Abstract
Regular running and strength training are the best ways to improve aerobic capacity and develop the size of skeletal muscles. However, uncontrolled physical activities can often lead to an undertraining or over-training syndrome. In particular, overtraining causes persistent fatigue and reduces physical performance due to changes in the various physiological and immunological factors. In this study, we gave an exhaustive submaximal endurance or resistance exercise to participants and investigated the relationship between physical stress (cortisol level in blood), oxidative stress (intracellular ROS accumulation), and adaptive immune response (CD4:CD8 ratio).
Materials and Methods
Ten male volunteers were recruited, and performed a submaximal endurance or resistance exercise with 85% of VO2max or 1-repetition maximum until exhaustion. Blood samples were collected at rest, and at 0 and 30 min after the exercise. Cortisol levels, oxidative stress, and immune cell phenotypes in peripheral blood were evaluated. Cortisol levels in the sera increased after the exhaustive endurance and resistance exercises and such increments were maintained through the recovery. Intra-cellular ROS levels also increased after the exhaustive endurance and resistance exercises. The ratio of CD4+ T cells to CD8+ T cells after each type of submaximal exercise decreased compared with that at the resting stage, and returned to the resting level at 30 min after the exercise. In this study, an exhaustive endurance or a resistance exercise with submaximal intensity caused excessive physical stress, intra-cellular oxidative stress, and post-exercise immunosuppression. This result suggests that excessive physical stress induced temporary immune dysfunction via physical and oxidative stress.
Keywords: CD4:CD8 ratio, Cortisol, Exercise, Immunosuppression, Reactive oxygen species
INTRODUCTION
Recently, an increasing number of people are participating in physical activities due to today’s increased level of income and leisure time. According to the “2013 National Survey on Physical Activity Participation”, 45.5% of the population perform physical exercise at least once a week and in terms of physical activities, aerobic exercise such as walking and resistance training such as bodybuilding are the most popular types of physical activities.
Aerobic exercise is a physical activity that meets the energy provision via the oxidative energy system during the exercise (Basavarajaiah et al., 2008). Regular aerobic exercise, also known as “cardio”, improves cardiovascular endurance by increasing the stroke volume, cardiac output, and arteriovenous oxygen difference. Furthermore, it indirectly reduces body fat and the incidence rate of cardiovascular disease and disorders (Takeshima et al., 2004). Resistance training improves muscular strength and muscular endurance by changing neural adaptation, muscle hypertrophy, and hyperplasia; it also has an effect on body composition changes by increasing the resting metabolic rate. However, regardless of exercise types, high-intensive exercise decreases physical ability due to ongoing fatigue. Intensive training causes chronic exposure to stress-induced hormones such as cortisol that can induce chronic fatigue (Prieto-Hinojosa et al., 2014). The level of reactive oxygen species (ROS), included in free radical and non-free radical oxygen intermediates, also increases during high-intensive exercise. A rapid increase of ROS surpasses the capacity of antioxidants that eliminate the ROS, thus causing an imbalance of the oxidants-antioxidants and damage to the cells and tissues, thereby causing oxidative stress (Radak et al., 2008). The oxidative stress contributes to fatigue during strenuous exercise (Reid, 2008). Elements of the immune system are temporarily depressed during high-intensive exercise. The post-exercise immunosuppression links to an increased susceptibility of infection such as upper respiratory tract infection. Immunological changes can be utilized as a tool for measuring physical stress such as exercise intensity, duration, and type during a certain exercise. Long-term regular physical exercise immediately increases adaptive immune response while intermittent high-intensive exercise rather decreases the adaptive immune response (Kwak et al., 2000; Walsh et al., 2011).
In this study, we gave an exhaustive submaximal endurance or resistance exercise to participants and investigated the relationship between physical stress (cortisol level in blood), oxidative stress (intracellular ROS accumulation), and adaptive immune response-a ratio of helper T cells to suppressor T cells (CD4:CD8 ratio).
MATERIALS AND METHODS
Subjects
Ten male volunteers who were all healthy and not currently taking any medication were recruited under conditions approved by the Institutional Review Board of Yonsei University for the protection of human subjects. Informed consent was obtained from all of the volunteers.
Scheme of study
In order to avoid interference among the tests, the participants inconsecutively visited the laboratory once a week for 4 weeks. (1) Basic physical characteristics (height, weight, percentage body fat, and VO2max) were measured at their first visit. (2) On the second visit, subjects performed a submaximal endurance exercise with 85% of maximal oxygen consumption (VO2max) until exhaustion. (3) Their maximal strength in 5 different weight training programs (bench press, seated long full, 45 degree sit-up, leg extension and leg curl) was measured at their third visit. (4) In the last visit, subjects performed a submaximal resistance exercise with 85% of one repetition maximum (1-RM) until exhaustion.
Preliminary measurement of baseline characteristics
All participants performed preliminary tests for analysis of their physical characteristics (height, weight, and percentage body fat). To measure maximal aerobic power (VO2max and HRmax), subjects performed a maximal test on a Q65 treadmill (Quinton, Seattle, WA, USA) according to the Bruce protocol (American College of Sports Medicine. 2010). The ratings of perceived exertion (RPE) (Borg’s RPE scale) were used as an indication of impending fatigue (Borg, 1982). VO2 and heart rate (HR) during exercise were measured using the MetaMax 3B (Cortex, Leipzig, Germany) and Polar T31 transmitter (Polor Electro, Lake Success, NY, USA), respectively. VO2 and HR were analyzed using the MetaSoft Ver. 3.9.5 (Cortex). The maximum strength in weight training was measured by calculating 1-RM, including five different stations of a resistance exercise: bench press, seated cable row, 45 degree sit-up, leg extension, and leg curl.
Submaximal endurance exercise with 85% of VO2max
According to the Bruce protocol, subjects ran on the treadmill until their oxygen intake reached 85% of VO2max. The gradient and speed of the 85% of VO2max were maintained until they were exhausted. The exercise intensity during the endurance exercise was fixed at 85% VO2max and was calculated using the HR reserve method (Karvonen et al., 1957). In order to predict signs of exhaustion, the oxygen consumption, HR, and Borg’s RPE scale were monitored every 5 min during the submaximal endurance exercise.
Submaximal resistance exercise with 85% of 1-RM
Following the 1-RM test, submaximal loads, which would elicit approximately 85% of maximal strength, were selected for the submaximal resistance exercise program. The submaximal resistance exercise was composed of five consecutive sets of exercise circuits: bench press, seated cable row, 45 degree sit-up, leg extension, and leg curl. Each exercise circuit was performed 8 times in 1 set followed by 30 sec of recovery until the participant was exhausted.
Blood sampling
Peripheral venous blood was collected from the antecubital vein of the subjects. Plain and heparin-containing vacuum tubes were used to isolate sera and peripheral blood lymphocytes (PBLs), respectively. To isolate PBLs, Ficoll-Hypaque density centrifugation was performed. The heparinized blood was diluted with the same amount of PBS, overlaid on a Ficoll, and centrifuged at 800×g continuously for 10 min. The interphase was transferred to another tube containing the PBS, mixed by inverting the tubes, and then centrifuged at 500×g continuously for 10 min. The supernatant was discarded and the cell pellet was suspended with PBS.
Blood cortisol levels
Blood samples were collected from each subject at rest, and at 0 and 30 min after the exercise. The sera isolated from the blood were analyzed using COBRA 5010 Quantum (Packard Inc., Meriden, CT, USA). Peripheral blood was spun at 1,000×g for 30 min at 4°C. The serum was separated and immediately stored at −70°C until analysis. The measurements of the blood cortisol levels were duplicated.
Flow cytometric analysis
To analyze the ROS positive cells and T cell phenotypes (CD4 or CD8 positive), the isolated PBLs were incubated with antibodies labeled with fluorochromes in a fluorescence-activated cell sorting (FACS) buffer (1% FBS and 0.05% sodium-azide contained PBS) for 30 min on ice, washed twice with the FACS buffer, measured using a BD LSR II (BD Bioscience, San Jose, CA, USA), and analyzed using BD FACS Diva (BD Biosciences) or FlowJo software (Three Star Inc., Ashland, OR, USA). The antibodies used for the immune cell phenotyping were as follows: FITC-anti-mouse-CD3, PE-anti-mouse-CD4, PerCP-Cy5.5-anti-mouse-CD8, and APC-anti-mouse CD45 (BD Pharmingen, SanDiego, CA, USA). The intracellular ROS production was estimated by fluorescence using 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA; Molecular Probes, Eugene, OR, USA) and expressed as a relative median fluorescence intensity (MFI) ratio, calculated relative to the “rest” stage.
Statistical analysis
The data are expressed as mean±standard deviation (SD). The significance of differences of the mean values among the groups were determined by one-way analysis of variance (ANOVA) followed by the Scheffé post-hoc test. Statistical significance was set at P<0.05.
RESULTS
Subjects suitable for high-intensity physical activities
Subjects who participated in this study were male college students majoring in physical education. They were all healthy adults in their early 20s who performed well in a high-intensity endurance and resistance exercise until exhausted. Their average levels of VO2max and HRmax were 53.99±6.34 mL/kg/min and 186.2±7.61 bpm, respectively (Table 1). The maximum strength in weight training involved five different stations of resistance exercises as follows: bench press, 65.5±11.47 kg; seated cable row, 148.5±12.03 kg; 45 degree sit-up, 15±4.41 kg; leg extension, 198.3±17.33 kg; and leg curl, 124±15.78 kg (Table 2).
Table 1.
Physical characteristics of the subjects
| Characteristics | Values* |
|---|---|
| Age (yr) | 20.8±1.4 |
| Height (cm) | 174.61±3.75 |
| Weight (kg) | 70.54±6.58 |
| %Body fat | 13.16±3.76 |
| VO2max (mL/kg/min) | 53.99±6.34 |
| HRrest (bpm) | 63.4±6.19 |
| HRmax (bpm) | 186.2±7.61 |
All values are mean±SD of 10 independent subjects.
Table 2.
Resistance training program*
| Variables | 1-RM (kg) | Resistance training program† | |
|---|---|---|---|
| Repeated time | Set‡ | ||
| Bench press | 65.5±11.47 | 8 | 5.6±1.78 |
| Seated long full | 148.5±12.03 | 8 | 7.8±2.20 |
| 45 degree sit-up | 15±4.41 | 8 | 5.1±0.57 |
| Leg extension | 198.3±17.33 | 8 | 9.9±3.67 |
| Leg curl | 124±15.78 | 8 | 7.4±2.72 |
All values are mean±SD of 10 independent subjects;
Resistance training was performed at 85% of 1-RM until
exhausted.
Exhaustive physical activities with high intensity are induced stress and fatigue
As an exhaustive endurance exercise model that would induce fatigue, the subjects performed a submaximal endurance exercise with 85% of VO2max until exhaustion. Their average running time was 28.85±4.74 min. Their heart rates dramatically increased during exercise, and hit a plateau 10 min after exercise (Fig. 1A). The cortisol levels in the sera also significantly increased after the exhaustive endurance exercise and such increments were maintained through the recovery (P<0.05; Fig. 1B).
Fig. 1.
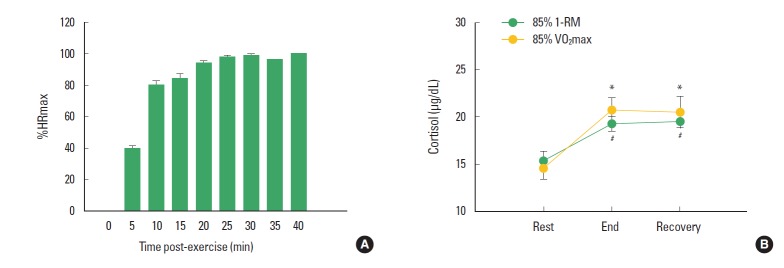
Physiological changes in the exhaustive submaximal endurance and resistance exercises. (A) Heart rate at rest and after the exhaustive submaximal endurance exercise. (B) Cortisol levels in the sera at rest, and 0 and 30 min after the submaximal endurance and resistance exercises. *represents statistical significance with “rest” (P<0.05). # represents statistical significance with “rest” (P<0.01). %HRmax: percent of maximal heart rate. 85% VO2max: 85% of maximal oxygen consumption. 85% 1-RM: 85% of one repetition maximum. Recovery: time point at 30 min after each exercise.
In the submaximal resistance exercise with 85% of 1-RM, the subjects performed 5 different weight training programs (bench press, seated long full, 45 degree sit-up, leg extension, and leg curl) until exhaustion, and the number of sets (8 times per set) is shown in Table 2. The cortisol levels in the sera also significantly increased after the exhaustive resistance exercise and such increments were maintained through the recovery (P<0.01; Fig. 1B).
Exhaustive physical activities causes a severe oxidative stress
In order to investigate whether the physical stress of such an exhaustive exercise induces cellular damage, the ROS levels in PBLs were measured during submaximal exercise with 85% intensity. The submaximal endurance exercise as well as the submaximal resistance exercise markedly increased the ROS levels in PBLs at the end of the exercises (P<0.05; Fig. 2).
Fig. 2.
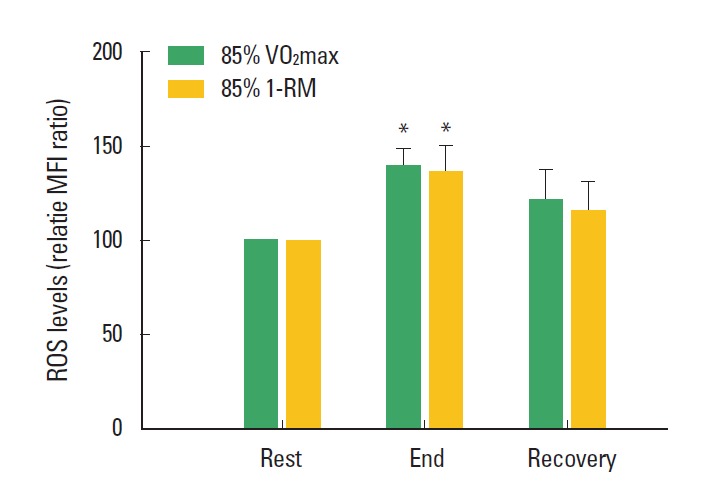
Changes of an intracellular oxidative stress during the exhaustive submaximal endurance and resistance exercises. The populations of the ROS positive peripheral blood lymphocytes were measured by flow cytometric analysis. *represents statistical significance with “rest” (P<0.05). ROS: reactive oxygen species. Relative MFI ratio: relative median fluorescence intensity to “rest” stage. 85% VO2max: 85% of maximal oxygen consumption. 85% 1-RM: 85% of one repetition maximum. Recovery: time point at 30 min after each exercise.
Physical stress alters T cell phenotypes
T cell phenotypes such as helper and cytotoxic T cells are important leukocytes in the adaptive immune system for maintaining homeostasis of an organism; the ratio of the T cell phenotypes is one of the criteria for determining the health condition. The exercises presented in this study were sufficient to induce physical stress, and were suitable for researching the relationship between the ratio of the T cell phenotypes and the health condition. Both the submaximal endurance exercise and the submaximal resistance exercise significantly increased the CD8+ T cell population compared with that in the resting stage (P<0.05 in the submaximal endurance exercise and P<0.01 in the submaximal resistance exercise), but did not affect changes in the CD4+ T cell population (Fig. 3A and B). The CD8+ T cell population returned to the resting level at 30 min after the exercises (P<0.01). The ratio of CD4+ T cells to CD8+ T cells (CD4:CD8 ratio) after each type of submaximal exercise decreased compared with that at the resting stage (P<0.05 in the submaximal endurance exercise). The CD4:CD8 ratio returned to the resting level at 30 min after the exercises (Fig. 3C).
Fig. 3.
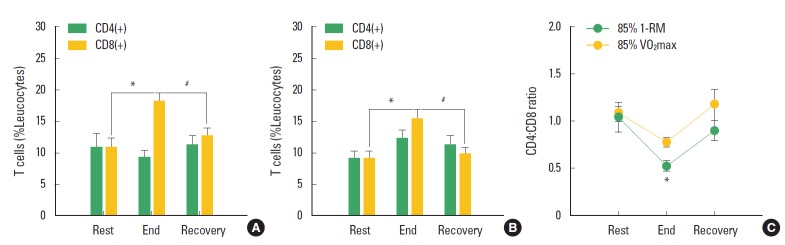
Changes of T cell phenotypes during the exhaustive submaximal endurance and resistance exercises. (A) CD4(+) and CD8(+) T cell populations in peripheral blood lymphocytes at rest, and 0 and 30 min after the submaximal endurance exercises. (B) CD4(+) and CD8(+) T cell populations in peripheral blood lymphocytes at rest, and 0 and 30 min after the submaximal resistance exercises. (C) CD4:CD8 ratio at rest, and 0 and 30 min after the submaximal endurance and resistance exercises. * represents statistical significance between “rest” and “end” (P<0.05). ** represents statistical significance between “rest” and “end” (P<0.01). # represents statistical significance between “end” and “recovery” (P<0.01). CD4(+): CD4 positive helper T cells. CD8(+): CD8 positive cytotoxic T cells. CD4:CD8 ratio: the ratio of CD4 (+) to CD8(+). 85% VO2max: 85% of maximal oxygen consumption. 85% 1-RM: 85% of one repetition maximum. Recovery: time point at 30 min after each exercise.
DISCUSSION
Aerobic exercise and resistance exercise are typical types in physical exercises. Proper exercises for strengthening the cardiovascular system and the skeletal muscles are needed by healthy people to improve and maintain their health (Haskell et al., 2007). However, inappropriate exercises negatively influence physiological health (Ho et al., 2012) as well as other body systems, such as endocrine (Davies et al., 1982) and immune systems (Gholamnezhad et al., 2014).
In this study, we confirmed experimentally that an exhaustive endurance or a resistance exercise with submaximal intensity caused excessive physical stress, intracellular oxidative stress, and post-exercise immunosuppression.
A number of studies have already been performed to determine whether the exercise regulates or causes physical stress. However, it is not clear how exercise changes the immune system. Several researchers have reported that light to moderate exercises enhance immunity but prolonged and severe exercises cause temporary immunosuppression (Kazeem et al., 2012; Zhao et al., 2012). T lymphocytes are part of the adaptive immune system and play an important role in maintaining self-regulation against the external environment. Clinically, abnormal T cell populations and dysfunction are often the cause of certain diseases, such as AIDS, hereditary deficiency of T cells, T-cell lymphoma, and T-cell exhaustion caused by sepsis and viral infection. Recent reports also demonstrated that the CD4:CD8 ratio does not reduce below 1.0 in healthy people (Nigam et al., 2011). However, in the case of AIDS patients (O’Gorman and Zijenah, 2008), as well as athletes such as elite swimmers (Mackinnon et al., 1997) or Taekwondo athletes (Lee et al., 2012), the CD4:CD8 ratio was inverted.
Our results also suggested that the exhaustive endurance or resistance exercise reduced the CD4:CD8 ratio to less than 1. Oxidative stress produced by the immune system participates in cellular and DNA damage, and causes immunomodulation. Previous reports showed that older individuals (> 60 yr) as well as elite Taekwondo athletes after competitions with inverted CD4:CD8 ratio also exhibited an excessive oxidative stress in their periphery (Lee et al., 2012; Muller et al., 2015). In our results, CD4:CD8 ratio as well as ROS levels in the PBLs decreased immediately after the exhaustive exercises, but returned to normal levels at recovery.
In conclusion, an exhaustive endurance or a resistance exercise with submaximal intensity caused excessive physical stress, intracellular oxidative stress, and post-exercise immunosuppression. This suggests that excessive physical stress induces temporary immune dysfunction via physical and oxidative stress.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2013R1A1A2061340).
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- Basavarajaiah S, Boraita A, Whyte G, Wilson M, Carby L, Shah A, Sharma S. Ethnic differences in left ventricular remodeling in highly-trained athletes relevance to differentiating physiologic left ventricular hypertrophy from hypertrophic cardiomyopathy. J Am Coll Cardiol. 2008;51:2256–2262. doi: 10.1016/j.jacc.2007.12.061. [DOI] [PubMed] [Google Scholar]
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- Gholamnezhad Z, Boskabady MH, Hosseini M, Sankian M, Khajavi Rad A. Evaluation of immune response after moderate and overtraining exercise in wistar rat. Iran J Basic Med Sci. 2014;17:1–8. [PMC free article] [PubMed] [Google Scholar]
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: Updated recommendation for adults from the american college of sports medicine and the american heart association. Med Sci Sports Exerc. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- Ho TJ, Huang CC, Huang CY, Lin WT. Fasudil, a rho-kinase inhibitor, protects against excessive endurance exercise training-induced cardiac hypertrophy, apoptosis and fibrosis in rats. Eur J Appl Physiol. 2012;112:2943–2955. doi: 10.1007/s00421-011-2270-z. [DOI] [PubMed] [Google Scholar]
- Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35:307–315. [PubMed] [Google Scholar]
- Kazeem A, Olubayo A, Ganiyu A. Plasma nitric oxide and acute phase proteins after moderate and prolonged xercises. Iran J Basic Med Sci. 2012;15:602–607. [PMC free article] [PubMed] [Google Scholar]
- Kwak YS, Lee SK, Paik IY. The effects of prolonged exercise on blood lipid levels and immune response in rico rat. Korean J Immunol. 2000;22:87–95. [Google Scholar]
- Lee YW, Shin KW, Paik IY, Jung WM, Cho SY, Choi ST, Kim HD, Kim JY. Immunological impact of taekwondo competitions. Int J Sports Med. 2012;33:58–66. doi: 10.1055/s-0031-1285926. [DOI] [PubMed] [Google Scholar]
- Mackinnon LT, Hooper SL, Jones S, Gordon RD, Bachmann AW. Hormonal, immunological, and hematological responses to intensified training in elite swimmers. Med Sci Sports Exerc. 1997;29:1637–1645. doi: 10.1097/00005768-199712000-00014. [DOI] [PubMed] [Google Scholar]
- Muller GC, Gottlieb MG, Luz Correa B, Filho IG, Moresco RN, Bauer ME. The inverted cd4:Cd8 ratio is associated with gender-related changes in oxidative stress during aging. Cell Immunol. 2015;296:149–154. doi: 10.1016/j.cellimm.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Nigam PK, Patra PK, Khodiar PK, Gual J. A study of blood CD3+, CD4+, and CD8+ T cell levels and CD4+:CD8+ ratio in vitiligo patients. Indian J Dermatol Venereol Leprol. 2011;77:111. doi: 10.4103/0378-6323.74993. [DOI] [PubMed] [Google Scholar]
- O’Gorman MR, Zijenah LS. CD4 T cell measurements in the management of antiretroviral therapy - A review with an emphasis on pediatric HIV-infected patients. Cytometry B Clin Cytom. 2008;74( Suppl 1):S19–S26. doi: 10.1002/cyto.b.20398. [DOI] [PubMed] [Google Scholar]
- Prieto-Hinojosa A, Knight A, Compton C, Gleeson M, Travers PJ. Reduced thymic output in elite athletes. Brain Behav Immun. 2014;39:75–79. doi: 10.1016/j.bbi.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med. 2008;44:153–159. doi: 10.1016/j.freeradbiomed.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Reid MB. Free radicals and muscle fatigue: Of ros, canaries, and the ioc. Free Radic Biol Med. 2008;44:169–179. doi: 10.1016/j.freeradbiomed.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Takeshima N, Rogers ME, Islam MM, Yamauchi T, Watanabe E, Okada A. Effect of concurrent aerobic and resistance circuit exercise training on fitness in older adults. Eur J Appl Physiol. 2004;93:173–182. doi: 10.1007/s00421-004-1193-3. [DOI] [PubMed] [Google Scholar]
- Walsh NP, Gleeson M, Shephard RJ, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P. Position statement. Part one: Immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- Zhao G, Zhou S, Davie A, Su Q. Effects of moderate and high intensity exercise on t1/t2 balance. Exerc Immunol Rev. 2012;18:98–114. [PubMed] [Google Scholar]


