 2009;9:962–972. doi: 10.1002/hipo.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Shirayama Y, Shin KH, Neve RL, Duman RS. Expression of the cAMP response element binding protein (CREB) in hippocampus produces an antidepressant effect. Biol Psychiatry. 2001;49:753–762. doi: 10.1016/s0006-3223(00)01114-8. [DOI] [PubMed] [Google Scholar]
- Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci. 2008;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Cowen PJ. Serotonin and depression: pathophysiological mechanism or marketing myth? Trends Pharmacol Sci. 2008;29:433–436. doi: 10.1016/j.tips.2008.05.004. [DOI] [PubMed] [Google Scholar]
- De Moor MH, Beem AL, Stubbe JH, Boomsma DI, De Geus EJ. Regular exercise, anxiety, depression and personality: a population-based study. Prev Med. 2006;42:273–279. doi: 10.1016/j.ypmed.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Dimeo F, Bauer M, Varahram I, Proest G, Halter U. Benefits from aerobic exercise in patients with major depression: a pilot study. Br J Sports Med. 2001;35:114–117. doi: 10.1136/bjsm.35.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Ying Z, Gómez-Pinilla F. Exercise influences hippocampal plasticity by modulating brain-derived neurotrophic factor processing. Neuroscience. 2011;192:773–780. doi: 10.1016/j.neuroscience.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Pathophysiology of depression: the concept of synaptic plasticity. Eur Psychiatry. 2002;3:306–310. doi: 10.1016/s0924-9338(02)00654-5. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- Goldstein AL, Walton MA, Cunningham RM, Trowbridge MJ, Maio RF. Violence and substance use as risk factors for depressive symptoms among adolescents in an urban emergency department. J Adolesc Health. 2007;40:276–279. doi: 10.1016/j.jadohealth.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Grant KE, Compas BE, Thurm AE, McMahon SD, Gipson PY. Stressors and child and adolescent psychopathology: measurement issues and prospective effects. J Clin Child Adolesc Psychol. 2004;33:412–425. doi: 10.1207/s15374424jccp3302_23. [DOI] [PubMed] [Google Scholar]
- Han JH, Kim DO, Yi JW, Park SW, Kang WJ, Choi YK, Kim SH, Ko IG, Jin JJ, Kim SE, Kim CJ. Dexmedetomidine, α2-adrenoceptor agonist, does not induce apoptosis in the brachial plexus of rats. Anim Cells Syst. 2014;18:407–415. [Google Scholar]
- Huang AM, Jen CJ, Chen HF, Yu L, Kuo YM, Chen HI. Compulsive exercise acutely upregulates rat hippocampal brain-derived neurotrophic factor. J Neural Transm. 2006;113:803–811. doi: 10.1007/s00702-005-0359-4. [DOI] [PubMed] [Google Scholar]
- Jeon JW, Lee JI, Shin HP, Cha JM, Joo KR, Kim SH, Ko IG, Jin JJ, Kim SE, Kim CJ. Adenosine A2A-receptor agonist polydeoxyribonucleotide promotes gastric ulcer healing in Mongolian gerbils. Anim Cells Syst. 2014;18:399–406. [Google Scholar]
- Kaltiala-Heino R, Fröjd S, Marttunen M. Involvement in bullying and depression in a 2-year follow-up in middle adolescence. Eur Child Adolesc Psychiatry. 2010;19:45–55. doi: 10.1007/s00787-009-0039-2. [DOI] [PubMed] [Google Scholar]
- Kim JE, Ji ES, Seo JH, Lee MH, Cho S, Pak YK, Seo TB, Kim CJ. Alcohol exposure induces depression-like behavior by decreasing hippocampal neuronal proliferation through inhibition of the BDNF-ERK pathway in gerbils. Anim Cells Syst. 2012;16:190–197. [Google Scholar]
- Kim TW, Kang HS, Park JK, Lee SJ, Baek SB, Kim CJ. Voluntary wheel running ameliorates symptoms of MK-801-induced schizophrenia in mice. Mol Med Rep. 2014;10:2924–2930. doi: 10.3892/mmr.2014.2644. [DOI] [PubMed] [Google Scholar]
- Kim TW, Lim BV, Baek D, Ryu DS, Seo JH. Stress-induced depression is alleviated by aerobic exercise through up-regulation of 5-hydroxytryptamine 1A receptors in rats. Int Neurourol J. 2015;19:27–33. doi: 10.5213/inj.2015.19.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Kim H, Park SH, Kim YK. Decreased plasma BDNF level in depressive patients. J Affect Disord. 2007;101:239–244. doi: 10.1016/j.jad.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Figueroa AL, Norton CS, López-Figueroa MO, Armellini-Dodel D, Burke S, Akil H, López JF, Watson SJ. Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biol Psychiatry. 2004;55:225–233. doi: 10.1016/j.biopsych.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Price JC, Mathis CA, Butters MA, Ziolko SK, Moses-Kolko E, Mazumdar S, Mulsant BH, Houck PR, Lopresti BJ, Weissfeld LA, Reynolds CF. Serotonin 1A receptor binding and treatment response in late-life depression. Neuropsychopharmacology. 2004;29:2258–2265. doi: 10.1038/sj.npp.1300556. [DOI] [PubMed] [Google Scholar]
- Michelsen KA, Prickaerts J, Steinbusch HW. The dorsal raphe nucleus and serotonin: implications for neuroplasticity linked to major depression and Alzheimer’s disease. Prog Brain Res. 2008;172:233–264. doi: 10.1016/S0079-6123(08)00912-6. [DOI] [PubMed] [Google Scholar]
- Sulser F. The role of CREB and other transcription factors in the pharmacotherapy and etiology of depression. Ann Med. 2002;34:348–356. doi: 10.1080/078538902320772106. [DOI] [PubMed] [Google Scholar]
- Sung YH, Kim SC, Hong HP, Park CY, Shin MS, Kim CJ, Seo JH, Kim DY, Kim DJ, Cho HJ. Treadmill exercise ameliorates dopaminergic neuronal loss through suppressing microglial activation in Parkinson’s disease mice. Life Sci. 2012;91:1309–1316. doi: 10.1016/j.lfs.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Willey JZ, Moon YP, Paik MC, Boden-Albala B, Sacco RL, Elkind MS. Physical activity and risk of ischemic stroke in the Northern Manhattan Study. Neurology. 2009;73:1774–1779. doi: 10.1212/WNL.0b013e3181c34b58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipfli B, Landers D, Nagoshi C, Ringenbach S. An examination of serotonin and psychological variables in the relationship between exercise and mental health. Scand J Med Sci Sports. 2011;21:474–481. doi: 10.1111/j.1600-0838.2009.01049.x. [DOI] [PubMed] [Google Scholar]


