Abstract
Many communication calls contain information about the physical characteristics of the calling animal. During maturation of the guinea pig purr call the pitch becomes lower as the fundamental frequency progressively decreases from 476 to 261 Hz on average. Neurons in the primary auditory cortex (AI) often respond strongly to the purr and we postulated that some of them are capable of distinguishing between purr calls of different pitch. Consequently four pitch-shifted versions of a single call were used as stimuli. Many units in AI (79/182) responded to the purr call either with an onset response or with multiple bursts of firing that were time-locked to the phrases of the call. All had a characteristic frequency ≤5 kHz. Both types of unit altered their firing rate in response to pitch-shifted versions of the call. Of the responsive units, 41% (32/79) had a firing rate locked to the stimulus envelope that was at least 50% higher for one version of the call than any other. Some (14/32) had a preference that could be predicted from their frequency response area while others (18/32) were not predictable. We conclude that about 18% of stimulus-driven cells at the low-frequency end of AI are very sensitive to age-related changes in the purr call.
1. Introduction
Species-specific communication calls provide ecologically relevant stimuli for investigating how the brain processes complex sounds. Some communication calls convey important information about the reproductive status, emotional state, age and or size of the animal producing the call (Clutton-Brock and Albon, 1979; Fitch and Hauser, 1995; Siemers et al., 2005; Charlton et al., 2009). This is also true of human speech: there is an almost linear relationship between age/weight and fundamental frequency (F0) of speech in adolescent boys between the ages of 12 and 15 years (Hollien et al., 1994). The F0 is related to glottal pulse rate. F0 and vocal tract length both have an effect on speech that is used by listeners to judge speaker size, sex and age (Smith and Patterson, 2005). Furthermore an increase in voice pitch has been shown in adult women just before they ovulate (Bryant and Haselton, 2009).
As a young animal increases in age and size, the vocal folds of the larynx become longer; this is associated with a lower voice pitch and a reduction in the F0 of harmonic calls (Charlton et al., 2009). The first section of this study investigated to what extent there are age or size dependent differences within a guinea pig communication call. We focused on the short purr call because it is a stereotyped call and is the one most reliably produced by both infant and adult animals (Berryman, 1976). This call has been termed the drrr call by Berryman (1976) or an alarm rumble by Rood (1972) and Arvola (1974). The short purr call is an alerting call that catches the attention of surrounding guinea pigs and often elicits a corresponding call from all the animals in the group (Berryman, 1976). We show that the F0 of the short purr call decreases significantly as an animal increases in age. With an understanding of how calls naturally vary with age, acoustic features of a single exemplar call can be manipulated so that the new versions appear to come from humans or animals of different ages (Smith and Patterson, 2005).
The main aim of this study was to investigate whether single neurons in AI were sensitive to the age-related changes in the pitch of the short purr call. Pitch-sensitive neurons have been shown to occur at the low-frequency border of AI and the rostral field of marmoset monkeys (Bendor and Wang, 2005) but there has not been any evidence of a homologous area in the guinea pig. There is also evidence of one or more pitch sensitive areas in the human belt cortex (Hall and Plack, 2009 and see review by Bizley and Walker, 2010) but not so far in any non-primate species. We targeted the low-frequency region of AI because cells there have been shown to be better at locking to the waveform envelope of the purr call than cells in other cortical areas (Wallace et al., 2005a,b). Preference for individual versions of a call is more than just a measure of whether a unit simply does, or does not respond. A unit responding with a simple, onset response to one sound may not carry as much information as the same unit responding with a long-lasting, multi-peaked response to another sound (Wang et al., 1995). It has been proposed that a unit will respond with a sustained response, rather than an onset response, to its preferred stimulus (Wang et al., 2005). Romanski et al. (2004) categorised the specificity of ventrolateral prefrontal cortex units for communication calls by measuring the differences in their overall firing rate for a battery of different calls. From this they calculated the ‘call preference index’ of a unit. Gourévitch and Eggermont (2007) proposed that in cat auditory cortex the differences between natural and adapted calls were coded by differences in the overall firing rate of a unit, or the type of temporal response given, or by the overall synchrony of responses. The importance of temporal information in coding the characteristics of a neural response to a vocalization were also emphasised by other authors (Wang et al., 1995; Šuta et al., 2007; Huetz et al., 2009). This study investigates whether these methods of coding may also be used by cortical units to code for differences between pitch-shifted versions of the same communication call that mimic the age-related changes.
2. Materials and methods
2.1. Recording vocalizations
Ten pigmented guinea pigs (Cavia porcellus) were used to study the development of the short purr call; seven male and three female. Vocalizations were recorded from between 1–3 animals at a time, placed in the centre of a sound attenuating room. When guinea pigs are isolated, young animals typically produce isolation calls, while the adults usually tend not to vocalize (Berryman, 1976). When the animals were frightened or stressed they would not produce a purr call and on any one day only a few of the animals would vocalize. Vocalizations were recorded using a single-diaphragm condenser microphone (Model, B-5 Behringer) and the signal was passed via a mixer (Eurorack UB802) and a sound blaster (Creative, SBO 490) to a lap-top computer and stored using Adobe Audition 1 software (stereo, 24 bit float, 48.8 kHz sample rate). Vocalizations were recorded from the animals at various postnatal ages between p9 and p100. Vocalizations were separated into four age groups for analysis: 1) young pups, below p10; 2) older pre-sexually mature pups of p20–p30; 3) adolescent animals of p31–p55; 4) adult guinea pigs of p100. P30 was used as a boundary related to changes in sexual maturity because in our colony females have become pregnant as early as p28. Similarly in some strains of males the average age of first mounting occurs at about p30 while intromission/ejaculation occurs at p50–p55 (Harper, 1976). Animals vocalized in response to the experimenter clicking their tongue against the roof of their mouth (see Fig. 1a). Fundamental frequencies were measured from the spectra obtained by fast Fourier transformation of the call waveforms (Fig. 1b). The call duration and inter-pulse interval were also compared across ages.
Fig. 1.
(a) Spectrogram of four tongue clicks followed immediately by a short purr from an adult animal. (b) Fast Fourier transform of an adult short purr showing the fundamental frequency (F0) at 277 Hz.
2.1.1. Surgical preparation and cortical recording
Neural recordings were made in 9 pigmented guinea pigs (5 male and 4 virgin female) weighing 600–1007 g (aged p71–p133 days), all of which were also being used for another study. Neurophysiological recordings were undertaken within a sound attenuated chamber on a floating table. Anaesthesia was induced with urethane (0.9 g/kg in a 20% solution, i.p.). To suppress bronchial secretions a single injection of 0.2 ml atropine sulphate (0.06 mg/kg s.c.) was administered. Supplementary analgesia was maintained using injections (i.m.) of between 0.2 and 0.3 ml Hypnorm (Fentanyl citrate 0.315 mg/ml, fluanisone 10 mg/ml, Janssen). Once surgical anaesthesia was established, the trachea was cannulated and the animal was artificially respired with 100% oxygen using a Harvard small animal ventilator model 683. Any wax in the ear canals was removed before the animal was placed into a stereotaxic frame so that its tympanic membranes were clearly visible through hollow Perspex speculae.
To prevent pressure building up in the middle ear, polyethylene tubing was inserted into the auditory bullae. A small incision was made in the connective tissue above the foramen magnum to release the pressure of the cerebro-spinal fluid. A craniotomy was performed on the right side, above AI. The dura was removed and the cortex was covered with agar solution to avoid desiccation. The animal’s respiratory rate, end tidal CO2 and core body temperature were monitored and maintained within normal limits.
To maximize data collection we used multi-channel electrode arrays. Arrays were custom-manufactured by attaching 4–8 glass-insulated tungsten electrodes (Bullock et al., 1988) to a circuit board that attached directly to a headstage amplifier (Medusa, Tucker-Davis Technologies, Alachua, Florida). Extracellular potentials were amplified and filtered (300–3000 Hz). Responses were collected using Brainware (v7.43, Jan Schnupp, Oxford University) and exported into Matlab for further analysis. The recorded spikes were sorted using the Plexon Offline Sorter: clusters of spikes which had similar waveform properties were grouped together as belonging to a single unit. Statistical analysis was undertaken using Multivariate ANOVA, followed by pairwise analysis to investigate whether the clusters differed significantly from one another and the background noise.
Stimuli were delivered diotically through sealed acoustic systems. One representative exemplar of the adult short purr call was chosen because it had an F0 that was typical for a p100 animal, it had a good signal-to-noise ratio and a regular rhythm of sound pulses (18 Hz) that was towards the upper end of the range of pulse frequencies. A stimulus. wav file was prepared by choosing a zero crossing point at 100 ms before the onset of the call. The background noise was ramped up over a 25 ms segment from this point. The call was then manipulated so that it would appear to be from animals of different ages. This was achieved by pitch-shifting the call so that its fundamental frequency changed, but the duration of the call remained the same. The Dirac LE computer program changes the formant frequencies of the sound without changing the temporal structure. The call was adapted so that it appeared to be from animals aged <p10 (476 Hz F0), aged between p20–p30 (381 Hz F0), aged between p31–p55 (333 Hz F0) and adult at 100 + days (281 Hz F0). Each of the four short purrs was presented 30 times in a pseudo randomised order with their peak level at 80 dB SPL. Pure tones (100 ms) were also presented at different frequencies and attenuations to create frequency response areas. Tones ranged between 50 Hz and 33,780 Hz in 1/5 octave steps, and attenuations ranged between 5 and 95 dB SPL in 5 dB steps. Tone pips had rise/fall times of 2 ms, and were presented at a rate of 2/second. The sound delivery system was calibrated in every experiment as described previously (Wallace and Palmer, 2008).
2.2. Analysis of responses
Unit activity displayed as a peristimulus time histogram (PSTH), with 5 ms time bins, was used to identify responses where there was an increase in firing rate related to the beginning of the call or to one of the discrete pulses of sound that composed it. A peak in the firing rate was taken to be significant if, at the highest point, it was at least two standard deviations above the base firing rate and had a minimum of 8 spikes in one bin. Responses were classified as one of two types in the same way as our previous work (Wallace et al., 2005b): onset or multi-peaked. Onset response PSTHs contained only one significant peak within 200 ms of the start of the vocalization. Multi-peaked responses are those with two or more significant peaks within the PSTH. The temporal accuracy of the peaked responses was measured by calculating a correlation value as follows. The waveform envelope of each version of the purr sampled at 5 ms intervals was cross correlated with the PSTHs of the responses to each version computed in 5 ms bins. The cross correlation was computed as the envelope was progressively delayed in 5 ms increments from 10 to 80 ms and the largest value taken. This value gave a measure of the accuracy with which the response was time-locked to the envelope of the purr version but did not give any indication of the strength of the response. Thus this correlation value was multiplied by the mean firing rate of the unit over the duration of the response (from 0 to 1000 ms) to give a “correlated firing rate”. A unit was considered to discriminate between different versions of the short purr call if it responded with a change in correlated firing rate (20 or 50% change between the response to the best version and the second best version).
Spectral analysis of each of the four versions of the short purr was made to determine the range of sound levels present at each frequency in each version. Each version contained a band of sound levels which were systematically shifted towards higher frequencies as the corresponding age of the animal decreased. These bands of sound energy were then imported into Matlab and used as filters to superimpose on the frequency response area data. All the spikes produced by tone pips at frequencies and sound levels within the energy bands were summed for each version of the call and this was used to predict which version of the call ought to give the highest firing rate.
All experiments were carried out in accordance with UK Home Office regulations and with approval by the local Ethical Review Committee.
3. Results
3.1. Changes in the short purr with age
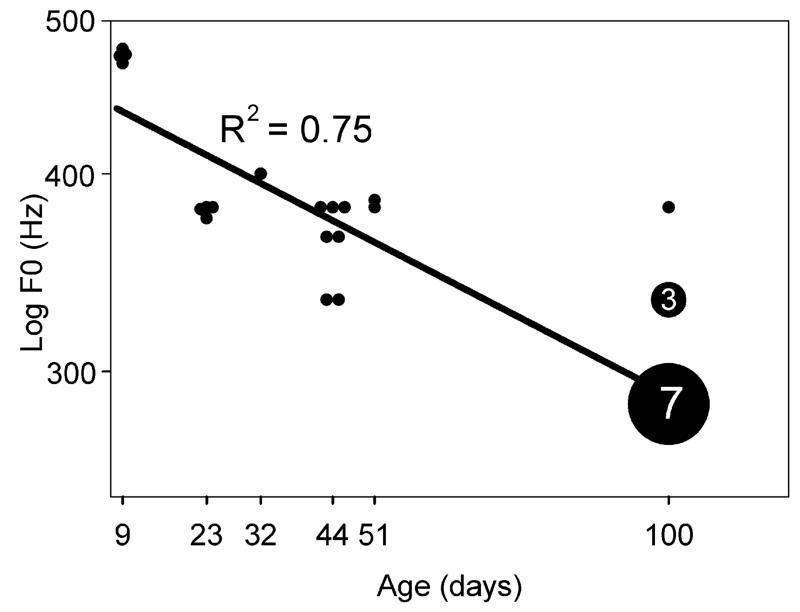
Stimulation with a clicking sound was a reliable way of producing the short purr call even in animals as young as 8 days old. At 8 days old, the short purr already had the distinctive rhythmic pulses of low-frequency sound at 13–20 Hz, but the pitch of the call was higher than that of adult animals. This is illustrated in Fig. 2 where the fast Fourier transforms of calls from a single animal at four different ages are compared. The F0 of the purr becomes progressively lower as the animal matures and there is also a general reduction in the relative energy levels at higher frequencies. The F0s of 30 recorded purr calls from animals aged 8–100 days and 28 calls from older animals were measured. Between the ages of 9 and 100 days there was a fall in F0 that was reasonably linear (R2 = 0.75) when the age was plotted on a log scale (Fig. 3). Beyond 100 days there was not any consistent change in F0 with increasing age. The calls were placed in four groups for further comparison. These groups were defined by the sexual maturity of the animals composing them. The mean F0 in the youngest, <p10, group was 476 Hz, in the older pup group, p2–ep30, it was 381 Hz, in the adolescent age group, p30–p55, it was 368 Hz and by p100 and above it was 297Hz. A one way ANOVA revealed a significant main effect of age on the F0 of the short purr calls for the four age groups: F (3, 57) = 130.6, p < 0.001). Post hoc tests using the Bonferroni correction revealed that the F0 of the calls from the young pup group, <p10, were significantly higher than those of the three groups of older animals (in each case p < 0.001). The mean F0 of the calls from the adolescent group, p31–p55, was not significantly different from the older, pre-weaned, pups. These post hoc tests also showed that purr calls from the adult, p100 and above age group were significantly lower frequency than the calls from younger guinea pigs (in each case p < 0.001).
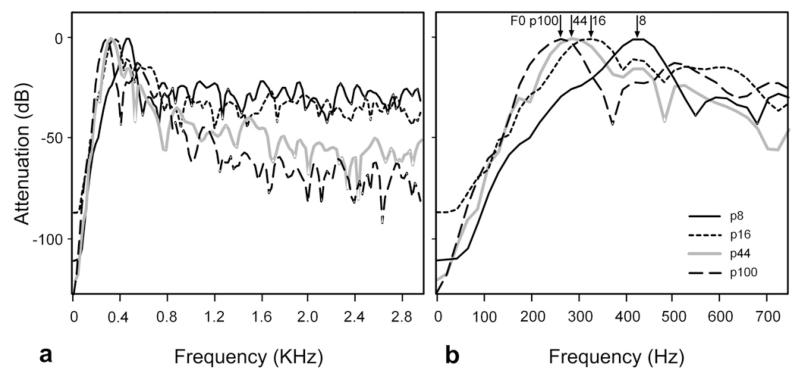
Fig. 2.
Spectral analysis of examples of the short purr call given by a single animal as it matured. (a) Fast Fourier transform of each call showing the relative energy at frequencies up to 3 kHz. (b) A more detailed analysis of the low frequencies showed that the F0 of the calls progressively fell between the ages of p8 and p100 (indicated by arrows).
Fig. 3.
Scatter plot showing the fundamental frequency (F0) of 30 examples of the short purr from animals of different ages. The larger symbols have greater numbers of examples – in this case 3 and 7. The linear regression line has a negative slope and a correlation value of 0.75. The F0 of the calls decreases as the animals increase in age/size.
The duration of the short purr call was very variable even for one animal at one age. Thus one p55 animal gave a series of short purrs during a single recording session that varied between 180 and 620 ms long. The inter-pulse interval and the duration of the individual pulses within the purr calls seemed to show as much variability between animals of the same age group as between animals of different age groups and we did not have sufficient data to make any reliable statistical analyses of these variables.
3.2. Efficacy of pitch-shifted versions of the short purr
It was not possible to find four perfectly matched examples of the short purr call from animals of different ages even when the same animal was used for recording. Calls varied in their duration, sound level and pulse rate even when obtained from the same animal during one recording session. Thus instead of matching four calls from animals of different ages we spectrally manipulated one adult call to produce four different pitches corresponding to the four different age groups studied. The spectra for these four versions are shown in Fig. 4. This illustrates the F0 changes for each of the versions and the associated increase in the range of frequencies making up the call. This higher range of frequencies was not detected in the calls from our youngest animals presumably because the purrs were produced at lower sound levels and the highest frequencies were too faint to be picked up by our recording system. In the original 281 Hz version there is a low peak in spectral energy at about 3 kHz whereas in the 476 Hz version the peak has shifted to about 5 kHz.
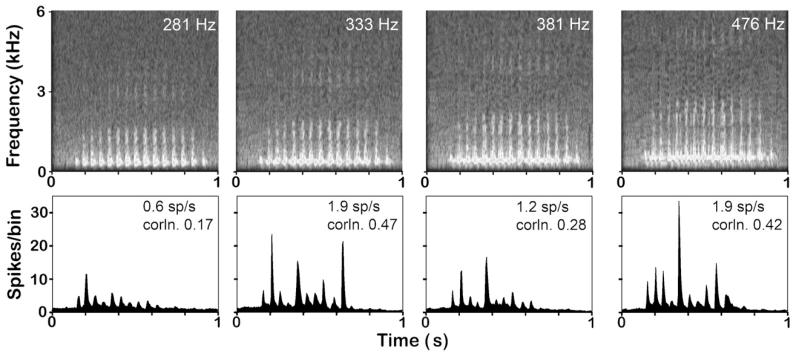
Fig. 4.
Spectrograms of pitch-shifted versions of the purr call. The 281 Hz version is the original recording of a short purr call from an adult animal which has been digitally manipulated to alter the frequencies of the sound waves making up the call without changing its temporal envelope. The 333 Hz version corresponds to the call from an animal of 44 days old, the 381 Hz version to a 16 day old and the 476 Hz version to an 8 day old animal. Underneath each panel is the population PSTH based on the responses of all 79 units that responded to the call. In the top right corner of each histogram is a number that gives the mean firing rate (spikes/s) and the mean correlation value between the PSTH and the corresponding version of the call.
The different versions of the call were presented in an interleaved fashion and the responses collected for comparison. To verify that none of the artificially manipulated versions was less salient as a stimulus than the others, we compared the population plots for the four versions (Fig. 4). The population response for each version was multi-peaked with peaks of various heights corresponding to each of the first 10 phrases of the purr. However, the mean firing rate varied between the different versions with the lowest F0 (281 Hz) version eliciting the lowest rate. The mean firing rate is given by the upper number in the right hand corner of each histogram. The mean firing rate was highest for the 333 and 476 Hz versions and intermediate for the 381 Hz version. Firing rate alone does not indicate how well matched the peaks in the PSTH are to the rhythmic pulses of the call. Therefore, we multiplied the mean firing rate by the mean correlation value to give the mean correlated firing rate. This value for each of the versions is shown as the lower number in the right hand corner of each histogram in Fig. 4. These values vary between the lowest for the 281 Hz version and the highest for the 333 Hz version. This showed that the artificially manipulated versions of the call were just as effective a stimulus as the original call (281 Hz) and indeed were more effective.
3.3. Location of purr responsive neurons
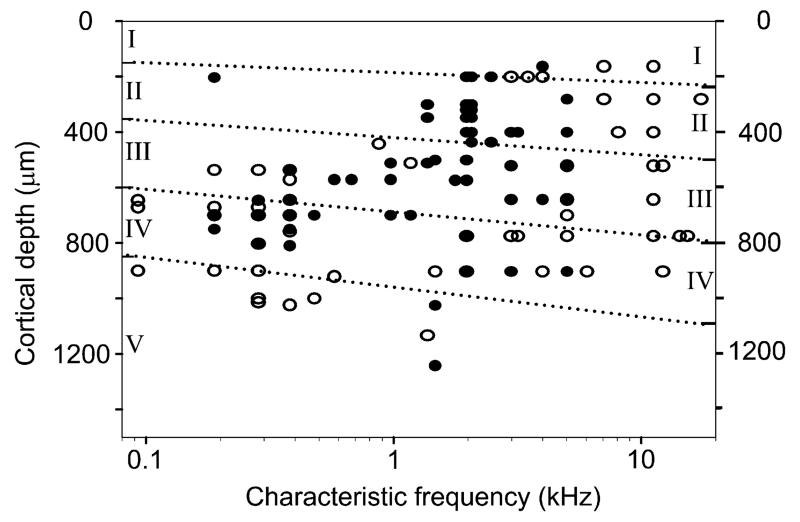
Our previous study of purr responsive neurons in AI had been restricted to the low-frequency end (≤3 kHz) (Wallace et al., 2005b). However purr responses have been described in 40% of units with CFs of 16–32 kHz in the inferior colliculus (Šuta et al., 2003) and in thalamic units with CFs of up to 15 kHz (Šuta et al., 2007). In this study we targeted a broad portion of AI and studied cells with CFs of 0.1–17 kHz (mean 3.5 kHz). A total of 182 sorted units were tested with the four versions of the purr and of these 79 responded to at least one version when using the peak measure. The responsive cells had a much smaller range of CFs which went from 0.2 kHz–5 kHz (mean 1.81 kHz, S.D. 1.3) as shown in Fig. 5. The non-responsive cells had a mean of 5.02 kHz, S.D. 4.8 and the two population means are significantly different (unpaired t(91) = 5.6, p < 0.001). By contrast, the laminar distribution of the responsive and non-responsive cells appeared to be very similar. The range of depths for non-responsive units was 100–1300 μm (mean 604 μm, S.D. 264). The range of depths of responsive units was 100–1242 μm (mean 534 μm, S.D. 207). The difference between the mean depths for the two populations is significant (unpaired t(174) = 2.05, p = 0.04) with the mean depth of the non-responsive cells being greater. However this appears to be due to the fact that many of the non-responsive cells were located closer to the high-frequency end of AI where the layers are thicker. No lesions were made to confirm the laminar position of recorded units but the depth of the laminae has been measured in previous studies for both the thick, high-frequency end of AI (Wallace and Palmer, 2008) and the thinner, low-frequency end of AI (Wallace et al., in press). The approximate depths of the laminar borders are indicated in Fig. 5 and they show that a large majority of the units were located in layers I–IV for both the responsive and non-responsive units.
Fig. 5.
Scatter plot showing the characteristic frequencies (CFs) and depths of recorded units. The solid black circles represent units that responded to at least one version of the purr while the clear circles represent units which did not give a significant response to any version of the purr. The thickness of AI varies along the tonotopic gradient and the thickness of the layers at the rostral, low-frequency end is indicated on the left axis while the high-frequency end is indicated on the right axis.
3.4. Ability of AI neurons to discriminate between different versions of the short purr
Clear pitch differences were easily discerned between the different versions of the purr by human listeners and there were also striking differences between the responses of some cortical cells in AI. Cells could potentially discriminate between different versions of the purr by changing either their overall firing rate or the strength with which their response was locked to the rhythmic pulses of sound in the purr. We had previously shown that cortical responses to the purr could be either onset or multi-peaked (Wallace et al., 2005b). Examples of both types were recorded in the present study and the same cell often gave different response types for different versions of the call.
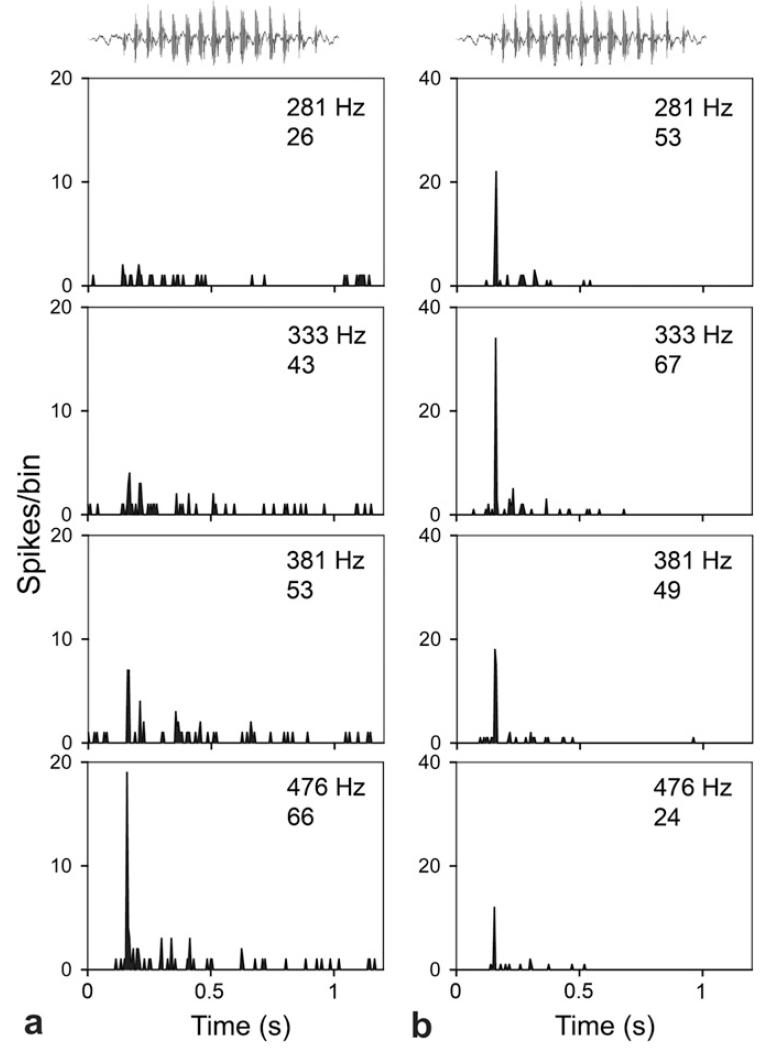
Examples of single units giving onset responses that showed large differences in firing rates between call versions are shown in Fig. 6. For the unit in Fig. 6a there was a clear response for the 476 Hz version of the call, but no significant responses to the other three versions based on the peak measure. The number of spikes fired during the duration of the call varied between 26 and 66 as shown by the lower number in each panel. The unit in Fig. 6b responded to all four versions of the call based on the peak measure, but the firing rate was highest for the 333 Hz version. The number of spikes fired during the call varied over a similar range from 24 to 67.
Fig. 6.
PSTHs showing the response to the four versions of the short purr by single units in AI. The waveform of the unmodified short purr is shown above the top panel. The numbers in the top corner of each panel indicates the version of the purr that was used as a stimulus and the number below shows the number of spikes recorded. (a) This onset unit only responds to the youngest (8 day) version. CF of unit is 4 kHz. (b) The firing rate for this onset unit is highest for the 44 day old version (333 Hz). CF of unit is 0.4 kHz.
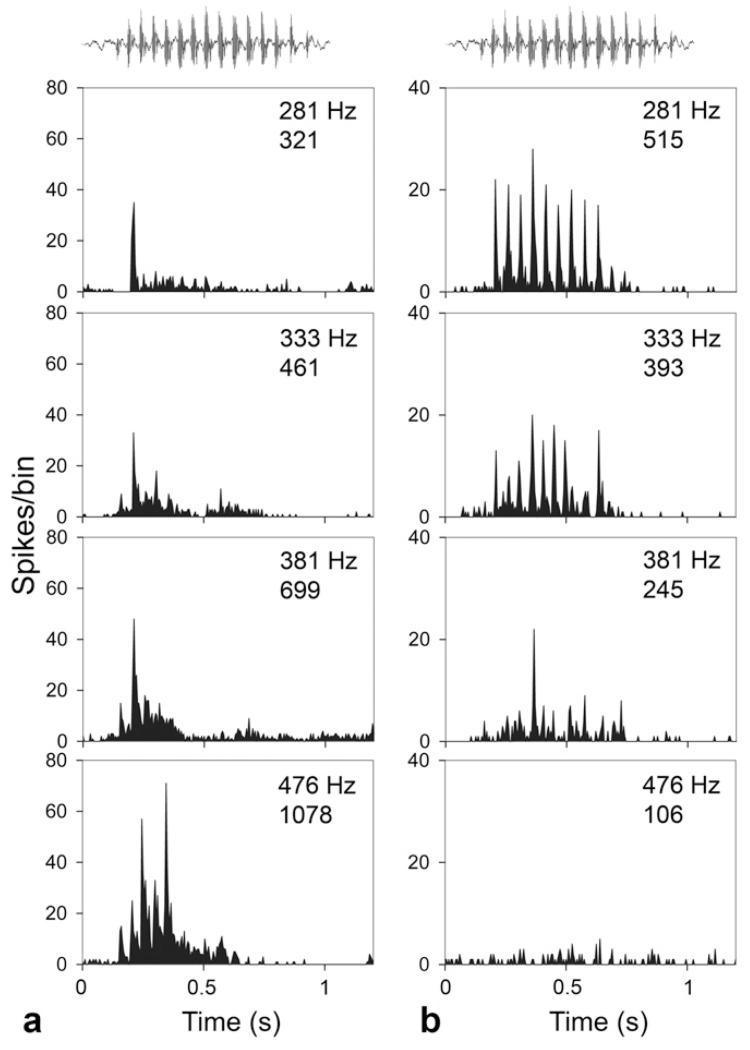
Examples of units giving multi-peaked responses to different versions of the call are shown in Fig. 7. In both cases, there is a monotonic relationship between the number of spikes produced and the F0 of the call. However one has a positive relationship between F0 and firing rate while the other has a negative relationship. The unit in Fig. 7a shows over three times as many spikes for the 476 Hz version as the 281 Hz version (positive slope) while the unit in Fig. 7b shows the opposite relationship with almost five times as many spikes for the 281 Hz version as the 476 Hz version (negative slope). Monotonic responses of either type were unusual (6/79 units) and the non-monotonic responses such as those shown in Fig. 6 were much more common (73/79). In Fig. 7a the response appears to change from an onset response to the 281 Hz version to a multi-peaked response to the 476 Hz version. However, the unit does give a small response to multiple components of the call even although they are not large enough to be classed as a peak by our criteria. The unit in Fig. 7b has an even more striking change in response pattern between the 281 and 476 Hz versions, as it goes from showing a rigorously time-locked response with multiple peaks to giving no response. The fact that the type of response could vary between different versions of the call meant that some units could not be classified uniquely as either onset or multi-peaked. The response to a particular version of the purr may be related to the unit’s frequency tuning (see Section 3.5).
Fig. 7.
PSTHs showing the response to the four versions of the short purr by multi-peaked units in AI in the same arrangement as in Fig. 6. (a) The response to the 281 Hz version is mainly an onset response whereas the response to the 476 Hz version is a multi-peaked response with a much higher firing rate. CF of unit is 2.5 kHz. (b) The firing pattern in this unit changes from a robust multi-peaked response to the 281 Hz version to a firing rate that is not significantly above background for the 476 Hz version. CF of unit 0.3 kHz.
The majority of units that responded to the short purr call on the peak measure responded to all 4 versions (53%, 42/79). Only 9% (7/79) were highly discriminatory on this measure responding to only one version of the short purr call. Many cells responded to 2 (19%, 15/79) or 3 versions, 19% (15/79). The majority of units that responded to multiple versions of the purr call responded with different response types to different versions (57%, 41/72).
The preference of units for a particular version of a call was calculated using a rate metric based on correlated firing rate. The correlation value was calculated for the response and then multiplied by the number of spikes. The majority of units showed a weak preference for one call (73%, 58/79) by responding to that version with a 20% higher firing rate than any other version. Further, 41% (32/79) showed a strong preference for one version, responding with a firing rate that was more than 50% higher than for any other version.
3.5. Relationship between version preference and spectral response characteristics
In this study, none of the responsive units had a CF above 5 kHz. However, within that group we expected that units with a preference for the lowest F0 (281 Hz) version of the purr to have a lower CF for pure tone stimulation than those with a preference for the highest F0 version (476 Hz). This was indeed the case. The 9 units that preferred the 281 Hz version (adult animal) had CFs ranging from 0.3 to 1 kHz and a mean of 0.42 kHz (S.D. 0.22). The 19 units that preferred the 476 Hz version (youngest age group) had CFs ranging from 0.2 to 5 kHz and a mean of 2.16 kHz (S.D. 1.13). Despite the high variance, these two populations still had significantly different means (unpaired t(21) = −6.46, p < 0.01). The mean CFs of the units preferring the two intermediate versions were not significantly different (unpaired t(8) = 1.7, p = 0.13). Both of these groups had a wide range of CFs: 0.4–3.2 kHz for version 2 and 0.2–3.5 kHz for version 3. Thus, although there was a trend for cells with the lowest CFs to be more responsive to the adult version of the call, we were unable to predict which version of a call a unit would prefer based solely on its CF.
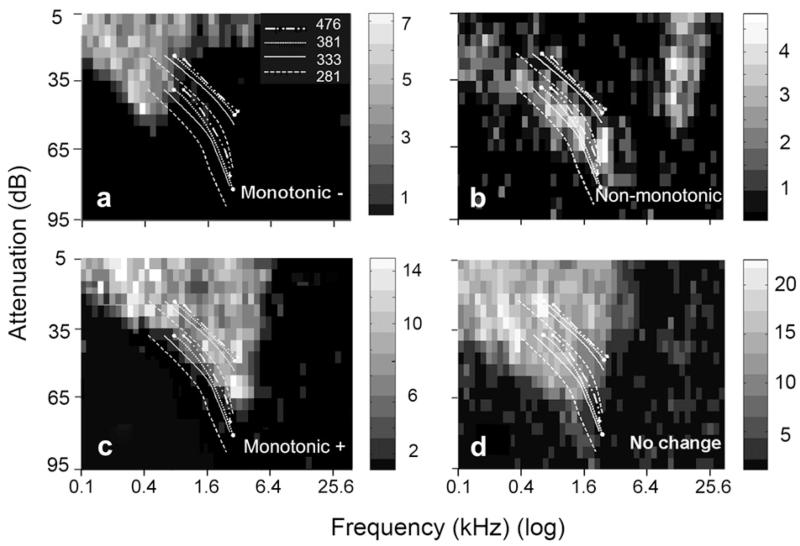
The CF is a convenient measure for use in comparing the spectral sensitivity of units but a more accurate method of assessment is provided by their frequency response area. By using the spectral range of each call version as a filter it was sometimes possible to correctly predict which version of the call would produce the greatest increase in firing rate. This is illustrated in Fig. 8 which shows the spectral energy for the four versions superimposed on the frequency response area for different types of unit. For 4/9 units with a CF of 300 Hz or lower the version with an F0 of 281 Hz had the greatest overlap and as the F0 increased there was a monotonic decrease in firing rate (see Fig. 8a which is the same unit as shown in Fig. 7b). Conversely for 13/37 units with a CF of 2–5 kHz the 281 Hz version had the least overlap and versions with increasing F0 had a monotonically increasing firing rate (see Fig. 8c which is the same unit as shown in Fig. 7a). Units which did not have a simple “V” shaped frequency response area could give a non-monotonic response to the call versions with one of the middle frequency versions having the highest firing rate (Fig. 8b). Other units, either with a “V” shaped or a more complex frequency response area, could have a fairly uniform firing rate over each of the four bands of spectral energy and showed less than a 20% change in firing rate between different versions (Fig. 8d). We found that in 43% of units (31/72) it was possible to correctly predict the rank order of firing rate for different versions of the call based purely on the frequency response area. Of the units that responded to one version with a 50% higher correlated firing rate than any other version, the best response was predictable by the frequency response area in 44% (14/32) of units. All the versions of the purr were presented at the same sound level and we made no attempt to study the effect of changing the sound levels on the responses. Changing the sound level of presentation has been shown to affect the responses to another low-frequency vocalization (chutter) in the guinea pig AI (Wallace and Palmer, 2009) and similar effects would be expected with the purr.
Fig. 8.
Frequency response areas from four units in AI in relation to the spectral energy bands of the four call versions. The four pairs of lines show the smoothed borders of the spectral energy band that corresponds to the version whose F0 is shown in the legend. (a) Low-frequency unit (same unit as in Fig. 7b) where the firing rate falls as the F0 of the purr version increases. (b) Double peaked unit where the firing rate does not change smoothly with increasing F0. (c) “V” shaped unit (same unit as in Fig. 7a) where the firing rate increases smoothly with increasing F0. (d) “V” shaped unit where the spectral energy bands are all within the frequency response area and all four versions of the purr produce a similar response. The grey level intensity of the rectangles forming the frequency response area indicates the firing rate of the unit measured in a 100 ms long recording window and indicated by the intensity chart at the side of each panel.
4. Discussion
4.1. Age-related changes in the short purr
The F0 of the short purr call was found to change significantly as the guinea pig increased in age. Calls from older animals were found to have a lower F0 than those of younger animals. This result is consistent with previous studies in some other animals (Charlton et al., 2009). Generally, younger animals have higher pitched communication calls (Darwin, 1871). The fundamental frequency of a call is determined by the length and stiffness of the vocal folds which determine the rate of the air pulses coming from the lungs (Fant, 1960). The formant frequencies of harmonic calls are then determined by the resonant frequencies of the cavities in the vocal tract (Smith and Patterson, 2005). As the vocal tract length increases the frequency gaps between these formant frequencies will decrease to give a reduction in formant dispersion (Fitch, 1997). In many animals, the vocal folds and the vocal tract length will grow at the same rate as an animal matures. In studying the purr, we chose to concentrate on the F0 of the call because it appears to be the dominant feature. Formants are not clearly defined in the purr call and in juvenile animals the purr call is very quiet and the upper formants are often not detectable on a spectrogram (Fig. 2). Despite this it is possible that changes in both F0 and formant dispersion within the purr call may be used by animals to estimate the age/size of the caller.
4.2. Behavioural relevance of the purr call
The short purr (drrr) is the most suitable for studying maturational changes because 1) it does not contain any noise bursts or frequency modulated sweeps; 2) it is easily elicited in young and old animals in response to a mild, non-distressing stimulus; 3) it’s F0 is not affected in any consistent way by altered stress levels; 4) it retains the same basic structure at all ages. The other main calls given by infants are isolation whistles, screams and tweets (Berryman, 1976). The tweet is a distinctive call that is not produced by adults. The infant isolation whistles appear to develop into the whistles and whines produced by adults but even in young animals the whistles are very variable. The mean frequency of isolation whistles has been shown to change significantly during a 15 min period of isolation (Monticelli et al., 2004). Screams are also variable and generally indicate that the animal is distressed.
The short purr is produced in response to a sudden, mildly alarming sound in the environment. We found that making one or more clicking sounds with the tongue could reliably elicit a purr providing the animal was not too frightened. When a group of animals are startled they will often all produce the short purr together (Arvola, 1974). This call may be important in the wild form of the guinea pig (Cavia aperea) as a means of keeping a family group together. Wild guinea pigs live in groups that normally stay within hearing range of each other and these alerting calls may be important in maintaining contact when the relatively tall vegetation obscures visual contact (Rood, 1972). The production of a short purr would allow other animals in the group to localize the caller. We have previously shown that there are columns of cells in cortical area AI that give multi-peaked responses to the purr call and are also sensitive to the interaural phase differences that may be the basis of localising low-frequency sounds in azimuthal space (Wallace and Palmer, 2009). Thus, some purr-sensitive columns are more sensitive to cues from ipsilateral or contralateral space and some may also be sensitive to auditory motion. In playback experiments using recorded vocalizations, Berryman (1981) showed that lactating females were particularly responsive to the purrs of juvenile animals and this is consistent with a maternal concern about the location of the young.
4.3. Sensitivity of cortical cells to different versions of the purr
Cortical cells showed surprising sensitivity to the different versions of the purr. Some cells only responded reliably to one version of the call (Fig. 6a) and many others had a strong preference for one version. This indicated that just using one exemplar of a call is not sufficient to determine whether or not a cell is responsive to a particular type of communication call. This is relevant to determining the selectivity of a cell to different call types and means that it will be difficult to develop a selectivity index for AI in the way that has been done for other auditory areas and the prefrontal cortex (Tian et al., 2001; Romanski et al., 2004). Another confounding factor is anaesthesia. All our animals were anaesthetised with urethane, but anaesthetics are known to have a potentially profound effect on neuronal responses to vocalizations and the responses in an awake guinea pig might have been very different (Syka et al., 2005).
In the guinea pig auditory thalamus (Philibert et al., 2005) and cortex (Wallace et al., 2005a,b) there are two main types of response to the purr. One is an onset response that marks the beginning of the call and the other is a multi-peaked response that involves a higher firing rate and a temporal representation of the rate of sound pulses within the call. One way of separating different aspects of the call at the level of AI would have been by using onset cells to code one type of information and the multi-peaked cells to code another type of information. However, our results suggest that both types of response may provide information relevant to animal size and that an individual cell can flip between the different coding strategies. Cells of both types were capable of showing large changes in firing rate in response to different versions of the call. Cells in AI are thought to give a sustained response when stimulated by a particularly relevant stimulus (Wang et al., 2005) and we have suggested that cells with a multi-peaked response have particular input filters that make them ideally suited to the purr, whereas cells with an onset response may be giving a more general response that is common across a potentially wide range of stimuli. The current results mean that we have to refine that hypothesis, and now suggest that the multi-peaked responses are sometimes only produced by a particular version of the purr rather than by purr as a particular class of vocalization. Our results suggest that many cells within a population (both onset and multi-peaked) may contain information about the size of the caller and that this could be coded by firing rate integrated over the first few phrases of the call. The multi-peaked cells may have an additional role in providing temporal information about the call. The purr is a multi-functional call as the long version of it (also called a rut rumble) is associated with sexual arousal and mating behaviour (Rood, 1972; Arvola, 1974; Berryman, 1976). Thus the multi-peaked responses may be of particular relevance in representing the duration or other aspects of the long purr that are relevant to mating behaviour.
4.4. Cortical representation of call significance
Studies of cortical responses to vocalizations are often made on cells in AI because they can be very sensitive to conspecific calls and are sensitive to morphological manipulations of a call in a variety of species including cat (Gehr et al., 2000) and monkey (Wang and Kadia, 2001). Thus, for the purr call, previous studies have shown responses in guinea pig AI that are as good or better than those in any of three surrounding areas (Wallace et al., 2005a,b; Syka et al., 2005) or the ventral medial geniculate body of the thalamus (Philibert et al., 2005; Šuta et al., 2007; Huetz et al., 2009). These assessments have been made both in terms of firing rate and temporal locking to the individual pulses of sound. However, a distinction has been made between perception and recognition so that some cells might respond well to a call (perception), but not be able to distinguish between different variants of it that had different behavioural significance (recognition). In one study of responses to mouse pup wriggling calls the cells that were able to discriminate between significant and non-significant versions of a call were only found in higher order areas and not in AI (Geissler and Ehret, 2004). However, in a study of which areas of the human brain were involved in processing the acoustic effect of size in speech sounds von Kriegstein et al. (2006) suggested that there was a two stage processing of the parameters relevant to source size. The initial stage may have involved the medial geniculate body while the second stage appeared to involve higher order areas of the cortex. In the present study, 41%(32/79) of purr-responsive cells in AI appeared to have a role in the recognition of different body sizes based on changes in the purr call. These formed two groups: one with a “simple”, predictable relationship between spectral tuning properties and version preference (14/32 units) and one with a more “complex” version preference where responses were not predictable by tuning properties alone (18/32 units). This implies that some cells in AI may represent a basic stage of processing similar to that found in the ventral medial geniculate body (Šuta et al., 2007; Huetz et al., 2009) while others have a more complex response that may indicate higher order processing that would be expected in a secondary area. Two levels of hierarchical complexity have already been found in the responses of cells in the mouse AI area in response to natural calls (Lin and Liu, 2010).
The low-frequency end of AI, where the purr-responsive cells are mainly located, has a distinctive set of functional properties (Wallace and Palmer, 2009). It also has projections to both bimodal (somato-sensory/auditory) and non-auditory areas of the parietal cortex (Wallace et al., 2002). As such, it has a different set of projections to the primate cortex where AI projects primarily to the adjacent belt areas (Kaas and Hackett, 1998) and the proisocortex area which may also be part of the medial belt (de la Mothe et al., 2006). Pitch-sensitive cells have been described at the low-frequency border between AI and the rostral area in the marmoset and these may have a role in analysing vocalizations (Bendor and Wang, 2005). However, although some of our units were very sensitive to the pitch-shifted versions of the purr this may just have indicated that the units were sensitive to the frequency differences at F0. We did not test them to determine if they were also sensitive to pitch differences in complex harmonic sounds with a missing fundamental. As a result we did not confirm that they were homologous to the neurons that are thought to represent pitch in the primate brain (Bendor and Wang, 2005). Nevertheless we can conclude that the anaesthetised guinea pig cortex is still a useful preparation for studying the mechanisms for processing the subtle signals that are contained in vocalizations.
Abbreviations
- AI
primary auditory cortex
- CF
characteristic frequency
- F0
fundamental frequency
- PSTH
peristimulus time histogram
- S.D.
standard deviation
References
- Arvola A. Vocalization in the guinea-pig, Cavia porcellus l. Ann. Zool. Fennici. 1974;11:1–96. [Google Scholar]
- Bendor D, Wang X. The neuronal representation of pitch in primate auditory cortex. Nature. 2005;436:1161–1165. doi: 10.1038/nature03867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryman JC. Guinea–pig vocalizations: their structure, causation and function. Z. Tierpsychol. 1976;41:80–106. doi: 10.1111/j.1439-0310.1976.tb00471.x. [DOI] [PubMed] [Google Scholar]
- Berryman JC. Guinea pig responses to conspecific vocalizations: playback experiments. Behav. Neural Biol. 1981;31:476–482. [Google Scholar]
- Bizley JK, Walker KMM. Sensitivity and selectivity of neurons in auditory cortex to the pitch, timbre, and location of sounds. The Neuroscientist. 2010;16(4):453–469. doi: 10.1177/1073858410371009. [DOI] [PubMed] [Google Scholar]
- Bryant GA, Haselton MG. Vocal cues of ovulation in human females. Biol. Lett. 2009;5:12–15. doi: 10.1098/rsbl.2008.0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock D, Palmer AR, Rees A. A compact and easy to use tungsten-in-glass microelectrode manufacturing workstation. Med. Biol. Eng. Comput. 1988;26:669–672. doi: 10.1007/BF02447511. [DOI] [PubMed] [Google Scholar]
- Charlton BD, Zhihe Z, Snyder RJ. The information content of giant panda, Ailuropoda melanoleuca, bleats: acoustic cues to sex, age and size. Anim. Behav. 2009;78:893–898. [Google Scholar]
- Clutton-Brock TH, Albon SD. The roaring of red deer and the evolution of honest advertising. Behaviour. 1979;69:145–170. [Google Scholar]
- Darwin C. The Descent of Man and Selection in Relation to Sex. Murray; London: 1871. [Google Scholar]
- de la Mothe LA, Blumell S, Kajikawa Y, Hackett TA. Cortical connections of the auditory cortex in marmoset monkeys: core and medial belt regions. J. Comp. Neurol. 2006;496:27–71. doi: 10.1002/cne.20923. [DOI] [PubMed] [Google Scholar]
- Fant G. Acoustic Theory of Speech Production. Mouton; The Hague: 1960. [Google Scholar]
- Fitch WT. Vocal tract length and formant frequency dispersion correlate with body size in rhesus macaques. J. Acoust. Soc. Am. 1997;102(2):1213–1222. doi: 10.1121/1.421048. [DOI] [PubMed] [Google Scholar]
- Fitch WT, Hauser MD. Vocal production in nonhuman primates: acoustics, physiology and functional constraints on “honest” advertising. Am. J. Primatology. 1995;37:191–219. doi: 10.1002/ajp.1350370303. [DOI] [PubMed] [Google Scholar]
- Gehr DD, Komiya H, Eggermont JJ. Neuronal responses in cat primary auditory cortex to natural and altered species-specific calls. Hear. Res. 2000;150:27–42. doi: 10.1016/s0378-5955(00)00170-2. [DOI] [PubMed] [Google Scholar]
- Geissler DB, Ehret G. Auditory perception vs. recognition: representation of complex communication sounds in the mouse auditory cortical fields. Eur. J. Neurosci. 2004;19:1027–1040. doi: 10.1111/j.1460-9568.2004.03205.x. [DOI] [PubMed] [Google Scholar]
- Gourévitch B, Eggermont JJ. Spatial representation of neural responses to natural and altered conspecific vocalizations in cat auditory cortex. J. Neurophysiol. 2007;97:144–158. doi: 10.1152/jn.00807.2006. [DOI] [PubMed] [Google Scholar]
- Hall DA, Plack CJ. Pitch processing sites in human auditory cortex. Cereb. Cortex. 2009;19(3):543–553. doi: 10.1093/cercor/bhn108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper LV. Behavior. In: Wagner JE, Manning PJ, editors. The Biology of Guinea Pig. Academic Press; New York: 1976. pp. 31–51. [Google Scholar]
- Hollien H, Green R, Massey K. Longitudinal research on adolescent voice change in males. J. Acoust. Soc. Am. 1994;96(5):2646–2654. doi: 10.1121/1.411275. [DOI] [PubMed] [Google Scholar]
- Huetz C, Philibert B, Edeline J-M. A spike timing code for discriminating conspecific vocalizations in the thalamocortical system of anesthetized and awake guinea pigs. J. Neurosci. 2009;29(2):334–350. doi: 10.1523/JNEUROSCI.3269-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas J, Hackett TA. Subdivisions of auditory cortex and levels of processing in primates. Audiol. Neurootol. 1998;3(2-3):73–85. doi: 10.1159/000013783. [DOI] [PubMed] [Google Scholar]
- Lin FG, Liu RC. Subset of thin spike cortical neurons preserve the peripheral encoding of stimulus onsets. J. Neurophysiol. 2010;104(6):3588–3599. doi: 10.1152/jn.00295.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli PF, Tokumaru RS, Ades C. Isolation induced changes in Guinea Pig Cavia porcellus pup distress whistles. Ann. Braz. Acad. Sci. 2004;76(2):368–372. doi: 10.1590/s0001-37652004000200027. [DOI] [PubMed] [Google Scholar]
- Philibert B, Laudanski J, Edeline J-M. Auditory thalamus responses to guinea-pig vocalizations: a comparison between rat and guinea-pig. Hear. Res. 2005;209:97–103. doi: 10.1016/j.heares.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Averbeck BB, Diltz M. Neural representation of vocalizations in the primate ventrolateral prefrontal cortex. J. Neurophysiol. 2004;93:734–747. doi: 10.1152/jn.00675.2004. [DOI] [PubMed] [Google Scholar]
- Rood JP. Ecological and behavioural comparisons of three genera of Argentine cavies. Anim. Behav. Monogr. 1972;5:1–83. [Google Scholar]
- Siemers BM, Beedholm K, Dietz C, Dietz I, Ivanova T. Is species identity, sex, age or individual quality conveyed by echolocation call frequency in European horseshoe bats? Acta Chiropterologica. 2005;7(2):259–274. [Google Scholar]
- Smith DRR, Patterson RD. The interaction of glottal-pulse rate and vocal-tract length in judgements of speaker size, sex, and age. J. Acoust. Soc. Am. 2005;118(5):3177–3186. doi: 10.1121/1.2047107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šuta J, Kvašňák E, Popelář J, Syka J. Representation of species-specific vocalizations in the inferior colliculus of the guinea pig. J. Neurophysiol. 2003;90:3794–3808. doi: 10.1152/jn.01175.2002. [DOI] [PubMed] [Google Scholar]
- Šuta D, Popelář J, Kvašňák E, Syka J. Representation of species-specific vocalizations in the medial geniculate body of the guinea pig. Exp. Brain Res. 2007;183:377–388. doi: 10.1007/s00221-007-1056-3. [DOI] [PubMed] [Google Scholar]
- Syka J, Šuta D, Popelář J. Responses to species-specific vocalizations in the auditory cortex of awake and anesthetised guinea pigs. Hear. Res. 2005;206:177–84. doi: 10.1016/j.heares.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Tian B, Reser D, Durham A, Kustov A, Rauschecker JP. Functional specialization in rhesus monkey auditory cortex. Science. 2001;292:290–293. doi: 10.1126/science.1058911. [DOI] [PubMed] [Google Scholar]
- von Kriegstein K, Warren JD, Ives DT, Patterson RD, Griffiths TD. Processing the acoustic effect of size in speech sounds. Neuroimage. 2006;32:368–375. doi: 10.1016/j.neuroimage.2006.02.045. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Palmer AR. Laminar differences in the response properties of cells in the primary auditory cortex. Exp. Brain Res. 2008;184:179–191. doi: 10.1007/s00221-007-1092-z. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Palmer AR. Functional subdivisions in low-frequency primary auditory cortex (AI) Exp. Brain Res. 2009;194:395–408. doi: 10.1007/s00221-009-1714-8. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Rutkowski RG, Palmer AR. Interconnections of auditory areas in the guinea pig neocortex. Exp. Brain Res. 2002;143:106–119. doi: 10.1007/s00221-001-0973-9. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Rutkowski RG, Palmer AR. Responses to the purr call in three areas of the guinea pig auditory cortex. Neuroreport. 2005a;16:2001–2005. doi: 10.1097/00001756-200512190-00006. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Shackleton TM, Anderson LA, Palmer AR. Representation of the purr call in the guinea pig primary auditory cortex. Hear. Res. 2005b;204:115–126. doi: 10.1016/j.heares.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Coomber B, Sumner CJ, Grimsley JMS, Shackleton TM, Palmer AR. Location of cells giving phase-locked responses to pure tones in the primary auditory cortex. Hear. Res. doi: 10.1016/j.heares.2010.05.012. in press. doi:10.1016/j.heares.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Wang X, Kadia SC. Differential representation of species-specific primate vocalizations in the auditory cortices of marmoset and cat. J. Neurophysiol. 2001;86:2616–2620. doi: 10.1152/jn.2001.86.5.2616. [DOI] [PubMed] [Google Scholar]
- Wang X, Merzenich MM, Beitel R, Schreiner CE. Representation of a species-specific vocalization in the primary auditory cortex of the common marmoset: temporal and spectral characteristics. J. Neurophysiol. 1995;74:2685–2706. doi: 10.1152/jn.1995.74.6.2685. [DOI] [PubMed] [Google Scholar]
- Wang X, Lu T, Snider RK, Liang L. Sustained firing in auditory cortex evoked by preferred stimuli. Nature. 2005;435:341–346. doi: 10.1038/nature03565. [DOI] [PubMed] [Google Scholar]