Abstract
Background
Cardiac magnetic resonance (CMR) is increasingly used to assess heart diseases. Relevant non-cardiac diseases may also be incidentally found on CMR images. The aim of this study was to determine the prevalence and nature of incidental extra-cardiac findings (IEF) and their clinical impact in non-selected patients referred for CMR.
Material/Methods
MR images of 762 consecutive patients (515 men, age: 56±18 years) referred for CMR were prospectively interpreted by 2 radiologists blinded for any previous imaging study. IEFs were classified as major when requiring treatment, follow-up, or further investigation. Clinical follow-up was performed by checking hospital information records and by calling referring physicians. The 2 endpoints were: 1) non-cardiac death and new treatment related to major IEFs, and 2) hospitalization related to major IEFs during follow-up.
Results
Major IEFs were proven in 129 patients (18.6% of the study population), 14% of those being unknown before CMR. During 15±6 month follow-up, treatment of confirmed major IEFs was initiated in 1.4%, and no non-cardiac deaths occurred. Hospitalization occurred in 8 patients (1.0% of the study population) with confirmed major IEFs and none occurred in the remaining 110 patients with unconfirmed/unexplored major IEFs (p<0.001).
Conclusions
Screening for major IEFs in a population referred for routine CMR changed management in 1.4% of patients. Major IEFs unknown before CMR but without further exploration, however, carried a favorable prognosis over a follow-up period of 15 months.
MeSH Keywords: Cardiac Imaging Techniques, Incidental Findings, Magnetic Resonance Imaging
Background
Cardiac magnetic resonance (CMR) is a highly reproducible method performed to assess myocardial morphology as well as global and regional heart function. It also provides relevant information regarding tissue characteristics such as viability, myocardial perfusion, storage diseases, and inflammation. CMR is thus increasingly used in daily practice [1,2] and demonstrated great efficacy in detecting coronary artery disease (CAD) as compared to stress echocardiography [3,4] or myocardial scintigraphy [5–7]. Therefore, CMR is recommended in current guidelines for the work-up of CAD [8] and heart failure [9]. In CMR examinations a scrupulous reading of non-cardiac structures may also detect relevant non-cardiac diseases. Indeed, some incidental extra-cardiac findings (IEFs) such as pneumonitis, pleurisy, pulmonary embolism or aortic diseases may also mimic cardiac symptoms. While substantial data exist on the prevalence of IEFs on CMR examinations [10–17], it is less clear to what extent these IEFs influence patient management and outcome. Reported prevalences of major and minor IEFs detected in CMR examinations ranged between 3–27% and 5–31%, respectively, depending on whether great vessels pathologies were considered [10–12,16,17]. Wyttenbach et al. [17] reported a prevalence of 34% for potentially significant IEFs, while Atalay et al. [10] found a prevalence of 10% for significant major IEFs when excluding the pathologies of the great vessels.
In view of the paucity of outcome data, this study was performed in a large population undergoing a CMR examination to determine the prevalence of major and minor IEFs, and in particular to assess the impact of these findings on patient management and outcome during the first year after CMR.
Material and Methods
Study protocol and CMR imaging
Seven hundred sixty-two patients referred to the Cardiac Magnetic Resonance Center of our University Hospital between August 2011 and September 2013 for clinically indicated CMR were consecutively included. The Institutional Review Board approved the study. All patients accepted participation and gave their written informed consent before CMR for the use of imaging results for clinical research purpose and for a follow-up phone call. All patients had a single CMR examination. For every patient, common cardiovascular risk factors and coronary angiography results were recorded.
All CMR studies were performed on a 1.5 Tesla (n=425, MAGNETOM Aera, Siemens Medical Imaging, Erlangen, Germany) or 3 Tesla (n=337, MAGNETOM Verio or Skyra, Siemens Medical Imaging, Erlangen, Germany) equipment. All sequences were part of the daily CMR protocol of our institution. Imaging protocols included multi-planar localizers (TR 290 ms, TR 1.13 ms, matrix 256×256, 8-mm slice thickness) and an axial T2-weighted half-Fourier single-shot turbo-spin echo (HASTE) (TR 750 ms, TE 40 ms, matrix 368×512, 8-mm slice thickness, field-of-view 350 mm), followed by short-axis (TR 49 ms, TE 1.3 ms, matrix 208×256, 8-mm slice thickness) and long-axis cine acquisitions (TR 37 ms, TE 1.3 ms, matrix 168×208, 6-mm slice thickness) for assessment of LV and RV morphology and function. After injection of contrast medium (0.2 mmol/kg IV Gadovist®, Bayer Healthcare, Germany) axial, coronal, and sagittal gadolinium enhanced fat-sat T1-weighted 3D-volumetric interpolated breath-hold acquisitions (VIBE) (TR 4.6 ms, TE 2.3 ms, matrix 260×320, slice thickness 3 mm, field-of-view 350 mm) were performed for extra-cardiac structures analysis.
CMR analysis
All sequences, including axial T2-weighted (HASTE) and gadolinium enhanced T1-weighted axial, sagittal, and coronal images (VIBE), were prospectively reviewed in consensus by 2 experienced thoracic radiologists (XB, CB-A) who were blinded for any previous imaging study and the patients’ previous history.
Incidental IEFs were categorized into major and minor findings. Major IEFs were defined as findings requiring treatment or follow-up, and findings of unclear nature needing further investigation. Minor IEFs were defined as findings considered as benign disease without the need for complementary examination nor follow-up or treatment. Imaging examinations performed previously to CMR and available on our institutional picture archiving and communications system were secondarily analyzed to classify IEFs as known or unknown. Additional imaging studies such as CT, ultrasonography, or X-ray mammography were suggested to the patient’s referring physician to confirm or exclude unknown major IEFs. Proven major IEFs included major IEFs known before CMR and major IEFs unknown before CMR but confirmed by subsequent investigations.
Follow-up of patients with unknown major IEFs
Clinical follow-up of patients with unknown major IEFs on CMR was subsequently performed from October 2013 to December 2013. For every patient, any new treatment related to these major IEFs was searched in the hospital information system and recorded. Major IEF-related non-cardiac deaths and hospitalizations related to major IEF were also registered. When data were not available in the patient charts, information was sought using a structured phone interview with the referring physician. The primary end-point during follow-up consisted of the composite of non-cardiac death related to major IEFs and initiation of a treatment of major IEFs. The secondary end-point was hospitalizations related to major IEFs during follow-up.
Statistics
All statistics were performed with Stata 13.0 software (Stata College, Texas, USA). Continuous variables are presented as mean ±SD. Categorical variables are reported as percentages. The prevalence of major IEFs was defined as the number of patients with major IEFs divided by the number of included patients. Taking into account all patients with proven major IEFs (patients with previously known and previously unknown but confirmed major IEFs), logistic regression analysis was performed to assess the relation between major IEF and sex, diabetes, hypertension, dyslipidemia, obesity, smoking, CAD (proven by coronary angiography), symptoms or type of equipment (1.5 vs. 3-Tesla scanner). Age comparison between patients with or without major IEFs was assessed by Student’s t-test. A p-value<0.05 was considered as statistically significant.
Results
Patient population
Between August 2011 and September 2013, 762 patients (mean age 56±18 y, range: 1–88 years) referred to our CMR center were included and consecutively read by 2 observers. The female-to-male ratio was 1: 2.1 (247 women: 515 men). One hundred five (18%) patients had diabetes, 301 (40%) had hypertension, 263 (35%) had dyslipidemia, 182 (24%) smoked, 92 (12%) had family history of CAD, and 222 patients (29%) had CAD proven by coronary angiography. Four hundred and eleven (54%) patients were asymptomatic.
Extra-cardiac findings
Of 762 patients, 440 (57.7%) had a total of 735 IEFs, 322 patients having no IEF (Table 1). Among all IEFs, 368/735 (50.1%) were categorized as unknown. Liver (138/735), kidney (109/735), and lung parenchyma (100/735) were the 3 organs most frequently concerned with major and minor IEFs.
Table 1.
Major extracardiac findings.
| Abnormalities | All (n) | Unknown (n) | Explored (n) | Confirmed (n) |
|---|---|---|---|---|
| Thyroid or thymic mass/nodule | 11 | 5 | 1 | 0 |
| Mediastinal adenopathy | 39 | 19 | 6 | 2 |
| Aortic dilatation/dissection/coarctation | 63 | 21 | 8 | 7 |
| Main pulmonary trunk dilatation | 11 | 4 | 1 | 0 |
| Esophageal wall thickening | 4 | 1 | 0 | 0 |
| Major parenchymal lung lesion | 44 | 21 | 13 | 2 |
| Pulmonary embolism/angiosarcoma | 15 | 13 | 8 | 5 |
| Pleural effusion with thickening/pleural nodule/chest wall abnormality | 3 | 0 | 0 | 0 |
| Breast nodule/mass | 25 | 19 | 9 | 0 |
| Axillary adenopathy | 2 | 0 | 0 | 0 |
| Hepatic mass/dilated biliary tree/cirrhosis/hepatomegaly | 45 | 31 | 11 | 3 |
| Splenomegaly/splenic lesion | 6 | 5 | 3 | 0 |
| Complex renal cyst/renal tumor/dilated renal pelvis | 13 | 9 | 6 | 2 |
| Adrenal mass/nodule | 4 | 4 | 1 | 0 |
| Retroperitoneal lesion | 3 | 2 | 1 | 0 |
| Vertebral body lesion | 15 | 9 | 3 | 0 |
| Muscular lesion | 1 | 0 | 0 | 0 |
| Total | 304 | 163 | 74 | 21 |
Dilated aorta >40 mm; Major parenchymal lung lesion: nodule >1 cm, multiple micronodules, consolidation, mass, metastasis, interstitial lung disease; Adenopathy >1 cm small axis.
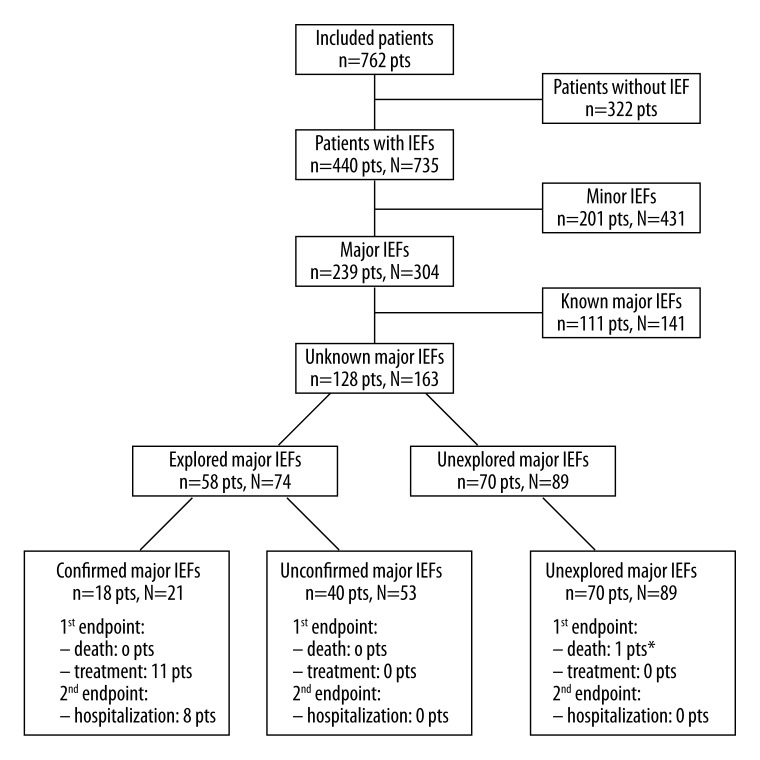
Of a total of 735 IEFs, 304 IEFs in 239 patients were classified as major (Table 1). There were 183 patients with 1 major IEF and 56 patients with more than 1 major IEF. Of 304 major IEFs, 163 (54%) in 128 patients were unknown. Besides aortic diseases (63/304; 20.7%), the 3 structures most frequently affected by major IEFs were the liver (45/304; 14.8%), the lungs (44/304; 14.3%), and the mediastinal lymph nodes (39/304; 12.7%). Seventy-four unknown major IEFs were explored and 21 were confirmed in 18 patients, the others being minor IEFs (Table 2). Eighty-nine (29.3%) major IEFs remained unexplored during follow-up. Among 163 unknown major IEFs, 21 new diagnoses were established (Figures 1 and 2), including aortic disease in 7 patients (4.3%), pulmonary embolisms in 5 patients (3.1%), and tumors in 3 patients (1.9%). Overall, the prevalence of proven major IEFs was 18.6% (111 patients with known major IEFs and 18 patients with confirmed unknown major IEFs divided by 762 patients minus 70 patients with unexplored major IEFs, see Figure 3), 14% (18/129) of them being unknown before CMR.
Table 2.
Minor extracardiac findings.
| Abnormalities | n |
|---|---|
| Thyroid goiter/cyst | 6 |
| Mediastinal lymph node | 9 |
| Aortic ectasia/anatomical variants | 18 |
| Main pulmonary trunk prothesis | 1 |
| Hiatus hernia/oesophagitis/esophageal dilation | 18 |
| Minor parenchymal lung lesion | 56 |
| Pleural effusion without thickening | 38 |
| Breast cyst/post-surgical status/gynecomastia | 9 |
| Diaphragmatic hernia | 8 |
| Axillary benign lesion (lipoma) | 1 |
| Hepatic cyst, hemangioma | 93 |
| Splenectomy/polysplenia/splenic cyst/accessory spleen | 19 |
| Simple renal cyst | 96 |
| Vertebral body angioma/discarthropathy/Schmorl hernia | 56 |
| Benign muscular lesion | 3 |
| Total | 431 |
Minor parenchymal lung lesion: stripe, atelectasia, solitary micronodule, azygous lobe, post-surgical status.
Figure 1.
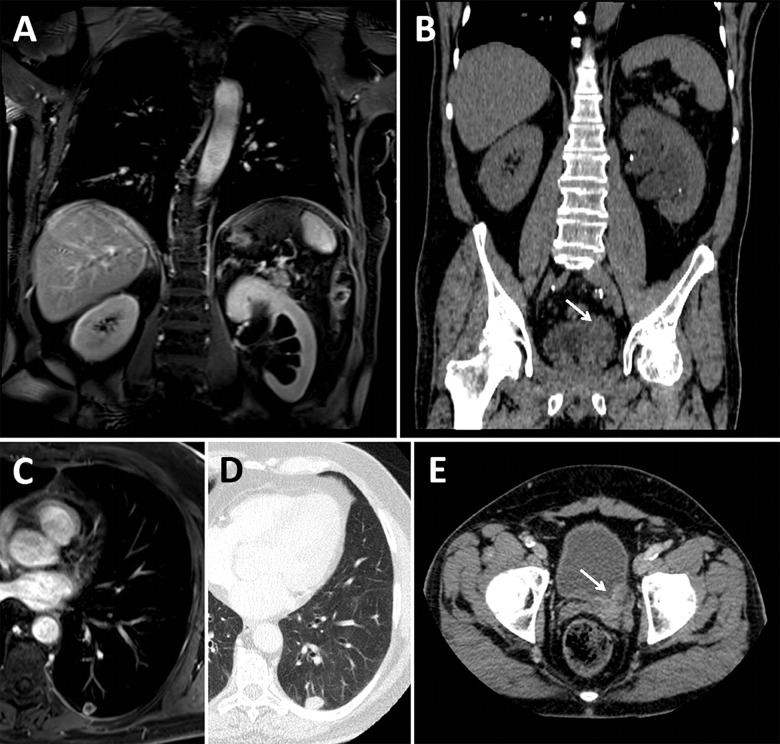
Patient with left pyelocaliceal dilation and pulmonary nodule that revealed bladder cancer. (A) Left pyelocaliceal dilation on fat-sat post-gadolinium T1-weighted coronal image. (B) Thickening of the left ureterovesical junction (arrow) on coronal unenhanced CT view. (C and D) Necrotic metastasis of the left posterobasal segment of the left lower lobe on axial fat-sat post-gadolinium T1-weighted and axial CT view respectively. (E) Thickening of the left ureterovesical junction (arrow) on axial enhanced CT view.
Figure 2.
Patient with confirmed new pulmonary embolism. (A and B) Vascular filing defect of the posterobasal segmental pulmonary artery of the right lower lobe (arrows) on axial fat-sat post-gadolinium T1-weighted and axial CT pulmonary angiography views, respectively. (C and D) Vascular filling defect of the laterobasal segmental pulmonary artery of the right lower lobe (arrows) on axial fat-sat post-gadolinium T1-weighted and coronal CT pulmonary angiography views, respectively.
Figure 3.
Patients’ IEFs flowchart. Non-cardiac deaths, treatments and hospitalizations related to major IEFs are reported for each category. By convention, we used in this figure the small letter n when describing the number of patients and the capital N when describing the number of IEF. * One patient died from end stage heart failure.
Patients with major IEFs were older than patients without (60±15 vs. 54±18 years, p<0.0001). Taking into account all patients with proven major IEFs, there was no significant relation between major IEFs and sex, diabetes, hypertension, dyslipidemia, smoking, symptoms, CAD, obesity, or type of MR imager (p>0.11).
A total of 431 minor IEFs were recorded in 201 patients (prevalence of 26.4%). Kidneys (96/431), liver (93/431), and lung parenchyma (56/431) were the 3 structures the most frequently concerned when considering minor and major IEFs (Table 2).
Follow-up of patients with unknown major IEFs
a) Primary end-point
The mean follow-up duration for patients with unknown major IEFs was 15±6 months. A total of 11 patients were treated for major IEFs and no death for non-cardiac reasons occurred. Thus, the primary end-point was observed in 1.4% of the study population (total n=762). Three of these 11 patients were treated for pathologies of the thoracic aorta and 5 patients for pulmonary embolism, corresponding to 0.4% and 0.7% of the total study population, respectively (Figure 3). Three patients (0.4% of the total population) were treated for extra-thoracic major IEFs (renal and bladder cancer, liver cirrhosis). Specifically, in 2 patients with thoracic aortic aneurysms, open-chest repair was performed and 1 patient with aortic dissection was treated by endoprosthesis placement. Anticoagulation therapy was started in 5 patients with pulmonary embolism. Among 128 patients with unknown major IEFs, only 1 patient died from end-stage heart failure, no deaths due to non-cardiac disease occurred during follow-up (Figure 3).
b) Secondary end-point
Patients’ follow-up revealed that 8 patients were hospitalized due to major IEFs found on CMR corresponding to 1.0% of the total study population (Figure 3). These 8 hospitalizations occurred in the 18 patients with a confirmation of major IEF by further investigations (CT in 16 patients, ultrasound in 2 patients) whereas no hospitalization occurred in the remaining 110 patients with major IEFs (44% vs. 0%, p<0.001), which were not confirmed in additional tests (n=40) or where no further testing was performed (n=70). Hospitalizations occurred more frequently in patients who underwent further testing after CMR (n=58) than in cases where no further testing was performed (n=70) (14% vs. 0%, respectively, p=0.001).
Discussion
The main findings of this study can be summarized as follows: 1) Proven major IEFs were found in 18.6% of the study population. These major IEFs were correlated with age but not with other cardiovascular risk factors and involved most frequently the thoracic aorta, the lungs, the mediastinal lymph nodes and the liver; 2) A new treatment was installed because of the detection of previously unknown major IEFs in 1.4% of the study population, and no death from major IEFs occurred; 3) In the 70 patients with previously unknown major IEFs on CMR, which were not further explored, the prognosis during follow-up however appeared favorable with no treatment initiated and no death for non-cardiac reasons.
Prevalence of proven major IEFs
In the current study, proven major IEFs were found in 18.6% of the population, which is in line with other recent studies that reported similar prevalences for major IEFs during CMR of 3–27% [10–12,16,17]. Except for the aorta, the 3 most frequently involved organs presenting with major IEFs were the liver (14.8% of major IEFs), lung parenchyma (14.5%), and mediastinal lymph nodes (12.8%), as described in previous large studies [11,13,16]. Also, as previously published [10,13], the prevalence of major IEFs correlated with age in the current study, but not with other cardiovascular risk factors.
It is important to mention that criteria for major and minor IEFs were used differently in the various studies, which can explain differences in prevalence. Khosa et al. [14] described thoracic lymphadenopathy of less than 1.5 cm as benign findings, while Wyttenbach et al. [17] included hiatal hernia (n=6), gallstones (n=8), or left superior vena cava (n=5) as significant findings, yielding a prevalence of 23%. Atalay et al. [10] reported a prevalence of 15% for significant non-cardiac findings but did not include aortic or main pulmonary artery disease, which may influence the prevalence, since we found that 93/735 (12.7%) IEFs concerned these structures in our study. These findings clearly highlight the need for a harmonization of the definition of minor and major IEFs for CMR.
There are also methodological and technical variations to consider when comparing the prevalences of IEFs reported in the literature. Hence, some authors only reported IEFs based on CMR report review [10,11,13], while others performed a complete image analysis [12,14–17], as we did. This can significantly influence the number of non-cardiac findings, as demonstrated by Wyttenbach et al. [17]. Irwin et al. [13] also reported that there was no statistical difference in reported IEF rate between cardiology and radiology readers on separate examinations. There is, however, no published data on the equality of performance to detect IEF between cardiologists and radiologists in a blinded fashion.
Pulmonary embolism and other lung pathologies
The most frequent extra-cardiac diagnosis was pulmonary embolism, which was suspected by CMR in 15 patients. Of those, 8 patients underwent further exploration, which confirmed the diagnosis in 5 patients, yielding a specificity of 63%. In our study, all pulmonary embolisms were diagnosed on contrast-enhanced T1-weighted VIBE images by detecting endoluminal hyposignals on at least 2 orthogonal sequences in order to exclude flow artifacts [18]. As no dedicated MR pulse sequences were used to detect pulmonary embolism and the T1-weighted VIBE images did not cover the entire lung parenchyma, this specificity is not surprising, but is rather low in comparison to previous findings [19–21]. Pulmonary nodules and other parenchymal lung lesions were detected in 5.8% of patients, but diagnoses were confirmed in only 2 of 13 patients (specificity of 15%). The prevalence of major parenchymal lung lesions was also lower in comparison to that reported by Horton et al. [22] and Onuma et al. [23] in cardiac MDCT studies, mainly due to the lower spatial resolution of MRI. These data suggest that further improvements of pulse sequences are needed to allow for a better assessment of lung tissue and a better estimation of the prevalence of IEFs in lung parenchyma during CMR. Overall, the prognosis of the patients with major unknown IEFs of the lung parenchyma was good, with no need for treatment and no death for extra-cardiac reasons during follow-up.
Impact of previously unknown major IEFs on patient management and outcome
Of the 128 patients with previously unknown major IEFs on CMR, treatment was initiated in 8.6% in11 patients or 1.4% of the screened study population. A major IEF of the thorax leading to a change in management was found in 8 patients (1.0% of the study population). In 5 patients anticoagulation was initiated due to the detection of pulmonary embolism confirmed by CT and in 3 patients the thoracic aorta was treated (2 open chest repair, one endoprosthesis). An abdominal pathology was found in 3 patients (1 renal cancer, 1 urinary bladder cancer, 1 liver cirrhosis). From the current study, we can only speculate on the potential benefit of the patients treated for peripheral pulmonary embolisms. In another 5 patients with this diagnosis on CMR but without any further testing or confirmation, no complications (no death, no treatment, no hospitalization) occurred during the follow-up period after CMR.
In 2 patients (0.3% of the study population), abdominal tumors were detected (kidney and urinary bladder). However, from these data it remains questionable whether a systematic reading of abdominal structures on CMR studies should be recommended.
As it was an observational study, confirmation or exclusion of majors IEFs found on CMR was not obtained in all patients. Whether reported major IEFs were subject to additional testing was at the discretion of the referring physician. Only 45% of the patients with newly diagnosed major IEFs on CMR underwent a further work-up and the CMR diagnosis was confirmed in 31% of these patients. In the remaining 110 patients, major IEFs were not confirmed or were not explored further. Interestingly, in these 110 patients, the outcome over the first 15 months after CMR was excellent without any IEF-related death or treatment change, and no IEF-related hospitalization. These results may indicate that major IEFs, if not accompanied by clinically suspicious symptoms or findings that trigger further testing, may have little or no impact on patient outcome, at least during the first 15 months after a CMR examination.
In summary, the presence of a previously unknown major IEF on CMR caused new treatment initiation in 1.4% of the study population and this decreased to 1% for IEF located in the thorax.
Limitations
The detection of minor and major IEFs was evaluated on all sequences performed in the daily CMR protocol of our institution, which includes T2-weighted HASTE and contrast-enhanced T1-weighted VIBE sequences. While other studies reported IEFs using either scout, steady-state free precession (SSFP), HASTE, or T1-weighted fast spin echo images, alone or in combination, it remains unclear whether major IEFs detection would benefit from dedicated fast or ultrafast sequences such as contrast-enhanced T1-weighted VIBE by using fast gradient echo respiration-triggered or 3D-contrast-enhanced sequences [19,24,25] especially for the detection of pulmonary embolism.
Eighty-nine unknown major IEFs remained unexplored, most likely because the decision to add further testing was at the discretion of the referring physician. With the current design of this study, we cannot assess what would be the impact of unknown major IEFs if all these lesions would have been further explored. At least, in this population without further testing of major IEFs, the prognosis was favorable with no treatment started, no death for non-cardiac reasons and no hospitalization related to major IEFs. The good prognosis of this patient subset also indicates that the presence of major IEFs per se is not associated with complications during follow-up. Further study is needed on the additional information that prompted treating physicians to further explore unknown major IEFs or information that cannot be extracted from the current data but would warrant further study.
We performed an early follow-up within 15±6 months. A longer follow-up period is however mandatory to evaluate the impact on the long term.
The detection of major IEFs modified patient management, i.e. it induced further testing (58 patients), additional treatment (11 patients) and hospitalization (8 patients). The cost for the acquisition of the T2-weighted and T1-weighted sequences, the reading of these images as well as the costs for down-stream additional testing and treatment was not assessed in this study. While data exist on the cost-effectiveness of CMR for the work-up of CAD [26,27] a prospective study should assess the economic impact of the detection of major IEFs by CMR.
Conclusions
Proven major IEFs were found in 18.6% of patients undergoing a routine CMR exam. The most commonly involved organs were the thoracic aorta, the liver, lungs, and the mediastinal lymph nodes. Pulmonary embolism accounted for 3.1% of unknown major IEF. Screening for major IEFs in a population referred for routine CMR changed management in 1.4% of patients, and in 1% in patients with unknown major IEF of the thorax. Major IEFs unknown before CMR but without further exploration however carried a favorable prognosis over a follow-up period of 15 months. Longer follow-up is needed to determine whether these major IEFs may influence long term patient survival.
Ackowledgments
The authors would like to acknowledge Maria-Gabriella Vincenti, MD, and all the technicians of the Cardiac MR Center of the Lausanne University Hospital for their help in performing CMR as well as Nathalie Lauriers, RN, for her help in performing patient follow-up.
Footnotes
Source of support: Self financing
Conflict of interest
The authors do not declare any financial competing interests regarding this study.
References
- 1.Bruder O, Wagner A, Lombardi M, et al. European Cardiovascular Magnetic Resonance (EuroCMR) registry – multi national results from 57 centers in 15 countries. J Cardiovasc Magn Reson. 2013;15:1–9. doi: 10.1186/1532-429X-15-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawecki D, Morawiec B, Monney P, et al. Diagnostic contribution of Cardiac Magnetic Resonance in patients with acute coronary syndrome and culprit-free angiograms. Med Sci Monit. 2015;21:171–80. doi: 10.12659/MSM.892296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagel E, Lehmkuhl HB, Bocksch W, et al. Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation. 1999;99(6):763–70. doi: 10.1161/01.cir.99.6.763. [DOI] [PubMed] [Google Scholar]
- 4.Hundley WG, Morgan TM, Neagle CM, et al. Magnetic resonance imaging determination of cardiac prognosis. Circulation. 2002;106(18):2328–33. doi: 10.1161/01.cir.0000036017.46437.02. [DOI] [PubMed] [Google Scholar]
- 5.Schwitter J, Wacker CM, van Rossum AC, et al. MR-IMPACT: Magnetic Resonance Imaging for myocardial perfusion assessment in coronary artery disease trial: comparison of perfusion CMR with single photon emission computed tomography for the detection of coronary artery disease in a multicenter, multivendor, randomized trial. Eur Heart J. 2008;29:480–89. doi: 10.1093/eurheartj/ehm617. [DOI] [PubMed] [Google Scholar]
- 6.Schwitter J, Wacker CM, Wilke N, et al. Superior diagnostic performance of perfusion-CMR versus SPECT to detect coronary artery disease: The secondary endpoints of the multicenter multivendor MR-IMPACT II. J Cardiovasc Magn Reson. 2012;14:61–71. doi: 10.1186/1532-429X-14-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaarsma C, Leiner T, Bekkers SC, et al. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2012;59(19):1719–28. doi: 10.1016/j.jacc.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 8.Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: The Task Force on the management of stable coronary artery disease of the ESC. Eur Heart J. 2013;34(38):2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 9.McMurray JJV, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur Heart J. 2012;(33):1787–847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 10.Atalay MK, Prince EA, Pearson CA, Chang KJ. The prevalence and clinical significance of noncardiac findings on cardiac MRI. Am J Roentgenol. 2011;196(4):W387–93. doi: 10.2214/AJR.09.3302. [DOI] [PubMed] [Google Scholar]
- 11.Chan PG, Smith MP, Hauser TH, et al. Noncardiac pathology on clinical cardiac magnetic resonance imaging. JACC Cardiovasc Imaging. 2009;2(8):980–86. doi: 10.1016/j.jcmg.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Dewey M, Schnapauff D, Teige F, Hamm B. Non-cardiac findings on coronary computed tomography and magnetic resonance imaging. Eur Radiol. 2007;17(8):2038–43. doi: 10.1007/s00330-006-0570-1. [DOI] [PubMed] [Google Scholar]
- 13.Irwin RB, Newton T, Peebles C, et al. Incidental extra-cardiac findings on clinical CMR. Eur Heart J Cardiovasc Imaging. 2013;14(2):158–66. doi: 10.1093/ehjci/jes133. [DOI] [PubMed] [Google Scholar]
- 14.Khosa F, Romney BP, Costa DN, et al. Prevalence of noncardiac findings on clinical cardiovascular MRI. Am J Roentgenol. 2011;196(4):W380–86. doi: 10.2214/AJR.09.3112. [DOI] [PubMed] [Google Scholar]
- 15.McKenna DA, Laxpati M, Colletti PM. The prevalence of incidental findings at cardiac MRI. Open Cardiovasc Med J. 2008;2:20–25. doi: 10.2174/1874192400802010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sohns JM, Schwarz A, Menke J, et al. Prevalence and clinical relevance of extracardiac findings at cardiac MRI. J Magn Reson Imaging. 2014;39(1):68–76. doi: 10.1002/jmri.24142. [DOI] [PubMed] [Google Scholar]
- 17.Wyttenbach R, Medioni N, Santini P, et al. Extracardiac findings detected by cardiac magnetic resonance imaging. Eur Radiol. 2012;22(6):1295–302. doi: 10.1007/s00330-011-2369-y. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira PF, Gatehouse PD, Mohiaddin RH, Firmin DN. Cardiovascular magnetic resonance artefacts. J Cardiovasc Magn Reson. 2013;15:41. doi: 10.1186/1532-429X-15-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang LJ, Luo S, Yeh BM, et al. Diagnostic accuracy of three-dimensional contrast-enhanced MR angiography at 3-T for acute pulmonary embolism detection: comparison with multidetector CT angiography. Int J Cardiol. 2013;168(5):4775–83. doi: 10.1016/j.ijcard.2013.07.228. [DOI] [PubMed] [Google Scholar]
- 20.van Beek EJ, Wild JM, Fink C, et al. MRI for the diagnosis of pulmonary embolism. J Magn Reson Imaging. 2003;18(6):627–40. doi: 10.1002/jmri.10421. [DOI] [PubMed] [Google Scholar]
- 21.Hosch W, Schlieter M, Ley S, et al. Detection of acute pulmonary embolism: feasibility of diagnostic accuracy of MRI using a stepwise protocol. Emerg Radiol. 2014;21(2):151–58. doi: 10.1007/s10140-013-1176-y. [DOI] [PubMed] [Google Scholar]
- 22.Horton KM, Post WS, Blumenthal RS, Fishman EK. Prevalence of significant noncardiac findings on electron-beam computed tomography coronary artery calcium screening examinations. Circulation. 2002;106(5):532–34. doi: 10.1161/01.cir.0000027136.56615.de. [DOI] [PubMed] [Google Scholar]
- 23.Onuma Y, Tanabe K, Nakazawa G, et al. Noncardiac findings in cardiac imaging with multidetector computed tomography. J Am Coll Cardiol. 2006;48(2):402–6. doi: 10.1016/j.jacc.2006.04.071. [DOI] [PubMed] [Google Scholar]
- 24.Kalb B, Sharma P, Tigges S, et al. MR imaging of pulmonary embolism: diagnostic accuracy of contrast-enhanced 3D MR pulmonary angiography, contrast-enhanced low-flip angle 3D GRE, and nonenhanced free-induction FISP sequences. Radiology. 2012;263(1):271–78. doi: 10.1148/radiol.12110224. [DOI] [PubMed] [Google Scholar]
- 25.Piccini D, Monney P, Sierro C, et al. Respiratory Self-navigated postcontrast whole-heart coronary MR angiography: initial experience in patients. Radiology. 2014;270:378–86. doi: 10.1148/radiol.13132045. [DOI] [PubMed] [Google Scholar]
- 26.Moschetti K, Muzzarelli S, Pinget C, et al. Cost evaluation of cardiac magnetic resonance imaging versus coronary angiography for the diagnostic work-up of coronary artery disease: Application of the European Cardiovascular Magnetic Resonance registry data to the German, United Kingdom, Swiss, and United States health care systems. J Cardiovasc Magn Reson. 2012;(14):35–44. doi: 10.1186/1532-429X-14-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker S, Girardin F, McKenna C, et al. Cost-effectiveness of cardiovascular magnetic resonance in the diagnosis of coronary heart disease: an economic evaluation using data from the CE-MARC study. Heart. 2013;99(12):873–81. doi: 10.1136/heartjnl-2013-303624. [DOI] [PubMed] [Google Scholar]