Abstract
Purpose
Accommodation can mask hyperopia and reduce the accuracy of non-cycloplegic refraction. It is therefore important to minimize accommodation to obtain as accurate a measure of hyperopia as possible. In order to characterize the parameters required to measure the maximally hyperopic error using photorefraction, we used different target types and distances to determine which target was most likely to maximally relax accommodation and thus more accurately detect hyperopia in an individual.
Methods
A PlusoptiX SO4 infra-red photorefractor was mounted in a remote haploscope which presented the targets. All participants were tested with targets at four fixation distances between 0.3m and 2m containing all combinations of blur, disparity and proximity/looming cues. 38 infants (6-44 wks) were studied longitudinally, and 104 children (4 -15 yrs (mean 6.4)) and 85 adults, with a range of refractive errors and binocular vision status, were tested once. Cycloplegic refraction data was available for a sub-set of 59 participants spread across the age range.
Results
The maximally hyperopic refraction (MHR) found at any time in the session was most frequently found when fixating the most distant targets and those containing disparity and dynamic proximity/looming cues. Presence or absence of blur was less significant, and targets in which only single cues to depth were present were also less likely to produce MHR. MHR correlated closely with cycloplegic refraction (r = 0.93,mean difference 0.07D,p=n.s.,95%CI ±<0.25D) after correction by a calibration factor.
Conclusion
Maximum relaxation of accommodation occurred for binocular targets receding into the distance. Proximal and disparity cues aid relaxation of accommodation to a greater extent than blur, and thus non-cycloplegic refraction targets should incorporate these cues. This is especially important in screening contexts with a brief opportunity to test for significant hyperopia. MHR in our laboratory was found to be a reliable estimation of cycloplegic refraction.
The motivation for this study was to determine how best to estimate a maximally hyperopic spherical refraction (MHR) using non-cycloplegic photorefraction. In our laboratory this is particularly important for our research into the development of accommodation because we test accommodative responses in typically developing infants at frequent intervals and we need to be able estimate refractive error without cycloplegia. Such estimates are a particular problem for infants and children because many are known to be hyperopic and this hyperopia may change rapidly in infancy 1, 2. In hyperopia, efforts to clear fixation targets often mean that accommodation may mask the maximum refractive error and make undilated measurements inaccurate. Cycloplegic refraction gives a “gold standard” measure of refractive error in children, but cycloplegic refraction is not practicable with frequently repeated sessions, unpopular with parents and arguably ethically questionable in typically developing infants, so we were keen to ascertain the most accurate non-cycloplegic estimate of refraction for our research.
Outside the research context, it is not practicable to use cycloplegic refraction in large-scale screening situations, and so non-cycloplegic autorefraction is commonly used for detecting and assessing the presence of significant refractive error. It is quick, acceptable to children and can be administered by less highly trained personnel. There is always, however, a risk of underestimation of hyperopia3 (and over estimation of myopia4, 5) if accommodation is active. Recent reports by Dahlman-Noor et al 3, 6 show that the Plusoptix SO4 photoscreener that we use in this study, if used in the screening mode as a stand-alone test, where the subject fixates the fixation LEDs at 1m with both eyes open, may underestimate refractive error and may miss significant clinical problems. Kaakinen and Ranta-Kemppainen 7, using a two-flash method, also reported false negatives, and under-referral of hyperopia, as did Ghose et al using a NR-1000F Auto Refractometer 8. Hyperopia is, however, arguably the most important refractive error to detect in young children because of its association with strabismus and amblyopia 9-13. Hyperopia is also reported to be associated with poor progress at school 14,15 and poorer motor skills 16. It is therefore important to develop screening paradigms which have the best chance of correctly detecting or excluding hyperopia, and therefore lead to more hyperopic children receiving prompt correction.
Commercially available photo screeners use a variety of target types and all have been the subject of numerous publications assessing sensitivity and specificity , often comparing one method with another or with cycloplegic or manifest refraction4, 17-25, but none have addressed fixation target type in particular. The Medical Technology & Innovations (MTI) photoscreener has been extensively tested on large numbers of children13, 26-28 and uses flashing fixation LEDs at a testing distance of 1m , testing both eyes simultaneously. The Nikon Retinomax Autorefractor 18, 20, 29 tests each eye separately, uses fixation lights visible as the subject looks into the hand-held instrument while the Welch Alleyn SureSight autorefractor 4, 24 uses a testing distance of 35cm, LED fixation lights and tests each eye sequentially, but with both eyes open during testing. The Shin-Nippon SRW-5000 30 is an open-field autorefractor and so fixation targets or distance are not fixed. Tthere is little consensus as to the best target to use for screening, with a variety of targets, testing distances, monocular / binocular, simultaneous or sequential measurements, and none include proximal or looming cues (except for the Retinomax which may introduce some consistent instrumental proximal cues).
In more general accommodation research it may be important to open the accommodation negative feedback loop to study the characteristics of motor responses. Most methods used are based on the assumption that blur is the main cue to accommodation and so providing a target with ambiguous or unreliable blur cues will open the loop and place a greater emphasis on any available disparity and proximity cues. In addition to the LEDs commonly used by commercial autorefractors, experimental situations have used pinholes or difference of Gaussian (DoG) targets 31 to open the accommodation loop. It would therefore be reasonable to use such targets in clinical situations where minimizing blur- driven accommodation is the aim.
Most childhood and clinical studies report non-cycloplegic refractions to be more variable and more myopic than those performed under cycloplegia 32-37. In optometric practice, the fogging technique is a common method used to minimize accommodation during refraction 38, 39 or biometry 40 and so minimize this difference. Queiros et al 41 used autorefraction to compare open field accommodative responses with non-fogged viewing, +2.00D fogging lenses, and responses with cycloplegia and found that fogging lenses helped relaxation of monocular accommodation, but Kee et al 40 found considerable inter-subject variation in response to the fogging lenses.
In terms of target distance, Suryakumar & Bobier 42 compared different types of autorefractor at the manufacturer’s recommended testing distances and also added a 3.5m DoG target. They found that a combination of farther testing distances and a DoG target aided detection of maximum hyperopia.
Many of the above studies only reported results from one eye 40, 41, even if both eyes are tested, the majority test each eye separately, and some did not specify whether the participants were occluded at the time of refraction 42, so it is difficult to compare studies in relation to binocular status. We 43, and others 44 have found in both infants and older subjects, that disparity cues have a large influence on accommodative responses, supporting views for a strong role for vergence accommodation 45-47, so disparity may also play a role in the relaxation, as well as the exercise of accommodation. From the outset of this study, therefore, we hypothesized that binocular status may be influential in accommodation responses.
Proximal cues also have a role in driving accommodative responses, and adult studies report a significant, but very variable, role in experimental situations 48-50. We have reported that, in our laboratory, proximal cues are a weak stimulus for influencing additional accommodation for near targets in older children and young adults when either presented in isolation, or removed in isolation from a rich stimulus43. It is possible that in early infancy, where not only may disparity detection be immature 51, 52, but blur cues unreliable due to poor acuity 53 or the high prevalence of refractive errors 1 , proximal cues may be more important than for adults. We were only able to find one relevant study of proximal cue use in children 53 which reported variable proximal accommodation responses in a small number of infants tested.
Although there have been reports comparing different photoscreening methods 54-57 and others comparing accommodative responses to some of the techniques commonly used to relax accommodation 38, 39, 41, there have been no reports specifically addressing a wide spectrum of target types during autorefraction in a within-subjects design with a range of participants and age-groups.
Our laboratory has been investigating accommodation and vergence responses to different combinations of the three main near cues of disparity, blur and proximity/looming using an autorefraction technique in a large group of participants from infancy to adulthood. We have used this dataset to establish the target type that maximizes hyperopic refraction within a testing session and have compared this estimate of refraction to that obtained from a “gold standard” cycloplegic retinoscopy (mean spherical equivalent (MSE)) in a subsample of participants. We have considered whether increasing target distance beyond 1 meter increases accuracy and whether statistical differences are large enough to be clinically significant. We have also examined the data to ascertain whether our findings are applicable across the age span. If we can demonstrate that they are, our findings may also help to improve accuracy of photoscreening and refraction in a wider context.
Methods
All studies adhered to the tenets of the Declaration of Helsinki and were scrutinized by University of Reading and UK National Health Service Ethics Committees. Adults and parents of children under 6 years gave fully informed consent. Parents of children older than 6 years gave fully informed consent and the children themselves gave informed assent appropriate to their age.
Our laboratory uses a remote haploscopic videorefractor (RHV) to measure vergence and accommodation responses in naturalistic conditions. This apparatus has been described in detail elsewhere 43 but is described briefly here. It combines two optical pathways, one for target presentation and manipulation and one for data capture (Figure 1). The participant sees the target approaching and receding in the mid-line, while infra-red photorefraction occurs in the same plane independent of target position.
Figure 1.
Remote haploscopic videorefractor. A. Motorised beam. B. Target monitor. C. Upper concave mirror. D. Lower concave mirror. E. Hot mirror. F. Image of participant’s eye where occlusion takes place. G. PlusoptiX SO4 PowerRef II. H. Headrest J. Raisable black cloth screen.
Photorefraction Pathway
We use a commercially available infra-red photorefractor (PlusoptiX S04, Plusoptix GmbH, Nurenberg, Germany). This is primarily marketed and used for child vision screening in the “C-Mode” but also incorporates a PowerRefII (“R-Mode”) that makes simultaneous recordings of accommodative state and gaze direction, which we are using to carry out our more detailed studies. In our laboratory the PlusoptiX S04 captures the image of the participant’s eyes via a large 600mm diameter “hot” mirror which reflects infra-red wavelengths but allows through visible light. As we are interested in binocular responses, the camera is mounted in the midline between the eyes and the fixation LEDs on the photorefractor are covered with opaque tape. When no target is presented, the infra-red sources can be seen subjectively as very faint red dots, but when any fixation target is on the target monitor, these are obscured by the brighter target elements and are invisible to the participant.
During the calibration phase of our studies we compared accommodation measured by the Monocular Estimate Method 58 with the raw refraction data provided by the PlusoptiXSO4 while a group of participants fixated the same target under the same conditions of illumination at 2m, 1m, 0.5m and 0.33m. While the PlusoptiXSO4 made a good estimate of refraction at its recommended testing distance of 1m (mean 0.127 D, paired t-test; t(58) = 0.13, ns), we consistently measured a smaller accommodative response (more hyperopic spherical refraction) to target demand with the RHV in comparison to dynamic retinoscopy, and this difference increased with increasing levels of accommodation, as found by Harb et al 59 using an earlier version of the PowerRefractor. We used a formula derived from the slope of the fitted line of a plot of the two sets of data as a correction function of 1.2385x+0.799, where x = accommodation measured by the PowerRefII mode in the PlusoptiXSO4, to adjust estimates of refraction in our lab on all participants. Plots of raw refraction data were made and representative vignettes of 25 consecutive and stable data points (1 sec of data) were selected for averaging once responses had settled after target movement stopped (within 1-2 secs of the target being stationary even in the infants).
Target Pathway
The targets are presented on a video monitor mounted on a motorized beam and viewed via two concave mirrors such that the image is placed optically at target positions between 0.25m and 2m from the participant. The target moves at approximately 1m per second. Targets are presented at five different fixation distances in a pseudo-random order (0.33m, 2m, 0.25m, 1m, 0.5m), representing 3D, 0.5D, 4D,1D & 2D demand. Data from the 0.25m target was discarded due to the unacceptable loss of data caused by small pupils in many participants, but the target position was retained in the presentation sequence because it meant that a distant target was always presented after a near one and vice versa. For the 2m and 1m targets, the target had been preceded by a near target (“anti-looming” if target movement was visible), while for the 0.25m and 0.5m targets it had been preceded by a more distant target (looming).
The advantages of the mirror system are that target presentation and photorefraction can occur in the same plane without the sensors obscuring the target, or vice versa, and also that disparity cues can be removed by occluding half of the upper mirror remote from the participant (F in Figure 1), so there is no distracting occluder visible to the participant.
Targets
The same range of targets was used for all participants, designed to maximize or minimize access to blur, disparity and proximity cues separately. Blur cues were made available by using a high contrast brightly colored clown target containing a wide range of spatial frequencies. Blur cues were minimized using a similar sized DoG target against a black background, which has been found to open the blur- driven accommodation loop 31. Each target had two versions which alternated at 1Hz to maintain attention of the youngest participants. The light background color of the DoG target alternated between yellow and green (chosen as both were towards the middle of the color spectrum) and the mouth and eyes of the clown target alternated between oval eyes and circle mouth and cross-shaped eyes and smiley mouth, while each of the concentric nose rings changed from red to blue and vice versa, but the black outlines of these nose elements remained constant. The level of detail available or position of any of the target elements did not change during the alternation of either type of target. Disparity cues were available when both eyes viewed the target, and eliminated by remote occlusion at the level of the upper mirror. The occlusion is invisible to the participant and even approximately 30% of adults were unaware that they had been monocular at times. Looming / proximity cues were made available by presenting the same physical size of the target at each fixation distance and allowing the participant to watch the monitor move between target positions (both the clown and the DoG targets subtended 3.15° at 2m and 18.26° at 33cm). Proximity cues were minimized by scaling the targets so that they subtended the same angular subtense at each fixation distance (3.15°), and also by obscuring the participants’ view of the screen with an opaque black cloth screen as it moved between fixation distances so that the target was only uncovered once the monitor had stopped moving and its position could not be guessed from changing size cues.
We were therefore able to present all combinations of the three main cues to vergence and accommodation. Although the monitor and camera are mounted within black painted shuttering, some residual minimal looming and blur cues are still available from the background luminance of the black screen background against the screen edge, despite efforts to mask this with graduated filters, so a “zero” cue condition was also included to assess the impact of residual cues we could not eliminate. Testing order was standardized across all participants and was designed to maximize infant data in our larger developmental study, where a full testing session with all cue combinations presented might exceed attention span, but where we were particularly interested in the relative use of the different cues. We, and others, have reported that infant attention reduces under monocular conditions 44, 60 and we anticipated that removing either of the other two cues could have similar effects, while removing two of the three cues might be even more disruptive. In order to maximize data in infants with limited attention, we chose to present the all-cue (blur, disparity & proximity (bdp – binocular, looming, clown)) condition first, followed by a block of the three conditions in which one-cue was removed (bd (binocular, scaled clown), bp (looming, occluded clown) or dp (binocular, looming DoG) with testing order counterbalanced across participants. If attention permitted, we then tested the three conditions in which one cue only was presented (b (occluded, scaled clown),d (binocular, scaled DoG) or p (occluded, looming DoG)), also counterbalanced between participants. A penultimate “zero cue” (occluded, scaled DoG) was presented next, followed by a final all-cue (bdp) condition. Repeating the all-cue condition at the end enabled us to assess whether waning attention was due to reducing cues or fatigue. All participants reported here were those who completed testing with all eight target conditions. With all except the youngest infants, testing was repeated within the testing session in a counterbalanced order.
Participants
Participants were recruited from the Infant Database and Psychology Undergraduate Research Panel at the University of Reading, as well as local hospital children’s eye clinic patients and their siblings. As we were interested in providing data that could be used to improve testing in unselected populations we have included all the participants tested in our laboratory who were able to complete testing with the full range of targets. We therefore did not select on the basis of visual acuity, refractive error or binocular status. Any participants showing refractions outside the operating range of the PowerRef II (−7.0D to +5.0D) at any time were excluded.
38 infants were able to provide a full dataset and were seen on between one and nine occasions (mean 3.05 visits) between the ages of 6 and 44 weeks as part of a longitudinal study of typical development. None have subsequently developed strabismus. As refractive error is known to change rapidly throughout early infancy we have included data from repeated testing sessions on the same infants. 104 children between 4 and 15 years were assessed (mean age 6.4yrs SD±1.9yrs). 52 of these were developing typically with visual acuity of better than 0.2 LogMAR in either eye and no strabismus. 52 children had a refractive error within the operating range of the PlusoptiX S04 and/or strabismus. Six had small angle constant strabismus with gross stereopsis (better than 100 secs arc on the Titmus stereotest) and 33 had intermittent eso- or exotropia with normal binocular vision (60 seconds of arc on the TNO stereotest ) when the deviation was controlled. We could have excluded the strabismic children on the basis that their responses to disparity stimuli might have been somewhat different, although these children all had good motor fusion ranges and so might be expected to have functioning disparity detection mechanisms, but if this data is to be used in an unselected screening context, this might have reduced its clinical usefulness. We checked for differences in their data in the analysis. For this study, measurements on all participants (whether spectacle wearers or not) were carried out without spectacles. We also tested 85 young adults between 19 and 25 years of age. All had had a recent subjective refraction. 59 of the adults did not wear a correction (refraction MSE within 0.75D of emmetropia) and the others had a range of refractive errors and were tested either with their own contact lenses (n=16) or without glasses if worn (n=10).
All non-infant children and adults were tested on only one visit but measurements were repeated within the session to assess for repeatability. As many of our studies are on infant development, we made strenuous efforts to ensure that our older participants were completely naïve to vision experiments and the theory of vision. None of the child or adult participants had been given orthoptic exercises that might have changed their habitual responses to blur or disparity cues.
Of this large group of infants and children, we were able to obtain recent cycloplegic refraction data on 59 participants. 19 were infants at 26 weeks of age, 28 were children between 4 and 9 years of age and the remaining 12 were adults. This testing was carried out 40 minutes after using 2 drops of cyclopentolate hydrochloride 1% in each eye, within 3 months of testing in the laboratory for the children and adults and within one month for the infants, who might be emmetropizing more rapidly.
In all cases MHR was defined as the most hyperopic refraction obtained with any target and any testing distance for each participant in each testing session i.e. which stimulus condition produced MHR on that visit. If two stimuli or testing distances produced similar refractions, only the very largest was used, even if the difference was small.
Analysis
Data were recorded and analyzed initially using Excel. Statistical analyses were carried out using SPSS 14.
Results
Data were available from 316 testing sessions with 227 participants. Because of the testing sequence used, all targets were tested at least once, but the bdp target was tested at the beginning and end of testing. Examination of repeated data (bdp at the start and end of each testing sequence, and repetition of all targets in a counterbalanced order if co-operation allowed) showed no significant differences in accommodation responses between testing early or late in the sequence (p>0.4 in all comparisons), i.e. there were no fatigue or attention-related effects.
For each participant, the target which produced the maximally hyperopic refraction during the session was determined. Figure 2 shows the percentage of MHR found for each target condition. There was a significant difference in the distribution of the MHR across targets (χ2= 110.0, df 7, p<0.00001). MHR was most frequently found when using the bdp (binocular, looming clown) and dp (binocular, looming DoG) targets. These two target conditions together contributed 49.8% of all maximum hyperopia / minimum myopias.
Figure 2.
Percentage of MHR found for each target condition
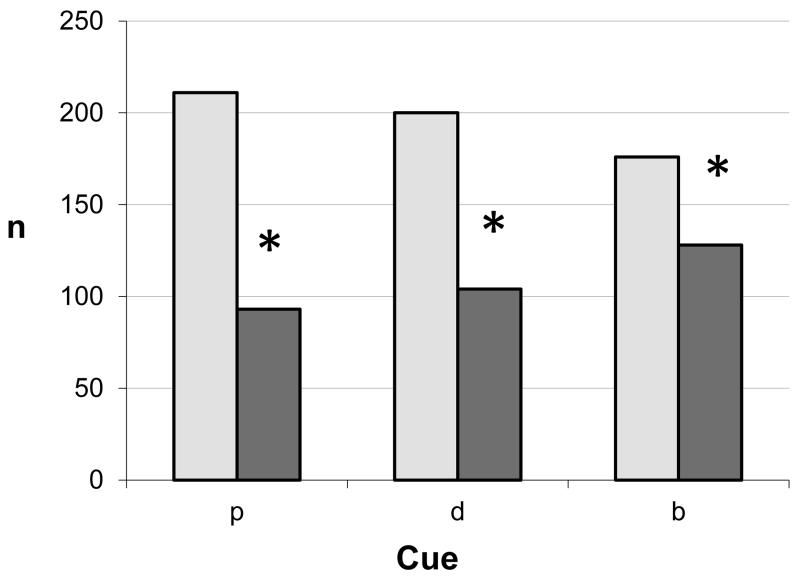
Figure 3 shows the numbers of MHR found if a target did, or did not contain an individual cue. Any target that contained proximal / looming clues (bdp, dp bp and p) was more effective in producing maximum hyperopic error than those that did not (χ2 = 111.6, df 1, p < 0.00001). A similar comparison between targets that contained disparity cues versus those without disparity showed that MHR was found more often when the target contained disparity cues (χ2 = 54.1, df 1, p<0.00001) but the effect for proximity was larger than for disparity. The MHR was also more likely to be found in targets that included blur as a cue to depth than those without (χ2 = 12.83, df 1, p<0.0003)
Figure 3.
Distribution of MHR according to whether an individual cue was present or absent in the target. Pale bars = cue present, dark bars = cua absent. p = proximity/looming(bdp,bp,dp,p targets vs. bd,b,d,o), d = disparity (bdp,bd,dp,d targets vs. bp,b,p,o), b = blur (bdp,bd,bp,b vs. dp,d,p,o). Asterisks denote significant differences p<0.0003 in all cases.
The data were then divided by age group. We grouped the data into 3 groups - infants, children between 4 & 15 years, who have passed the most active phases of the visual critical period 61, 62 but who would be expected to have the highest accommodative ability due to their high accommodative amplitude 63, 64 in comparison to adults, and adults (Figure 4).
Figure 4.
Distribution of MHR across age groups and target. There were no significant differences between age groups.
There were no significant differences in the distribution of the target which produced the MHR between age groups. The largest age difference was in the dp condition, where infants showed proportionally more MHR than children or adults, but even this difference failed to reach significance (χ2=1.89, df 2, p=0.38).
Figure 5 shows the percentage of MHR found at each target distance with Figure 5a showing the results for all participants and Figure 5b showing the results for only those participants with an MHR greater than +2.00D. When all participants were considered together, the MHR was overwhelmingly found for the most distant target (χ2=305.2, df 3, p,0.0001). When examined by age group this pattern remained stable (p<0.0001 in all cases). When the higher refractive errors (>+2.00DS) were examined separately, MHR’s were found almost equally at the 0.5 and 1D targets (χ2=0.02, df 1, p=0.88). Although small numbers limited statistical analysis of these hyperopes by age, it was noticeable that of the 19 hyperopic participants over 4 yrs of age there appeared to be less association between target distance and MHR (n=3,7,6,3 at 0.5D, 1D, 2D and 3D demand respectively).
Figure 5.
Target distances where MHR found (percentages). a) all participants (n = 316) b) hyperopes >2.00D only (n = 55)
We considered whether the significant difference in refraction between fixation at 1m and 2m (as found by Suryakumar & Bobier 42) was large enough to be clinically meaningful and whether it differed across targets. Mean accommodation at 0.5D demand was significantly less than that at 1D across all target conditions (mean difference 0.23D, 95%CI ± 0.05D (F=159.7, df1,292, p<0.0001) but with no significant interaction with target type (F(7,2044)=1.3,9=0.22)(Fig 6). The variance in these data was remarkably similar. There was a small difference in variance between target type (F(7,4832)=2.41,p=0.019), with the bdp target having the smallest variance, but there were no difference between the variances for the 0.5D and 1D target distances (F(7,4838)=2.46,p=0.116), and, overall, these differences in standard error (between ±0.125 and 0.156D) were not large enough to be clinically significant.
Figure 6.
Accommodation responses at 2m (0.5D) and 1m (1D) fixation distances. NB. Includes a wide range of refractive errors and ages. Note similar size standard error bars in every cue condition.
We next considered how MHR compared with other actual and extrapolated measures of refraction we had available in our dataset. In previous studies, we have used the y-intercept of the accommodative response against demand as an estimate of refraction at infinity, and therefore maximum refractive error 60. Although in the past we have only tested all-cue and disparity-free conditions, in the current study, both accommodation y-intercept and maximum hyperopic refraction found for all cues were available, so we compared y-intercept with MHR within individuals and targets.
As with the MHR counts, the maximally hyperopic intercepts for most individuals were found with the bdp and dp targets, but even the most hyperopic of the mean y-intercepts ( found in the bdp condition) is 0.32D less hyperopic than the mean MHR for the same condition(t=9.94, df 315, p<0.00001).(Figure 7)
Figure7.
y-intercepts of mean accommodation (response against target demand) by target type (dotted line = mean across all targets). Minimum (most hyperopic) y-intercepts also found in the bdp and dp conditions, but always less hyperopic than mean MHR (dashed line) in the same particpants.
Finally, we were able to compare MHR and mean spherical equivalent (MSE) derived from cycloplegic retinoscopy on the 59 participants for whom we had recent data (Figure 8).
Figure 8.
MHR and y-intercept of accommodation against demand compared with refraction obtained from cycloplegic (cyclo)refraction (mean spherical equivalent). Filled points and solid fit line = MHR vs. cyclo (linear coefficients : y = 1.057x - 0.056, r2 = 0.870) . Open points and dotted fit line = y-intercept vs. cyclo (linear coefficient: y = 0.495x - 0.218, r2 = 0.300).
Mean cycloplegic retinoscopy was only 0.07D (±95%CI of 0.23D) more hyperopic than MHR, with a high correlation co-efficient of 0.87 in this very heterogeneous group. Although our laboratory findings, involving an additional calibration factor used with the PlusoptiX SO4, cannot be directly transferred to a clinical screening setting, if, as an example, MHR is compared with the “gold standard” cycloplegic retinoscopy, using a criterion of +2.0 for a marginally clinically significant refractive error, MHR showed a sensitivity of 83.3% and a specificity of 91% in detecting refractive error of >2.00D, comparing very favorably with other methods 55, 56, 65. If the same comparison is made with y-intercept of accommodation against demand using the bdp target (which we found the most accurate of the estimates of refraction in the past 60, 66 (open data points in Figure 8), sensitivity falls to 45% indicating that some hyperopes would not be detected by using y-intercept, although specificity remained high at 95%.
When the data from 39 children with intermittent strabismus or microtropia were examined separately, although numbers in the cue groups were too small for statistical analysis there were no noticeable differences in the pattern of distribution MHR between the cues.
Discussion
The primary motivation for this analysis was to determine how best to estimate refractive error in a group of infants we are studying in our laboratory. In doing so we have also collected data from participants of all ages, using a repeated measures design, the same equipment and lighting conditions and a minimal instruction set. The only experimental manipulation was the target type and position. Our findings, therefore, have wider clinical applications
In general, more cues are better than fewer when assessing maximal hyperopic error. Despite literature suggesting that minimizing blur cues helps relax accommodation 38-41, more MHRs were found with targets containing target detail than those which did not. While all three cues appear significantly associated with helping to relax accommodation, including proximity (dynamic “anti-looming”) and disparity in the target appears the most effective in relaxing accommodation. The target most likely to elicit maximum hyperopia or minimum myopia for an individual was not necessarily a blurred target, as might be expected from the common clinical use of fogging lenses to relax accommodation. Presence or absence of blur was the least influential of the three near cues we tested, but clear targets produced more MHRs than did blurred ones. Removing blur from the 3-cue condition (bdp vs dp), or adding blur as a single cue (b vs o condition) made little difference to the proportions of MHR found between these categories. This intuitively surprising finding differs from the findings of Queiros et al 41 who found that fogging lenses helped relax accommodation. Suryakumar & Bobier 42 found that refraction using a DoG target was more hyperopic than using a LED fixation target. However in their study the different fixation distances used with these two targets make it difficult to differentiate the effect of the target from that of fixation distance. They also state, in an appendix to the paper, that a pilot study failed to find differences between LEDs and high contrast accommodative targets at the same fixation distance. It is possible that the differences in our data may be explained by our DoG target being too blurred or qualitatively different in comparison to the usual +2.00D fogging lens, and so induce some pseudo-myopia 67 rather than relaxing accommodation, but Chiu et al 38 have suggested that the amount of fogging is of relatively little importance, so this explanation seems unlikely.
It is also possible that in the children with higher refractive errors, who may have blurred vision anyway without their spectacles, alteration of blur in the target would make little difference to retinal image clarity (although Chen et al 68 found no differences in the response to blur between myopes and emmetropes). If this was the case, the data from these individuals might skew the data and so cause an underestimation of the overall role of blur in typical individuals. However, in a screening situation, where the object is to detect significant refractive errors, from these data it seems clear that where blur may be an unreliable cue in the target group, disparity and proximity are even more strongly the best cues to drive relaxation of accommodation and help reveal maximum hyperopia.
Recent findings published by our laboratory and others in both infants and adults 43, 44, 60, reporting a very strong role for disparity cues and a relatively weak one for blur cues, made us unsurprised by our finding a significant role for disparity. Reducing disparity seems here to help relax accommodation in the distance as much as increasing disparity drives it for near. This was especially true when considering the large difference in number of MHRs between bdp vs bp conditions, although alone disparity seems to have little effect (the d vs o condition).
The strong effect of proximity / looming was less expected. Like disparity, it seems to have a weak effect as a single cue (p vs o condition), but in combination with disparity it was the cue which predicted the highest proportion of MHRs. We have reported that proximity is an extremely weak cue in comparison to disparity 43 in driving both vergence and accommodation to near targets in naïve adults (as opposed to in those with some knowledge of vision experiments as studied by others 69). Hung et al 70, also suggested that proximity played a small part under naturalistic conditions, but here, in combination with disparity in a relatively naturalistic setting, the dynamic “negative looming” of the target as it moves from near to distance seems to help in relaxing accommodation in the distance, even if it is a weak cue in driving it for near.
Although some studies have looked at the best target and testing distance to help relax accommodation 41, 42, none have looked systematically at target type and distance in the same participants. Our findings largely support those of others 42 in that distant targets relax accommodation more than nearer ones, but we suggest that additional hyperopia can be revealed in many individuals by using a binocular, visibly receding target.
The bdp and dp targets together resulted in MHR in only around 50% of the participants, and MHR could be found in any of the other target conditions. We accept that there is considerable inter-individual variability which needs further study, and ideally a range of targets should be used in unselected populations. Our testing used repeated presentations and there may also be some random distribution of MHR between targets or other influential factors such as fatigue or alertness. By using randomized testing orders, and checking for order effects, we have tried to control this as much as possible and despite this, have still found significant effects. In the context of developing screening paradigms, also subject to such variability, pragmatic decisions must be made to increase the probability of a single target producing the most accurate result, which this study might help.
It is not surprising that the most distant target produced most MHRs, and we found that the difference in refraction between the 2m and 1m targets remained relatively constant across targets. Suryakumar & Bobier 42 also found that the farthest distant targets relax accommodation the most, but also found that responses were less variable at these greater fixation distances. We found non-significant differences in the variance between the two most distant fixation targets in any of the cue conditions. In participants with refraction > + 2.0D, MHR occurred less reliably at the 2m target (more equally distributed between 2m and 1m), possibly suggesting more variability or less sensitivity to target distance in these individuals, which would benefit from further study.
When analyzed by age group and refractive error, we found no systematic age differences, so our findings are useful not only in our laboratory, but also in clinical settings. The inaccuracy of non-cycloplegic methods of estimating refraction, especially of large populations, has been a major cause of loss of sensitivity and specificity in screening, especially of the major target group of hyperopic pre- and school entry children 3, 8, 71. This study has shown that changing target type can help to produce more non-cycloplegic measures that are closer to those made under cycloplegia.
In the past we have used y-intercept of accommodation response slopes as a proxy measure of refraction in our laboratory 60, 66, 72 and Hainline et al 73 have used a slightly different, but also fitted, curve function. This was based on the assumption that a measure derived from multiple data points that did not include a measure at infinity would be the most accurate estimate of refraction at infinity in the absence of cycloplegia. We have become increasingly aware that, in the variable responses of hyperopes and infants especially, this is clearly not always the case and we have shown here that MHR at any time within a session is a more reliable estimate of true refraction in our laboratory, as demonstrated by the close correlation with cycloplegic refraction (with narrow confidence limits of less than ±0.25D). It is unfortunate that the scope for statistical analysis of our categorical data was somewhat limited and so further corroborative research may be necessary.
A further area for future research is to consider groups that would not be expected to have normal disparity detection mechanisms e.g. the very youngest infants under 12-16 weeks, before stereopsis has fully developed 51, and strabismic older children with constant suppression. Our numbers were too small here, and we had no participants with total absence of binocularity, but we would predict that disparity cues would be less influential in these individuals and responses may differ depending on the strength of binocularity or suppression. Such groups also have a high prevalence of refractive error, so they may rely even more heavily on proximal cues.
These data have some wider clinical implications. In terms of refractive errors, while myopia may be more of a problem with older children, hyperopia is arguably the most pressing condition for younger children. As well as reducing visual acuity, hyperopia is co-morbid with strabismus, amblyopia and failure at school 74, 75 and needs more prompt referral to avoid amblyopia and loss of binocularity. It may, however, remain undetected or underestimated if accommodation is exerted at the time of testing. Picking a target that increases the chances of detecting maximum hyperopia is clearly preferable in young children.
It has been argued that it may not be important to detect well-compensated hyperopia 7 but there are some reports of increased accommodative lag in hyperopic infants 73 and children 76 . In our laboratory we see some hyperopic children who accommodate appropriately, but only for near targets while not accommodating for distance (thus giving steep accommodation response slopes) while others retain a consistent amount of lag throughout, staying hyperopic in comparison to target demand whatever the fixation distance (and so with appropriate response slopes). This latter group may be disadvantaged when reading, but “pass” screening as low hyperopes, despite struggling against higher errors. As Mutti 77 suggests, there are many clinical unknowns in the correction of hyperopia that need further research, with a “gray zone” around the lower levels of hyperopia of 2-3D. Although a young child may manage with the visual stress of additional accommodation, or blur, imposed by their hyperopia, it is still not known which if these children will be educationally disadvantaged or put at risk of strabismus by its lack of correction.
In the absence of cycloplegia, there are many optometric techniques available to reveal maximum hyperopia during a detailed subjective refraction within a comprehensive and skilled examination. We did not assess the sustained responses that are necessary for such testing and so our findings may not necessarily transfer to these situations. Autorefraction screening situations, however, often use unskilled personnel in a “one off” event and using a pass/fail criterion. We have found that changing the target increases the chances of revealing a maximum hyperopia which is very close to that of a cycloplegic refraction. Our findings appear to be consistent across all the participants tested, so may be useful in developing techniques to reduce false positives in the case of myopia and false negatives in the case of hyperopia. We found that MHR correlated extremely well with cycloplegic refraction, although in our laboratory setting during the calibration stages of our equipment we found a small systematic underestimation of accommodation in relation to dynamic retinoscopy in adults. Others have also reported such discrepancies 59. We were unable to make individual calibrations on our child and infant participants and so have used adult group mean adjustments throughout. The correction factor we used in our laboratory setting would need to be tested in less artificial conditions (i.e. in daylight and not through mirrors) if the PlusoptiX SO4 output was to be adjusted for free-space testing. Whether or not a correction factor is used, the high correlation coefficient of MHR with cycloplegic retinoscopy of 0.87 is impressive, so using a target that is most likely to reveal this MHR should be the aim of any photoscreening testing paradigm.
No one target always produces MHR, and MHR can be found with any target, so non-cycloplegic autorefraction still risks missing some hyperopic children. Nevertheless, a binocular target, visibly receding into the distance, whether blurred or clear, increases the probability of maximum accommodative relaxation, so increasing sensitivity & specificity in detecting hyperopia. Adding a dynamic looming component to a binocular fixation target may also aid subjective refraction in office situations and may be a fruitful topic for future clinical research.
Acknowledgements
This research was supported by a UK Department of Health Research Capacity Development Fellowship Award PDA 01/05/031 to AMH.
References
- 1.Ehrlich D, Braddick O, Atkinson J, Anker S, Weeks F, Hartley T, et al. Infant Emmetropization: Longitudinal changes in refraction components from nine to twenty months of age. Optom Vision Sci. 1997;74:822–43. doi: 10.1097/00006324-199710000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Gwiazda J, Thorn F, Bauer J, Held R. Emmetropisation and the progression of manifest refraction in children followed from infancy to puberty. Clin Vis Sci. 1993;8:337–44. [Google Scholar]
- 3.Dahlmann-Noor AH, Vrotsou K, Kostakis V, Brown J, Heath J, Iron A, et al. Vision screening in children by Plusoptix Vision Screener compared with gold-standard orthoptic assessment. Br J Ophthalmol. 2009 Mar;93(3):342–5. doi: 10.1136/bjo.2008.138115. [DOI] [PubMed] [Google Scholar]
- 4.Iuorno JD, Grant WD, Noel LP. Clinical comparison of the Welch Allyn SureSight handheld autorefractor versus cycloplegic autorefraction and retinoscopic refraction. J Aapos. 2004 Apr;8(2):123–7. doi: 10.1016/j.jaapos.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Buchner TF, Schnorbus U, Grenzebach UH, Busse H. Examination of preschool children for ametropia: first experiences using a new hand-held autorefractor. Strabismus. 2004 Jun;12(2):111–7. doi: 10.1080/09273970490517854. [DOI] [PubMed] [Google Scholar]
- 6.Dahlmann-Noor AH, Comyn O, Kostakis V, Misra A, Gupta N, Heath J, et al. Plusoptix Vision Screener: the accuracy and repeatability of refractive measurements using a new autorefractor. Br J Ophthalmol. 2009 Mar;93(3):346–9. doi: 10.1136/bjo.2008.138123. [DOI] [PubMed] [Google Scholar]
- 7.Kaakinen K, Ranta-Kemppainen L. Screening of infants for strabismus and refractive errors with two-flash photorefraction with and without cycloplegia. Acta Ophthalmol (Copenh) 1986;64(5):578–82. doi: 10.1111/j.1755-3768.1986.tb06976.x. [DOI] [PubMed] [Google Scholar]
- 8.Ghose S, Nayak BK, Singh JP. Critical evaluation of the NR-1000F Auto Refractometer. Br J Ophthalmol. 1986 Mar;70(3):221–6. doi: 10.1136/bjo.70.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sjostrand J, Abrahamsson M. Can we identify risk groups for the development of amblyopia and strabismus? (in German) Klin Monatsbl Augenheilkd. 1996;208(1):23–6. doi: 10.1055/s-2008-1035163. [DOI] [PubMed] [Google Scholar]
- 10.Atkinson J, Braddick O, Robier B, Anker S, Ehrlich D, King J, et al. Two infant vision screening programmes: prediction and prevention of strabismus and amblyopia from photo- and videorefractive screening. Eye. 1996;10(( Pt 2)(10)):189–98. doi: 10.1038/eye.1996.46. [DOI] [PubMed] [Google Scholar]
- 11.Atkinson J, Braddick O. Infant precursors of later visual disorders: Correlation or causality. In: Yonas A, editor. 20th Minnesota Symposium on Child Psychology; 1988; Lawrence Erlbaum Associates; 1988. pp. 35–65. [Google Scholar]
- 12.Williams C, Northstone K, Howard M, Harvey I, Harrad RA, Sparrow JM. Prevalence and risk factors for common vision problems in children: data from the ALSPAC study. Br J Ophthalmol. 2008 Jul 1;92(7):959–64. doi: 10.1136/bjo.2007.134700. 2008. [DOI] [PubMed] [Google Scholar]
- 13.Donahue SP, Johnson TM, Ottar W, Scott WE. Sensitivity of photoscreening to detect high-magnitude amblyogenic factors. J Aapos. 2002 Apr;6(2):86–91. doi: 10.1067/mpa.2002.121168. [DOI] [PubMed] [Google Scholar]
- 14.Williams WR, Latif AH, Hannington L, Watkins DR. Hyperopia and educational attainment in a primary school cohort. Arch Dis Child. 2005 Feb;90(2):150–3. doi: 10.1136/adc.2003.046755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shankar S, Evans MA, Bobier WR. Hyperopia and emergent literacy of young children: pilot study. Optom Vis Sci. 2007 Nov;84(11):1031–8. doi: 10.1097/OPX.0b013e318157a67a. [DOI] [PubMed] [Google Scholar]
- 16.Atkinson J, Nardini M, Anker S, Braddick O, Hughes C, Rae S. Refractive errors in infancy predict reduced performance on the movement assessment battery for children at 3 1/2 and 5 1/2 years. Dev Med Child Neurol. 2005 Apr;47(4):243–51. doi: 10.1017/s0012162205000472. [DOI] [PubMed] [Google Scholar]
- 17.Allen PM, Radhakrishnan H, O’Leary DJ. Repeatability and validity of the PowerRefractor and the Nidek AR600-A in an adult population with healthy eyes. Optom Vis Sci. 2003 Mar;80(3):245–51. doi: 10.1097/00006324-200303000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Cordonnier M, De Maertelaer V. Comparison between two hand-held autorefractors: the Sure-Sight and the Retinomax. Strabismus. 2004 Dec;12(4):261–74. doi: 10.1080/09273970490886594. [DOI] [PubMed] [Google Scholar]
- 19.Davies LN, Mallen EA, Wolffsohn JS, Gilmartin B. Clinical evaluation of the Shin-Nippon NVision-K 5001/Grand Seiko WR-5100K autorefractor. Optom Vis Sci. 2003 Apr;80(4):320–4. doi: 10.1097/00006324-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Gole GA, Schluter PJ, Hall J, Colville D. Comparison of the Retinomax autorefractor with hand-held retinoscopy in 1-year-old infants. Clin Experiment Ophthalmol. 2003 Aug;31(4):341–7. doi: 10.1046/j.1442-9071.2003.00668.x. [DOI] [PubMed] [Google Scholar]
- 21.Kimura S, Hasebe S, Ohtsuki H. Systematic measurement errors involved in over-refraction using an autorefractor (Grand-Seiko WV-500): is measurement of accommodative lag through spectacle lenses valid? Ophthalmic Physiol Opt. 2007 May;27(3):281–6. doi: 10.1111/j.1475-1313.2007.00466.x. [DOI] [PubMed] [Google Scholar]
- 22.Pappas CJ, Anderson DR, Briese FW. Is the Autorefractor reading closest to manifest refraction? A comparison of the patient’s previous spectacles and the 6600 Autorefractor reading. Arch Ophthalmol. 1978 Jun;96(6):997–8. doi: 10.1001/archopht.1978.03910050521002. [DOI] [PubMed] [Google Scholar]
- 23.Pappas CJ, Anderson DR, Briese FW. Clinical evaluation of the 6600 Autorefractor. Arch Ophthalmol. 1978 Jun;96(6):993–6. doi: 10.1001/archopht.1978.03910050517001. [DOI] [PubMed] [Google Scholar]
- 24.Rogers DL, Neely DE, Chapman JB, Plager DA, Sprunger DT, Sondhi N, et al. Comparison of the MTI Photoscreener and the Welch-Allyn SureSight autorefractor in a tertiary care center. J Aapos. 2008 Feb;12(1):77–82. doi: 10.1016/j.jaapos.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Virgili G, Angi M, Heede S, Rodriguez D, Bottega E, Molinari A. PowerRefractor versus Canon R-50 Autorefraction to assess refractive error in children: a community-based study in ecuador. Optom Vis Sci. 2007 Feb;84(2):144–8. doi: 10.1097/OPX.0b013e318031b65d. [DOI] [PubMed] [Google Scholar]
- 26.Donahue SP, Baker JD, Scott WE, Rychwalski P, Neely DE, Tong P, et al. Lions Clubs International Foundation Core Four Photoscreening: results from 17 programs and 400,000 preschool children. J Aapos. 2006 Feb;10(1):44–8. doi: 10.1016/j.jaapos.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Donahue SP, Johnson TM, Leonard-Martin TC. Screening for amblyogenic factors using a volunteer lay network and the MTI photoscreener. Initial results from 15,000 preschool children in a statewide effort. Ophthalmology. 2000 Sep;107(9):1637–44. doi: 10.1016/s0161-6420(00)00298-0. discussion 45-6. [DOI] [PubMed] [Google Scholar]
- 28.Donahue SP, Lorenz S, Johnson T. Photo screening around the world: Lions Club International Foundation experience. Semin Ophthalmol. 2008 Sep-Oct;23(5):294–7. doi: 10.1080/08820530802506078. [DOI] [PubMed] [Google Scholar]
- 29.Steele G, Ireland D, Block S. Cycloplegic Autorefraction Results in Pre-School Children Using the Nikon Retinomax Plus and the Welch Allyn SureSight. Optometry & Vision Science. 2003;80(8):573–7. doi: 10.1097/00006324-200308000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Mallen EA, Wolffsohn JS, Gilmartin B, Tsujimura S. Clinical evaluation of the Shin-Nippon SRW-5000 autorefractor in adults. Ophthalmic Physiol Opt. 2001 Mar;21(2):101–7. [PubMed] [Google Scholar]
- 31.Kotulak J, Schor C. The effects of optical vergence, contrast, and luminance on the accommodative response to spatially bandpass filtered targets [published erratum appears in Vision Res 1988;27(10):361] Vision Res. 1987;27(10):1797–806. doi: 10.1016/0042-6989(87)90108-8. [DOI] [PubMed] [Google Scholar]
- 32.Abrahamsson M, Ohlsson J, Bjorndahl M, Abrahamsson H. Clinical evaluation of an eccentric infrared photorefractor: the PowerRefractor. Acta Ophthalmol Scand. 2003 Dec;81(6):605–10. doi: 10.1046/j.1600-0420.2003.0149.x. [DOI] [PubMed] [Google Scholar]
- 33.Bujara K, Schulz E, Haase W. [Retinoscopy under cycloplegic and noncycloplegic conditions in children comparison of measurements of three examiners (author’s transl)] Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1981;216(4):339–43. doi: 10.1007/BF00455041. [DOI] [PubMed] [Google Scholar]
- 34.Choong YF, Chen AH, Goh PP. A comparison of autorefraction and subjective refraction with and without cycloplegia in primary school children. Am J Ophthalmol. 2006 Jul;142(1):68–74. doi: 10.1016/j.ajo.2006.01.084. [DOI] [PubMed] [Google Scholar]
- 35.Hodi S, Wood IC. Comparison of the techniques of videorefraction and static retinoscopy in the measurement of refractive error in infants. Ophthalmic Physiol Opt. 1994 Jan;14(1):20–4. doi: 10.1111/j.1475-1313.1994.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 36.Nayak BK, Ghose S, Singh JP. A comparison of cycloplegic and manifest refractions on the NR-1000F (an objective Auto Refractometer) Br J Ophthalmol. 1987 Jan;71(1):73–5. doi: 10.1136/bjo.71.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saunders K, Westall C. Comparison between near and cycloplegic retinoscopy in the refraction of infants and children. Optom Vision Sci. 1992;69:615–22. doi: 10.1097/00006324-199208000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Chiu NN, Rosenfield M, Wong LC. Effect of contralateral fog during refractive error assessment. J Am Optom Assoc. 1997 May;68(5):305–8. [PubMed] [Google Scholar]
- 39.Ward PA, Charman WN. An objective assessment of the effect of fogging on accommodation. Am J Optom Physiol Opt. 1987 Oct;64(10):762–7. doi: 10.1097/00006324-198710000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Kee CS, Do TC, Lai RY, Wong G, Lam AK. Could a cycloplegic agent be replaced by a fogging or a corrective lens in the biometric measurement of the crystalline lens? Ophthalmic Physiol Opt. 1998 Nov;18(6):521–6. [PubMed] [Google Scholar]
- 41.Queiros A, Gonzalez-Meijome J, Jorge J. Influence of fogging lenses and cycloplegia on open-field automatic refraction. Ophthalmic Physiol Opt. 2008 Jul;28(4):387–92. doi: 10.1111/j.1475-1313.2008.00579.x. [DOI] [PubMed] [Google Scholar]
- 42.Suryakumar R, Bobier WR. The manifestation of noncycloplegic refractive state in pre-school children is dependent on autorefractor design. Optom Vis Sci. 2003 Aug;80(8):578–86. doi: 10.1097/00006324-200308000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Horwood A, Riddell P. The use of cues to convergence and accommodation in naïve, uninstructed participants. Vision Research. 2008;48(15):1613–24. doi: 10.1016/j.visres.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bharadwaj SR, Candy TR. Cues for the control of ocular accommodation and vergence during postnatal human development. Journal of Vision. 2008 Dec 22;8(16):1–16. doi: 10.1167/8.16.14. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fincham E, Walton J. The reciprocal actions of accommodation and vergence. J Physiol. 1957;137:488–508. doi: 10.1113/jphysiol.1957.sp005829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Judge S. How is binocularity maintained during convergence and divergence? Eye. 1996;10(Pt 2):172–6. doi: 10.1038/eye.1996.43. [DOI] [PubMed] [Google Scholar]
- 47.Stark L. Normal and abnormal vergence. In: Schor C, Cuiffreda K, editors. Vergence Eye Movments. Butterworth; Woburn,MA: 1983. pp. 3–14. [Google Scholar]
- 48.Rosenfield M, Ciuffreda KJ. Proximal and cognitively-induced accommodation. Ophthalmic Physiol Opt. 1990 Jul;10(3):252–6. [PubMed] [Google Scholar]
- 49.Rosenfield M, Ciuffreda KJ. Effect of surround propinquity on the open-loop accommodative response. Invest Ophthalmol Vis Sci. 1991 Jan;32(1):142–7. [PubMed] [Google Scholar]
- 50.Schor CM. A dynamic model of cross-coupling between accommodation and convergence: simulations of step and frequency responses. Optom Vis Sci. 1992 Apr;69(4):258–69. doi: 10.1097/00006324-199204000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Birch E, Gwiazda J, Held R. Stereoacity development for crossed and uncrossed disparities in human infants. Vision Res. 1982;22:507–13. doi: 10.1016/0042-6989(82)90108-0. [DOI] [PubMed] [Google Scholar]
- 52.Birch E, Shimojo S, Held R. Preferential-looking assessment of fusion and stereopsis in infants aged 1-6 months. Invest Ophthalmol Vis Sci. 1985;26(3):366–70. [PubMed] [Google Scholar]
- 53.Currie DC, Manny RE. The development of accommodation. Vision Res. 1997 Jun;37(11):1525–33. doi: 10.1016/s0042-6989(97)85022-5. [DOI] [PubMed] [Google Scholar]
- 54.Kennedy R, Sheps S. A comparison of photoscreening techniques for amblyogenic factors in children. Can J Ophthalmol. 1989;24:259–64. [PubMed] [Google Scholar]
- 55.Enzenauer RW. The Efficacy of Photoscreening for Amblyopiagenic Factors in a High Risk Population. Binocular Vision And Strabismus Quarterly. 2003;18(4):233–41. [PubMed] [Google Scholar]
- 56.Hamer R, Norcia A, Day S, Haegerstrom-Portnoy G, Lewis D, Hsu-Winges C. Comparison of on- and off-axis photorefraction with cycloplegic retinoscopy in infants. J Pediatric Ophthalmol & Strab. 1992;29:232–9. doi: 10.3928/0191-3913-19920701-11. [DOI] [PubMed] [Google Scholar]
- 57.Erdurmus M, Yagci R, Karadag R, Durmus M. A comparison of photorefraction and retinoscopy in children. J AAPOS. 2007 Dec;11(6):606–11. doi: 10.1016/j.jaapos.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 58.Eskridge JB. Clinical objective assessment of the accommodative response. J Am Optom Assoc. 1989 Apr;60(4):272–5. [PubMed] [Google Scholar]
- 59.Harb E, Thorn F, Troilo D. Characteristics of accommodative behavior during sustained reading in emmetropes and myopes. Vision Res. 2006 Aug;46(16):2581–92. doi: 10.1016/j.visres.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turner JE, Horwood AM, Houston SM, Riddell PM. Development of the response AC/A ratio over the first year of life. Vision Res. 2002 Oct;42(22):2521–32. doi: 10.1016/s0042-6989(02)00268-7. [DOI] [PubMed] [Google Scholar]
- 61.Fawcett S, Wang Y-Z, Birch E. The Critical Period for Susceptibility of Human Stereopsis. Investigative Ophthalmology & Vision Science. 2005;46(2):521–5. doi: 10.1167/iovs.04-0175. [DOI] [PubMed] [Google Scholar]
- 62.Levi D, Carkeet A. Amblyopia: a consequence of abnormal visual development. In: Simons K, editor. Early Visual Development , Normal & Abnormal. Oxford University Press; New York: 1993. pp. 391–408. [Google Scholar]
- 63.Breinin GM, Chin NB. Accommodation, convergence and aging. Doc Ophthalmol. 1973 Feb 21;34(1):109–21. doi: 10.1007/BF00151800. [DOI] [PubMed] [Google Scholar]
- 64.Donders F. In: On the Anomalies of Accommodation and Refraction of the Eye. Moore WD, translator. Sydenham Society; London: 1864. [Google Scholar]
- 65.Cordonnier M, Dramaix M. Screening for abnormal levels of hyperopia in children: a non-cycloplegic method with a hand held refractor. Br J Ophthalmol. 1998;82(11):1260–4. doi: 10.1136/bjo.82.11.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Horwood A, Riddell P. Can misalignments in typical infants be used as a model for infantile esotropia? Invest Ophthalmol Vis Sci. 2004;45(2):714–20. doi: 10.1167/iovs.03-0454. [DOI] [PubMed] [Google Scholar]
- 67.Jones R. Physiological pseudomyopia. Optom Vis Sci. 1990 Aug;67(8):610–6. doi: 10.1097/00006324-199008000-00010. [DOI] [PubMed] [Google Scholar]
- 68.Chen J, Kim A, Barone S, Ottar-Pfeiffer W, Holgado S, Scott W, et al. Screening for Amblyopia in Preverbal Children with Photoscreening Photographs:Correlation of Hyperopia with Crescent Sizes. ARVO. 2002 2002. [Google Scholar]
- 69.Rosenfield M, Ciuffreda KJ. Accommodative responses to conflicting stimuli. J Opt Soc Am A. 1991 Feb;8(2):422–7. doi: 10.1364/josaa.8.000422. [DOI] [PubMed] [Google Scholar]
- 70.Hung GK, Ciuffreda KJ, Rosenfield M. Proximal contribution to a linear static model of accommodation and vergence. Ophthalmic Physiol Opt. 1996 Jan;16(1):31–41. [PubMed] [Google Scholar]
- 71.Kaakinen K, Kaseva H, Kause ER. Mass screening of children for strabismus or ametropia with two-flash photoskiascopy. Acta Ophthalmol (Copenh) 1986 Feb;64(1):105–10. doi: 10.1111/j.1755-3768.1986.tb06882.x. [DOI] [PubMed] [Google Scholar]
- 72.Horwood A. Too much or too little: neonatal ocular misalignment frequency can predict later abnormality. Brit J Ophthalmol. 2003;87(9):1142–5. doi: 10.1136/bjo.87.9.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hainline L, Riddell P, Grose Fifer J, Abramov I. Development of accommodation and convergence in infancy. Behavioural Brain Research. 1992;49:33–50. doi: 10.1016/s0166-4328(05)80192-5. [DOI] [PubMed] [Google Scholar]
- 74.Atkinson J, Braddick O, Nardini M, Anker S. Infant hyperopia: detection, distribution, changes and correlates-outcomes from the cambridge infant screening programs. Optom Vis Sci. 2007 Feb;84(2):84–96. doi: 10.1097/OPX.0b013e318031b69a. [DOI] [PubMed] [Google Scholar]
- 75.Roch-Levecq A-C, Brody B, Thomas R, Brown S. Ametropia, Preschoolers’ Cognitive Abilities and Effects of Spectacle Correction at 6-Month Follow-Up. Invest Ophth Vis Sci. 2008;49 doi: 10.1001/archophthalmol.2007.36. e-abstract 1427. [DOI] [PubMed] [Google Scholar]
- 76.Ingram R, Gill L, Goldacre M. Emmetropisation and accommodation in hypermetropic children before they show signs of squint--a preliminary analysis. Bull Soc Belge Ophtalmol. 1994;253:41–56. [PubMed] [Google Scholar]
- 77.Mutti DO. To emmetropize or not to emmetropize? The question for hyperopic development. Optom Vis Sci. 2007 Feb;84(2):97–102. doi: 10.1097/OPX.0b013e318031b079. [DOI] [PubMed] [Google Scholar]