Abstract
Background
We aimed to measure the levels of inflammatory markers and neopterin in obese and non-obese patients with PCOS by using 2 separate control groups with matching body mass index (BMI).
Material/Methods
A total of 60 women of reproductive age with (n=30) and without (n=30) PCOS were included in this study. Based on their BMI, patients with PCOS were divided into 2 groups as obese (n=15) and non-obese (n=15) PCOS groups. In addition, 2 BMI-matched control groups were formed. Neopterin, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), C-reactive protein (CRP), neutrophil-to-lymphocyte ratio (N/L ratio), and vitamin B12 were assessed by complete blood count.
Results
No significant difference was found between patients with PCOS and control subjects in neopterin, IL-6, TNF-α, and CRP levels. However, N/L ratio levels were significantly higher (p 0.045) and vitamin B12 levels were significantly lower (p 0.033) in patients with PCOS compared to control subjects.
No statistically significant difference was found between obese and non-obese patients with PCOS and control subjects in neopterin, IL-6, TNF-α, and N/L ratio levels. However, CRP levels were significantly higher in obese patients with PCOS compared to obese control subjects (p 0.007).
Conclusions
It can be concluded that inflammatory activity is increased in patients with PCOS, can lead to an increased risk for atherosclerosis, and this increase is not caused by obesity but rather by the polycystic ovary syndrome itself. However, studies with larger sample sizes are needed in this area.
MeSH Keywords: Inflammation; Obesity, Abdominal; Polycystic Ovary Syndrome
Background
Polycystic ovary syndrome (PCOS), also known as Stein-Leventhal syndrome, is a common endocrine disorder that occurs in 5–10% of women of reproductive age [1]. According to the 2003 Rotterdam criteria [2], it is usually defined by presence of at least 2 out of the following 3 features: i) oligoovulation or anovulation, ii) clinical and/or biochemical signs of hyperandrogenism (presence of at least 2 signs), or iii) polycystic ovaries (ovaries with many small cysts no larger than 8–10 mm; they occur in 15–20% of women).
PCOS has significant and various clinical implications, including reproductive, endocrine, and metabolic disorders such as hyperandrogenism and obesity [3]. Obesity, especially abdominal obesity, is an independent factor aggravating PCOS endocrine abnormalities, as subcutaneous abdominal adipose tissues and the liver tissues contribute to extragonadal aromatization [4].
As individuals become obese and their adipocytes enlarge, adipose tissue undergoes molecular and cellular changes affecting systemic metabolism. Fasting body FFA and glycerol discharge from adipocytes is increased in obese women compared with lean women [5].
The term “low-grade inflammation” refers to a condition characterized by an increase in levels of various inflammatory markers such as C-reactive protein (CRP), fibrinogen, tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), interleukin-6 (IL-6), and neutrophil-to-lymphocyte ratio (N/L ratio) [6,7]. Vitamin B12 is considered as antiinflammatory [8]. Recognition of the impact of inflammation on atheromatous plaque generation and rupture leads to interest in novel inflammatory markers and determining the best assay to define the relevant cardiovascular diseases (CVDs) [9].
Immunological processes are frequently debated in neurological and cardiovascular diseases. In this respect, cellular immune activation can easily and sensitively be monitored by measurement of neopterin levels in biological fluids. Biosynthetically derived from guanosine triphosphate, neopterin [2-amino-4-hydroxy-6-(D-erythro-1′,2′,3′-trihydroxypropyl)-pteridin] belongs to the class of pteridines [10].
Patients with PCOS are considered to be at high risk for cardiovascular diseases because of hyperandrogenism, insulin resistance, glucose intolerance, type II diabetes, and obesity which might accompany PCOS. However, there is no study in the literature reporting a direct relationship between increased/early cardiovascular mortality or morbidity and PCOS [11].
The purpose of this study was to make a comparison between obese and non-obese patients with PCOS and control subjects with regard to concentrations of inflammatory markers (neopterin, IL-6, TNF-α, N/L ratio, and CRP) and vitamin B12, which might pose a risk for atherosclerosis in patients with PCOS, and to investigate the correlation between the examined concentrations and the hormonal anthropometric and cardiometabolic parameters.
Material and Methods
This study was conducted in the Obstetrics and Gynecology Clinic of Dicle University and approved by the Local Ethics Committee of the university.
A total of 30 patients between 18 and 33 years of age (mean 26.2±4.0 years) who presented to Dicle University Faculty of Medicine Clinic of Obstetrics and Gynecology between June 2013 and July 2014 and were diagnosed with PCOS based on their clinical and endocrinological data were included in this study. According to the revised Rotterdam diagnostic criteria, PCOS was defined by the presence of at least 2 out of the following 3 features: oligo-anovulation (menstrual cycle length >45 days or less than 6 menstrual cycles a year), clinical or biochemical hyperandrogenism (Ferriman-Gallowey (FG) score >8 or elevated serum testosterone levels), and polycystic ovaries on transvaginal USG (TVUSG) (enlarged ovaries with increased stromal volume and more than 10 follicles which measure 2–8 mm in diameter and localize along the periphery of the ovary in a way to form ‘a pearl necklace’ appearance. Thyroid functions, luteinizing hormone/follicle-stimulating hormone (LH/FSH), prolactin, dehydroepiandrosterone sulfate (DHEAS), 17-hydroxyprogesterone (17-OHP), androstenedione, and total and free testosterone were checked in patients with PCOS and control subjects, and those with thyroid diseases, hyperprolactinemia, Cushing’s disease, or congenital adrenal hyperplasia and those who were administered agents such as hormonal agents, ovulation inducing agents, glucocorticoids, anti-androgens, or anti-hypertensives over the last 6 months prior to the study were excluded from the study. Fifteen patients with body mass index (BMI) >25 and 15 patients with BMI <25 were included in the PCOS groups and 15 healthy control subjects with BMI >25 and 15 healthy control subjects with BMI <25 who were between 17 and 35 years of age (mean 27.6±4.3 years) were included in the control groups. They were all clinically normal normo-ovulatory women who were selected for the study based solely on their BMI. All the participants, who were given detailed information about the study, gave their informed consent. Patients did not receive vitamin supplements before or during the study. Detailed histories were taken and weight and height measurements were made. BMI was calculated in all participants using the following formula: weight (kg)/height (m2).
The current cutoffs underestimate risk in the Asian and South Asian population. Thus, in the WHO and NIH guidelines for Asians, overweight is a BMI between 23 and 24.9 kg/m2 and obesity a BMI >25 kg/m2 [12,13]. Waist and hip circumferences were measured, and those with waist-to-hip ratio (WHG) >0.85 were regarded as android obese. Waist circumference was measured at the minimum circumference between the iliac crest and rib cage, and hip circumference was measured at the maximum circumference between waist and thighs [14].
Hirsutism score was calculated according to the FG score system. Nine anatomic regions were assessed on a 4-point scale where 0 meant no growth of terminal hair and 4 meant maximum hair growth. Scores below 8 were regarded as normal and scores between 8 and 36 were regarded as pathological. Scores rose in parallel to the severity of hirsutism [15].
Clinical assessment
In addition to family history of metabolic disorders, a detailed history including menstrual cycle pattern, temporal or situated in the temples of the head profile, severity of unwanted hair growth, and drug intake was taken at the time of enrollment. Clinical examination included measurement of body weight (kg), height (cm), and waist and hip circumferences (cm) as well as FG scoring. FG scoring was done by a single observer, and scores above 8 were taken as significant. A single observer performed trans-abdominal or transvaginal ultrasonography to reveal findings of polycystic ovarian morphology (presence of at least 10 follicles around the ovary that measure 2–8 mm in size, with increased ovarian volume and/or echogenic ovarian stroma) [16]. Ultrasonography was used to assess central obesity [17].
Laboratory tests
Blood samples were taken from the patients at early follicular phase (between the third and the fifth days of the spontaneous or gestagen-induced menstrual cycle). Venous blood was taken from the forearm between 08.00–10.00 am after 8 h of fasting.
Blood samples were immediately centrifuged, and sera were kept at −80°C until laboratory testing. Serum neopterine, TNF-α, and IL 6 levels were determined using enzyme-linked immunosorbent assay method (SunRed Biotechnology Company, Shanghai, China) according to the manufacturer’s protocols.
Serum triglyceride (TAG) and total cholesterol (Total-C) levels were measured using an Architect C 16000 autoanalyzer (Abbott Laboratories, Abbott Park, IL, USA).
Vitamin B12, follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol 2 (E2) levels were determined by electrochemiluminescence immunoassay using a Cobas e 601 analyzer (Roche Diagnostics, Mannheim, Germany).
Serum-free testosterone (f.test.) levels were analyzed by means of RadioImmunoAssay (RIA) using a RIA testosterone commercial kit (Immunotech, Beckman Coulter).
Serum CRP levels were measured using immunochemistry system nephelometric method (Image 800, Beckman Coulter, USA).
For N/L ratio measurements, complete blood counts of patients with PCOS and control subjects were studied using an automated hematology analyzer (Cell-dyn Ruby-Abbott Diagnostics, USA).
Statistical analysis
Statistical Package for the Social Science (SPSS 10.0) was used for data analyses. Data are expressed as mean and standard deviation. T test and nonparametric Mann-Whitney U test were used for comparison of clinical and biochemical data between the groups. Whether intra-group variables demonstrated a normal distribution or not was determined by Kolmogorov-Smirnov test. Spearman’s correlation analysis was used for investigation of correlation between the values. A p value smaller than 0.05 was considered statistically significant.
Results
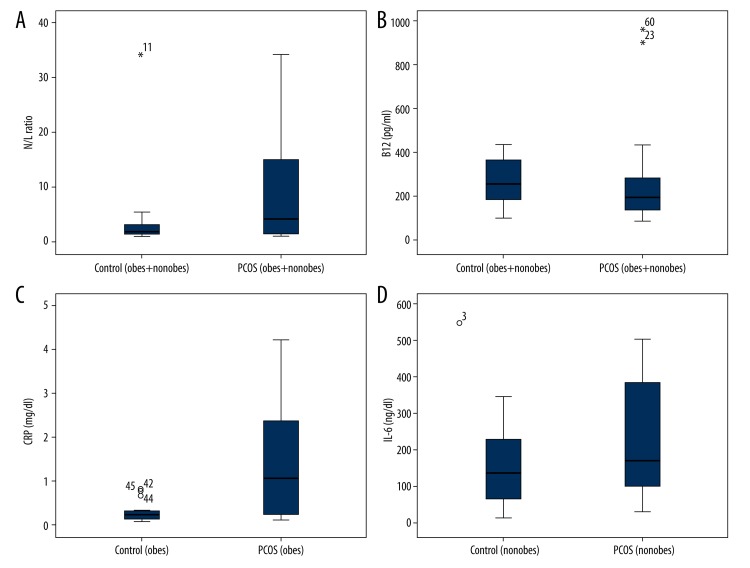
In this study, no statistically significant difference was found between patients with PCOS and control subjects in age and BMI (p 0.395, p 0.760). WHR and FG scores were significantly higher in patients with PCOS compared to control subjects (p 0.001 and p 0.000). Fasting blood glucose levels and TAG were significantly higher in patients with PCOS compared to control subjects (p 0.001, p 0.014). LH/FSH ratio and F.Test were significantly higher in patients with PCOS compared to control subjects (p 0.002, p 0.014) (Table 1). In this study, no statistically significant difference was found between patients with PCOS and control subjects in neopterin, IL-6, TNF-α and CRP levels. However, N/L ratio levels were significantly higher (p: 0.045) and vitamin B12 levels were significantly lower (p: 0.033) in patients with PCOS compared to control subjects (Table 1, Figure 1).
Table 1.
Comparison of inflammatory markers and biochemical parameters of PCOS between patients with PCOS and control subjects.
| n: 30 | Mean ±SD | P value | |
|---|---|---|---|
| Neopterin (nmol/L) | PCOS | 12.1±11 | 0.464 |
| Control | 11±9.4 | ||
|
| |||
| TNF-α (ng/L) | PCOS | 262.2±246.0 | 1.000 |
| Control | 254.1±263 | ||
|
| |||
| IL-6 (ng/L) | PCOS | 173.2±143.4 | 0.818 |
| Control | 165.5±119.3 | ||
|
| |||
| CRP (mg/dl) | PCOS | 1±1.1 | 0.121 |
| Control | 1±1.4 | ||
|
| |||
| N/L Ratio | PCOS | 9±9.4 | 0.045 |
| Control | 3.3±56 | ||
|
| |||
| B12 (pg/ml) | PCOS | 256.3±203.3 | 0.033 |
| Control | 265±99 | ||
|
| |||
| F. Test. (uıU/ML) | PCOS | 1.5±1.0 | 0.014 |
| Control | 1±1 | ||
|
| |||
| LH/FSH (muı/ml) | PCOS | 2.2±1.3 | 0.002 |
| Control | 1.3±0.7 | ||
|
| |||
| Total-C (mg/dl) | PCOS | 192±51.1 | 0.099 |
| Control | 170.5±47 | ||
|
| |||
| TAG (mg/dl) | PCOS | 1356±54.1 | 0.014 |
| Control | 115.3±81.2 | ||
|
| |||
| E2 (pg/ml) | PCOS | 123.3±145.4 | 0.093 |
| Control | 58±44 | ||
|
| |||
| Fasting (mg/dl) B.G. | PCOS | 104±18 | 0.001 |
| Control | 91.5±14.3 | ||
|
| |||
| Age | PCOS | 26.2±4 | 0.395 |
| Control | 28±4.3 | ||
|
| |||
| BMI (kg/m2) | PCOS | 24±4 | 0.760 |
| Control | 24±4 | ||
|
| |||
| WHR (cm) | PCOS | 0.9±0.1 | 0.001 |
| Control | 0.8±0.1 | ||
|
| |||
| FG score | PCOS | 12±3.1 | 0.000 |
| Control | 7±1 | ||
Data are presented as mean ± standard deviation. There are used student t test and nonparametric Mann-Whitney U test. p values: p values denoting the outcomes of comparison of the parameters of biochemical PCOS and Control groups. p<0.05 was considered to be statistically significant. CRP – C-reactive protein; TNF-α – tumor necrosis factor-α; IL-1 – interleukin-1; IL-6 – interleukin-6; NLR – neutrophil to lymphocyte ratio; TAG – triglyceride; Total-C – total cholesterol; FSH – follicle-stimulating hormone; LH – luteinizing hormone; E2 – Estradiol2; F.test – free testosterone; BMI – Body Mass Index; WHG – waist-to-hip ratio; FG score – Ferriman-Gallowey score; Fasting B.G. – fasting blood glucose.
Figure 1.
(A) N/L ratio levels in patients with PCOS and control subjects. Data are presented as mean ± standard deviation. (B) Vitamin B12 levels in patients with PCOS and control subjects. Data are presented as mean ± standard deviation. (C) CRP levels in obese patients with PCOS and obese control subjects. Data are presented as mean ± standard deviation. (D) IL-6 levels in non-obese patients with PCOS and non-obese control subjects. Data are presented as mean±standard deviation.
WHR and FG scores, which were used for rating of hirsutism, were significantly higher in both obese and non-obese patients with PCOS compared to control subjects (p 0.000 and p 0.000; p 0.000 and p 0.024, respectively). The comparison between obese patients with PCOS and obese control subjects showed that fasting blood glucose levels were significantly higher in obese patients with PCOS (p: 0.001). Total-C and TAG levels and LH/FSH ratio were significantly higher in the non-obese group with PCOS compared to control subjects (p 0.001, p 0.007 and p 0.024, respectively). CRP levels were significantly higher in obese patients with PCOS compared to control subjects (p 0.007). In the non-obese groups, IL-6 levels were significantly higher in patients with PCOS compared to control subjects (p 0.021) (Table 2, Figure 1).
Table 2.
Comparison of inflammatory markers and biochemical parameters of PCOS between obese and non-obese patients with PCOS and obese and non-obese control subjects.
| Cont.-BMI <25 | PCOS-BMI <25 | p | Cont.-BMI >25 | PCOS-BMI >25 | p | |
|---|---|---|---|---|---|---|
| Neopterin (nmol/L) | 10.3±9.2 | 15±10 | 0.191 | 11.0±10.1 | 11.2±11.5 | 0.772 |
| IL-6 (ng/L) | 172.5±140 | 232±148.1 | 0.269 | 159±100 | 115±101 | 0.241 |
| TNF-α (ng/L) | 294±292.2 | 313±248 | 0.901 | 214.5±233 | 212±242.1 | 0.772 |
| CRP (mg/dl) | 1.0±2.0 | 0.5±0.5 | 0.633 | 0.3±0.3 | 1.5±1.3 | 0.007 |
| NLR | 4.5±8.3 | 10.3±11.0 | 0.120 | 2.2±1.0 | 7.3±8 | 0.152 |
| B12 (pg/ml) | 258.3±94.4 | 257±194.5 | 0.407 | 271±106 | 256±219 | 0.206 |
| Total-C (mg/dl) | 140.4±26 | 196.5±54.3 | 0.001 | 200.2±45 | 187.1±49.2 | 0.395 |
| TAG (mg/dl) | 76.0±18.3 | 116±41.3 | 0.007 | 155±100.1 | 156±59 | 0.309 |
| Fasting B.G (mg/dl) | 92.1±16.4 | 101±19.5 | 0.177 | 91±12.5 | 107.1±16 | 0.001 |
| E2 (pg/ml) | 69±58.3 | 157.1±186 | 0.178 | 46.4±17 | 90±83.1 | 0.221 |
| FG Score | 6.0±1.0 | 11.3±3.3 | 0.000 | 7.3±0.6 | 12.0±3.1 | 0.000 |
| F.test. (uıU/ML) | 1.1±1 | 1.4±1 | 0.395 | 1±1 | 2±1.2 | 0.074 |
| LH/FSH (muı/ml) | 1.3±1 | 2.3±1.3 | 0.024 | 1.3±1 | 2±1.5 | 0.065 |
| WHR (cm) | 1±0.1 | 1±0.1 | 0.000 | 0.8±0.1 | 0.9±0.02 | 0.024 |
| Age | 26± 3 | 24.4± 4 | 0.347 | 30 ±4.5 | 28±4 | 0.149 |
| BMI | 20.4 ±2 | 20.3 ±2.2 | 0.763 | 27±1 | 27.3± 2 | 0.412 |
Data are presented as mean ± standard deviation. There are used student t test and nonparametric Mann-Whitney U test. p values: p values denoting the outcomes of comparison of the parameters of biochemical obese patients with PCOS and obese control, non-obese patients with PCOS and non-obese control groups. p<0.05 was considered to be statistically significant. CRP – C-reactive protein; TNF-α – tumor necrosis factor-α; IL-1 – interleukin-1; IL-6 – interleukin-6; NLR – neutrophil to lymphocyte ratio; TAG – triglyceride; Total-C – total cholesterol; FSH – follicle-stimulating hormone; LH – luteinizing hormone; E2 – Estradiol2; F.test – free testosterone; BMI – Body Mass Index; WHG – waist-to-hip ratio; FG score – Ferriman-Gallowey score; Fasting B.G. – fasting blood glucose.
WHR were significantly higher in obese patients with PCOS compared to non-obese patients with PCOS (p 0.047). Triglyceride and CRP levels were significantly higher in obese patients with PCOS compared to non-obese patients with PCOS (p 0.029 and 0.018, respectively) (Table 3).
Table 3.
Comparison of inflammatory markers and biochemical parameters of PCOS between obese and non-obese patients with PCOS.
| PCOS-BMI <25 | PCOS-BMI >25 | p | |
|---|---|---|---|
| Neopterin (nmol/L) | 15±10 | 11.2±11.5 | 0.372 |
| IL-6 (ng/L) | 232±148.1 | 115±101 | 0.021 |
| TNF-a (ng/L) | 313±248 | 212±242.1 | 0.290 |
| CRP (mg/dl) | 0.5±0.5 | 1.5±1.3 | 0.018 |
| N/L Ratio | 10.3±11 | 7.3±8 | 0.520 |
| B12 (pg/ml) | 257±194.5 | 256±218.5 | 0.678 |
| Total-C (mg/dl) | 196.5±54.3 | 187.1±49.1 | 0.709 |
| TAG (mg/dl) | 116±41.3 | 156±59 | 0.029 |
| Fasting B.G. (mg/dl) | 101±19.5 | 107.1±16 | 0.164 |
| E2 (pg/ml) | 157.1±186 | 90±83.1 | 0.310 |
| FG Score | 11.3±3.3 | 12±3.1 | 0.640 |
| F.test. (uıU/ML) | 1.4±1 | 2±1.1 | 0.534 |
| LH/FSH (muı/ml) | 2.3±1.2 | 2±1.4 | 0.309 |
| WHR (cm) | 0.8±0.1 | 0.9±0.02 | 0.047 |
Data are presented as mean ± standard deviation. There are used t test and nonparametric Mann-Whitney U test. p values: p values denoting the outcomes of comparison of the parameters of biochemical obese patients with PCOS and non-obese patients with PCOS. p<0.05 was considered to be statistically significant. CRP – C-reactive protein; TNF-α – tumor necrosis factor-α; IL-1 – interleukin-1; IL-6 – interleukin-6; NLR – neutrophil to lymphocyte ratio; TAG – triglyceride; Total-C – total cholesterol; FSH – follicle-stimulating hormone; LH – luteinizing hormone; E2 – Estradiol2; F.test – free testosterone; BMI – Body Mass Index; WHG – waist-to-hip ratio; FG score – Ferriman-Gallowey score; Fasting B.G. – fasting blood glucose.
N/L ratio levels were found to be significantly higher in non-obese patients with PCOS compared to obese control subjects (p: 0.042). In addition, IL-6 levels were significantly higher in non-obese patients with PCOS compared to obese patients with PCOS. Furthermore, CRP levels were significantly higher in obese patients with PCOS compared to non-obese patients with PCOS (p: 0.018) (Table 3).
In addition, vitamin B12 was negatively correlated with LH/FSH ratio, and no correlation was found between vitamin B12 and the inflammatory markers (Table 4).
Table 4.
Correlation analysis in patients with PCOS.
| Positive cor. | r | p | Negative cor. | r | p | |
|---|---|---|---|---|---|---|
| Neopterin (nmol/L) | TNF-α | 0.636** | 0.000 | AGE | −0.540** | 0.002 |
| IL-6 | 0.645** | 0.000 | ||||
| E2 | 0.612** | 0.000 | ||||
| LH/FSH | 0.443* | 0.014 | ||||
|
| ||||||
| TNF-α (ng/L) | Neopterin | 0.636** | 0.000 | AGE WHR |
−0.558** −0.499* |
0.001 0.005 |
| IL-6 | 0.632** | 0.000 | ||||
| E2 | 0.431* | 0.001 | ||||
|
| ||||||
| IL-6 (ng/L) | Neopterin | 0.645** | 0.000 | AGE | −0.438* | 0.015 |
| TNF-α | 0.632** | 0.000 | ||||
| LH/FSH | 0.373* | 0.042 | ||||
|
| ||||||
| CRP (mg/dl) | BMI | 0.410* | 0.025 | |||
| WHR | 0.403* | 0.027 | ||||
| TAG | 0.790** | 0.000 | ||||
| Total-C | 0.446* | 0.014 | ||||
|
| ||||||
| B12 (pg/ml) | LH/FSH | −0.365* | 0.047 | |||
|
| ||||||
| N/L ratio | Fasting B.G. | 0.369* | 0.045 | |||
|
| ||||||
| TAG (mg/dl) | CRP | 0.790** | 0.000 | |||
| Total-C | 0.390* | 0.033 | ||||
| BMI | 0.486* | 0.007 | ||||
| WHR | 0.448* | 0.013 | ||||
| LH/FSH ratio | 0.496* | 0.005 | ||||
|
| ||||||
| Total-C (mg/dl) | CRP | 0.446* | 0.014 | |||
| TAG | 0.390* | 0.033 | ||||
|
| ||||||
| BMI (kg/m2) | CRP | 0.410* | 0.025 | |||
| TAG | 0.486* | 0.007 | ||||
| WHR | 0.429* | 0.018 | ||||
|
| ||||||
| WHR (cm) | CRP | 0.403* | 0.027 | TNF-α | −0.499* | 0.005 |
| TAG | 0.448* | 0.013 | ||||
| BMI | 0.429* | 0.018 | ||||
|
| ||||||
| LH/FSH (muı/ml) | Neopterin | 0.443* | 0.014 | B12 | −0.365* | 0.047 |
| IL-6 | 0.373* | 0.045 | ||||
| E2 | 0.607** | 0.000 | ||||
| TAG. | 0.496* | 0.005 | ||||
The (Spearman) correlation coefficients (r) are given in the table. p values: p values denoting the outcomes of correlation of the parameters of biochemical patients with PCOS and control. p<0.05 was considered to be statistically significant. CRP – C-reactive protein; TNF-α – tumor necrosis factor-α; IL-1 – interleukin-1; IL-6 – interleukin-6; NLR – neutrophil to lymphocyte ratio; TAG – triglyceride; Total-C – total cholesterol; FSH – follicle-stimulating hormone; LH – luteinizing hormone; E2 – Estradiol2; F.test – free testosterone; BMI – Body Mass Index; WHG – waist-to-hip ratio; FG score – Ferriman-Gallowey score; Fasting B.G. – fasting blood glucose.
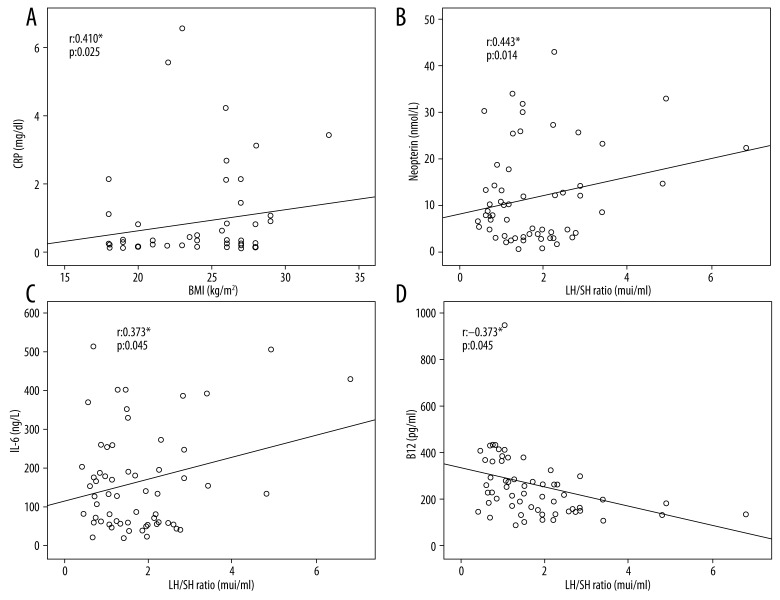
The correlation analysis was performed in the present study demonstrated that TAG and Total-C were positively correlated with CRP. We found that neopterin, IL-6, TNF-α, and N/L ratio were positively correlated with the PCOS parameters. Furthermore, we demonstrated that neopterin and IL-6 were positively correlated with LH/FSH ratio, a PCOS biomarker (r: 0.443* p 0.014 and r 0.373* p 0.042, respectively). Neopterin and TNF-α, on the other hand, was positively correlated with E2 (r 0.612** p 0.000 and r 0.431* p 0.001, respectively). In addition, vitamin B12 levels were found to be significantly lower in patients with PCOS. The correlation analysis revealed a negative correlation between vitamin B12 and LH/FSH ratio (Figure 2).
Figure 2.
(A) Correlation between BMI and CRP. The (Spearman) correlation coefficients (r) are given in the figure. (B) Correlation between Neopterin and LH/FSH. The (Spearman) correlation coefficients (r) are given in the figure. (C) Correlation between IL-6 and LH/FSH. The (Spearman) correlation coefficients (r) are given in the figure. (D) Correlation between B12 and LH/FSH. The (Spearman) correlation coefficients (r) are given in the figure.
The correlation analysis that was performed between CRP, BMI, WHR, TAG, and Total-C levels found a positive correlation between CRP levels and BMI, WHR, TAG, and Total-C levels (Table 4, Figure 2).
Discussion
Several studies reported an increased risk for cardiovascular diseases in patients with PCOS; however, the reason for this increased cardiovascular risk has not been clarified yet. Chronic low-grade inflammation, such as is seen in individuals with an excess of visceral/ectopic fat, plays an important role in cardiovascular disorders, in addition to atherosclerosis, which is strongly influenced by the inflammatory components of visceral obesity [18]. Several proinflammatory factors are manufactured in adipose tissue with increasing obesity. Compared with that of lean individuals, adipose tissue in obese persons shows higher expression of proinflammatory proteins, including TNF-α, interleukin 6 (IL-6), transforming growth factor β1, monocyte chemotactic protein 1, inducible nitric oxide synthase, procoagulant proteins such as tissue factor, and factor VII plasminogen activator inhibitor type 1 [19].
The purpose of this study was to investigate inflammatory markers in PCOS as well as the impact of obesity on the inflammatory markers. As a result, it was found that N/L ratio levels were significantly higher in patients with PCOS compared to control subjects. N/L ratio levels were found to be significantly higher in non-obese patients with PCOS compared to obese control subjects (p 0.042). Differences between the neopterin, CRP, IL-6, and TNF-α levels were not statistically significant in patients with PCOS compared to control subjects. This result might have been caused by the limited number of participants included in this study. We used ultrasound to assess central obesity; however, to evaluate central obesity magnetic resonance (MR), computed tomography (CT), and dual energy X-ray absorptiometry (DEXA) may also used [20]. Patients as well as control subjects were divided into 2 groups as those with BMI >25 and those with BMI <25, and it was found that only 1 inflammatory marker – CRP – was significantly higher in obese patients compared to obese control subjects.
The increased inflammation in patients with PCOS has not been clearly explained yet, and it is still unknown whether it is caused by PCOS itself or the accompanying obesity [21]. A meta-analysis reported increased WBC and CRP levels in patients with PCOS, and the increase was found to be independent of obesity. However, the meta-analysis had the limitation of inadequate BMI matching concerning the studies included [22]. Many researchers found increased CRP levels in obese and non-obese patients with PCOS compared to BMI-matched control subjects [23,24]. In addition, CRP levels were reported to be significantly higher in a PCOS group with BMI >25 kg/m2 compared to BMI-matched control subjects. However, there was no significant difference in CRP levels between non-obese insulin-resistant PCOS group and non-obese control subjects [25]. The results of the present study were in agreement with those of the abovementioned studies, showing that CRP might be affected by obesity.
Studies showed that N/L ratio correlated with CRP in several diseases with chronic inflammation such as hypertension and diabetes mellitus [26]. In this study was found that fasting blood glucose positively correlated with the N/L ratio. CRP was positively correlated with BMI, WHR, TAG, and Total-C.
Orio et al. studied leukocytes as an inflammatory marker, and found that neither patients with PCOS nor control subjects had leukocytosis; however, leukocyte count was significantly higher in patients with PCOS. They reported significantly higher levels of CRP, as well. These results were interpreted as a potential explanation for the increased cardiovascular risk in patients with PCOS [27]. In the present study, N/L ratio levels were found to be significantly higher in patients with PCOS compared to control subjects. In addition, CRP levels were significantly higher in obese patients with PCOS (p 0.018). It can be inferred from these results that increased inflammation is responsible for the increased risk for cardiovascular diseases and NIDDM in patients with PCOS. As to the comparison between non-obese patients with PCOS and obese control subjects, CRP levels were higher in patients with PCOS but the difference was not statistically significant. We also found that CRP was positively correlated with BMI and therefore was affected by obesity. However, it could increase independent of obesity in patients with PCOS but the increase would be more significant in obese patients compared to non-obese patients.
High serum neopterin levels in patients with atherosclerotic diseases revealed that immunological mechanisms might be responsible for development of atherosclerosis and related complications [28]. It was reported that neopterin, a key molecule taking part in organization of immune response, was released from active macrophages of atheromatous plaque and could be helpful for cardiovascular risk stratification [29]. In this study, neopterin levels were higher in patients with PCOS, but the difference was not statistically significant, perhaps due to the limited number of cases in our study. This finding suggests that macrophage activation occurs in women with PCOS, as has been reported in cardiovascular disease.
Barutcuoglu et al. found that increased concentrations of sCRP, neopterin, and WBC might be a contributory factor in increased risk for cardiovascular diseases in patients with PCOS. In addition to the widely known risk factors for CVDs (such as hypertension, dyslipidemia, insulin resistance, and obesity), PCOS comes to the foreground as a new risk factor for CVDs. It was shown for the first time by this study that patients with PCOS had significantly higher serum neopterin levels compared to healthy women with regular menstrual cycles [30]. In the present study, N/L ratio levels were significantly higher in patients with PCOS compared to control subjects. Increased concentrations of N/L ratio might be a contributory factor in increased risk for cardiovascular diseases in patients with PCOS.
Subclinical inflammation is considered to be a cause of atherogenesis, and IL-6 and TNF-α are also regarded as classic cardiovascular risk factors [31]. The most widely known and perhaps the most important systemic effect of IL-6 is the effect on acute-phase proteins, particularly C-reactive protein (CRP). Increase in serum IL-6 levels occurs in parallel with the increase in CRP levels [32]. PCOS is a proinflammatory disorder, and several studies on PCOS demonstrated increased levels of circulatory inflammatory markers such as plasminogen activator inhibitor-1, IL-18, TNF-α, IL-6, and hsCRP [33]. Gonzalez et al. found that serum TNF-α and IL-6 levels were increased in patients with PCOS and that the excess of adipose tissue was the most probable source[34]. In this study, IL-6 levels were significantly higher in non-obese patients with PCOS compared to obese patients with PCOS. It was demonstrated that IL-6 is positively correlated with LH/FSH ratio, a PCOS biomarker. TNF-α was positively correlated with E2. Our study supports the studies in the literature showing that inflammation markers increase in PCOS, independent of obesity. Increased inflammation is a risk factor for atherosclerosis.
It was shown that inflammatory markers that indicate low-grade chronic inflammation such as CRP and IL-6 were important predictors of NIDDM development. CRP levels were found to be elevated in patients with PCOS, and this finding was considered to be supportive of the hypothesis that elevated CRP levels triggered chronic inflammation, leading to an increased risk for NIDDM and CVDs in PCOS [35]. Kurt et al. found significantly increased levels of CRP, leukocytes, and neutrophils, which are inflammatory markers, and the increase was reported to be independent of obesity [36]. Although closely related to hyperglycemia, TNF-α is produced independently of obesity [37]. In the present study, when obese patients with PCOS were compared to the non-obese patients with PCOS, we found that IL-6 levels were significantly higher in non-obese patients (p 0.021). In addition, IL-6 and neopterin were positively correlated with LH/FSH ratio, a PCOS biomarker, and TNF-α was negatively correlated with WHR. Therefore, we conclude that IL-6 and TNF-α are positively correlated with PCOS biomarkers, independent of obesity.
In the present study, WHR was found to be significantly higher in patients with PCOS (both obese and non-obese) compared to control subjects, which led to the consideration that central body fat deposition occurred in a pathophysiological mechanism that was independent of obesity in patients with PCOS.
Hosseinzadeh and Rocha-Gonzalez found that cyanocobalamin may have anti-inflammatory effect (38, 39). Kaya et al. found significantly lower serum vitamin B12 levels in obese patients with PCOS compared to obese control subjects [40]. In the present study, as well, serum vitamin B12 levels were significantly lower in patients with PCOS (p 0.033). However, unlike the abovementioned study, no significant difference was found between obese and non-obese patients with PCOS. Therefore, B12 can say that independently of obesity decreased in PCOS. In the present study, B12 was significantly lower as anti-inflammatory marker in PCOS.
Conclusions
Inflammatory activity is increased in patients with PCOS, can lead to an increased risk for atherosclerosis, and this increase is not caused by obesity but rather by the polycystic ovary syndrome itself. However, larger studies are needed in this area with more participants.
Footnotes
Source of support: Departmental sources
Conflict of interest
We declare that we have no conflicts of interest.
References
- 1.Cho LW, Randeva HS, Atkin SL. Cardiometabolic aspects of polycystic ovarian syndrome. Vasc Health Risk Manag. 2007;3(1):55–63. [PMC free article] [PubMed] [Google Scholar]
- 2.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–31. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 4.Cupisti S, Kajaia N, Dittrich R, et al. Body mass index and ovarian function are associated with endocrine and metabolic abnormalities in women with hyperandrogenic syndrome. Eur J Endocrinol. 2008;158:711–19. doi: 10.1530/EJE-07-0515. [DOI] [PubMed] [Google Scholar]
- 5.Horowitz JF, Klein S. Whole body and abdominal lipolytic sensitivity to epinephrine is suppressed in upper body obese women. Am J Physiol Endocrinol Metab. 2000;278:E1144–52. doi: 10.1152/ajpendo.2000.278.6.E1144. [DOI] [PubMed] [Google Scholar]
- 6.Repaci A, Gambineri A, Pasquali R. The role of low-grade inflammation in the polycystic ovary syndrome. Mol Cell Endocrinol. 2011;335:30–41. doi: 10.1016/j.mce.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Orio F, Palomba S, Cascella T, et al. The increase of leukocytes as a new putative marker of low-grade chronic inflammation and early cardiovascular risk in polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:2–5. doi: 10.1210/jc.2004-0628. [DOI] [PubMed] [Google Scholar]
- 8.Vetter G, Brüggemann G, Lettko M, et al. Shortening diclofenac therapy by B vitamins. Results of a randomized double-blind study, diclofenac 50 mg versus diclofenac 50 mg plus B vitamins, in painful spinal diseases with degenerative changes. Zeitschrift fur Rheumatologie. 1988;47(5):351–62. [PubMed] [Google Scholar]
- 9.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice. A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann G, Schobersberger W. Neopterin: A mediator of the cellular immune system. Pteridines. 2004;15(3):107–12. [Google Scholar]
- 11.Legro RS. Polycystic ovary syndrome and cardiovascular disease: a premature association? Endocr Rev. 2003;24:302–12. doi: 10.1210/er.2003-0004. [DOI] [PubMed] [Google Scholar]
- 12.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 13.Speroff L, Class RH, Kase NG. Chapter 12, Anovulation and the polycystic ovary. 2005. Clinical gynecologic endocrinology and infertility; pp. 465–91. [Google Scholar]
- 14.Sermez Y, Türk T, Yaren A, et al. The relationship between QT interval and lipid metabolim in premenopausal android and gynoid obese women. Turkiye Klinikleri J Cardiol. 2000;13(1):15–18. [Google Scholar]
- 15.Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–47. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 16.Balen AH, Laven JS, Tan SL, Dewailly D. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update. 2003;9:505–14. doi: 10.1093/humupd/dmg044. [DOI] [PubMed] [Google Scholar]
- 17.Armellini F, Zamboni M, Rabbi R, et al. Total and intraabdominal fat measurements by ultrasound and computerized tomography. Int J Med. 1993;17:209–14. [PubMed] [Google Scholar]
- 18.Mathieu P, Pibarot P, Larose E, et al. Visceral obesity and the heart. Int J Biochem Cell Biol. 2008;40:821–36. doi: 10.1016/j.biocel.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–50. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 20.Schlemmer A, Hassager C, Haarbo J, et al. Direct measurement of abdominal fat by dual photon absorptiometry. Int J Obes. 1990;14:603–11. [PubMed] [Google Scholar]
- 21.Zahorska-Markiewicz B, Janowska J, Olszanecka-Glinianowicz M, Zurakowski A. Serum concentrations of TNF-alpha and soluble TNF-alpha receptors in obesity. Int J Obes Relat Metabol Disord: J Int Assoc Study Obes. 2000;24:1392–95. doi: 10.1038/sj.ijo.0801398. [DOI] [PubMed] [Google Scholar]
- 22.Escobar-Morreale HF, Luque-Ramirez M, Gonzalez F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and meta analysis. Fertil Steril. 2011;95(1048–58):e1–e2. doi: 10.1016/j.fertnstert.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boulman N, Levy Y, Leiba R, et al. Increased C-reactive protein levels in the polycystic ovary syndrome: a marker of cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2160–65. doi: 10.1210/jc.2003-031096. [DOI] [PubMed] [Google Scholar]
- 24.Fenkci V, Fenkci S, Yilmazer M, Serteser M. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil Steril. 2003;80:123–27. doi: 10.1016/s0015-0282(03)00571-5. [DOI] [PubMed] [Google Scholar]
- 25.Talbott EO, Zborowski JV, Bourdeaux MY, Mc Hugh-Pemu KP. The relationship between C-reactive protein and carotid intima-media wall thickness in middle-aged women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:6061–67. doi: 10.1210/jc.2003-032110. [DOI] [PubMed] [Google Scholar]
- 26.Imtiaz F, Shafique K, Mirza SS, et al. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int Arch Med. 2012;5:2. doi: 10.1186/1755-7682-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orio F, Jr, Palomba S, Cascella T, et al. The increase of leukocytes as a new putative marker of low grade chronic inflammation and early cardiovascular risk in the polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90(1):2–5. doi: 10.1210/jc.2004-0628. [DOI] [PubMed] [Google Scholar]
- 28.Vera R, Anita S, Uldis K, et al. Association between increased serum neopterin and homocysteine concentrations as well as pyridoxal-5-phosphate deficiency in patients with coronary heart disease. Pteridines. 2001;12(3):130–34. [Google Scholar]
- 29.Ray KK, Morrow DA, Sabatine MS, et al. Long-term prognostic value of neopterin: a novel marker of monocyte activation in patients with acute coronary syndrome. Circulation. 2007;115(24):3071–78. doi: 10.1161/CIRCULATIONAHA.106.666511. [DOI] [PubMed] [Google Scholar]
- 30.Barutcuoglu B, Bozdemir AE, Dereli D, et al. Increased serum neopterin levels in women with polycystic ovary syndrome. Ann Clin Lab Sci. 2006;36(3):267–72. [PubMed] [Google Scholar]
- 31.Blake GJ, Ridker PM. Novel clinical markers of vascular wall inflammation. Circ Res. 2001;89:763–71. doi: 10.1161/hh2101.099270. [DOI] [PubMed] [Google Scholar]
- 32.Madhok R, Crilly A, Watson J, Capell HA. Serum interleukin 6 levels in rheumatoid arthritis: correlation swith clinical and laboratory indices of disease activity. Ann Rheum Dis. 1993;52:232–34. doi: 10.1136/ard.52.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly CC, Lyall H, Petrie JR, et al. Low grade chronic inflammation in women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2001;86:2453–55. doi: 10.1210/jcem.86.6.7580. [DOI] [PubMed] [Google Scholar]
- 34.González F, Sia CL, Stanczyk FZ, et al. Hyperandrogenism exerts an anti-inflammatory effect in obese women with polycystic ovary syndrome. Endocrine. 2012;42:726–35. doi: 10.1007/s12020-012-9728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohlig M, Spranger J, Osterrhof M, et al. The polycystic ovary syndrome per se is not associated with increased chronic inflammation. Eur J Endocrinology. 2004;150:525–32. doi: 10.1530/eje.0.1500525. [DOI] [PubMed] [Google Scholar]
- 36.Kurt RK, Okyay AG, Hakverdi AU, et al. The effect of obesity on inflammatory markers in patients with PCOS: a BMI-matched case-control study. Arch Gynecol Obstet. 2014;290:315–19. doi: 10.1007/s00404-014-3199-3. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez F, Rote NS, Minium J, Kirwan JP. Increased activation of nuclear factor kappaB triggers inflammation and insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(4):1508–12. doi: 10.1210/jc.2005-2327. [DOI] [PubMed] [Google Scholar]
- 38.Hosseinzadeh H, Moallem SA, Moshiri M, et al. Anti-nociceptive and Anti-inflammatory effects of cyanocobalamin (Vitamin B12) against acute and chronic pain and inflammation in mice. Arzneimittelforschung. 2012;62(07):324–29. doi: 10.1055/s-0032-1311635. [DOI] [PubMed] [Google Scholar]
- 39.Rocha-González HI, Terán-Rosales F, Reyes-García G, et al. B vitamins increase the analgesic effect of diclofenac in the rat. Proc West Pharmacol Soc. 2004;47:84–87. [PubMed] [Google Scholar]
- 40.Kaya C, Cengiz SD, Satiroğlu H. Obesity and insulin resistance associated with lower plasma vitamin B12 in PCOS. Reprod Biomed Online. 2009;19(5):721–26. doi: 10.1016/j.rbmo.2009.06.005. [DOI] [PubMed] [Google Scholar]