Abstract
A 55-year-old woman underwent radiosurgery for a left cerebral hemisphere arteriovenous malformation (AVM) and developed radiation-induced necrosis causing a massive edema in the surrounding brain tissues. Despite various therapies, the edema expanded to the ipsilateral hemisphere and induced neurological symptoms. The radiation-induced necrotic lesion was surgically removed 4 years after radiosurgery. While the preoperative FDG PET revealed severe hypometabolism in the left cerebrum, the necrotomy significantly ameliorated the brain edema, glucose metabolism (postoperative FDG PET), and symptoms. This case indicates that radiation necrosis-induced neurological deficits may be associated with brain edema and hypometabolism, which could be reversed by appropriate necrotomy.
Keywords: Intracranial arteriovenous malformation, stereotactic radiosurgery, radiation-induced necrosis, brain edema, F-18 fluorodeoxyglucose, positron emission tomography
Introduction
Stereotactic radiosurgery (SRS) is an effective treatment for cerebral arteriovenous malformations (AVM). As a late complication, the incidence of radiation-induced necrosis after SRS for AVM has been reported to be in the range of 5.0–7.4% (1). Brain necrosis sometimes causes edema, which may result in delayed clinical deterioration. We present a case in which SRS for AVM developed radiation-induced necrosis causing brain edema and severe brain hypometabolism revealed with F-18 fluorodeoxyglucose (FDG)-positron emission tomography (PET). The symptoms and glucose metabolism were significantly improved after surgical removal of the necrotic core.
Case report
A 55-year-old woman presented with increasing episodes of focal seizures. The initial magnetic resonance imaging (MRI) revealed a mass lesion with signal void in the left frontal supplementary motor cortex (Fig. 1a). Digital subtraction angiography (DSA) confirmed the diagnosis of an intracranial AVM in the left frontal supplementary motor cortex (Fig. 1b). She underwent SRS using X-ray beams of 6 mV with a prescription dose of 25 Gy at the isocenter in a single fraction.
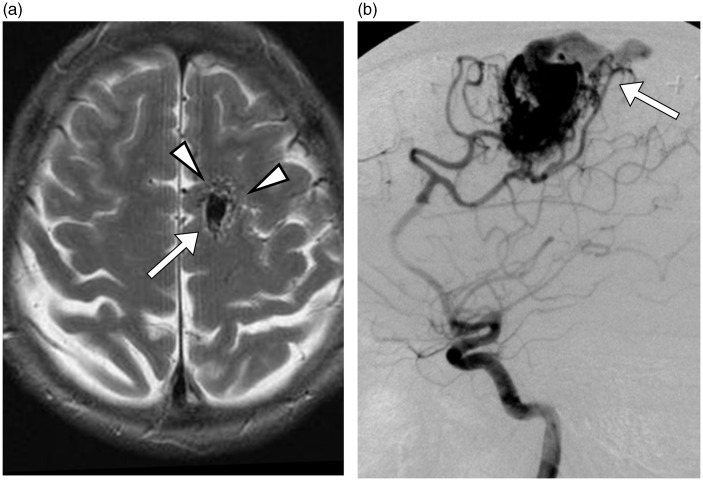
Fig. 1.
Initial radiologic studies of AVM. (a) An axial T2W MRI showed a tangle of tubular structures (arrowheads) representing the nidus, and an ectatic draining vein (arrow). (b) A lateral left internal carotid angiogram showed cerebral AVM with early drainage into the superior sagittal sinus.
The follow-up MRIs demonstrated gradual obliteration of the AVM. Two years after the SRS, she developed recurrent headaches, and right-sided lower limb paresthesia. Contrast-enhanced T1-weighted (T1W) MRI showed an enhanced lesion at the previous AVM site, indicating brain necrosis (Fig. 2a). T2-weighted (T2W) MRI showed a massive brain edema around the necrotic lesion (Fig. 2b). Despite steroids therapy and hyperbaric oxygen therapy, she suffered from repeated focal seizures and developed progressive hemiparesis. During the next 2 years, she was admitted to our hospital six times for symptoms of raised intracranial pressure, and treated with steroids. Four years after SRS, she was admitted to our hospital because of tonic seizure. Computed tomography (CT) scanning revealed microbleeding foci in the necrotic tissue (Fig. 3a). T2W MRI showed a necrotic mass and surrounding massive edema in the left hemisphere (Fig. 3b and c). On DSA, neither residual AVM nor early draining vein was observed, which confirmed complete obliteration (2).
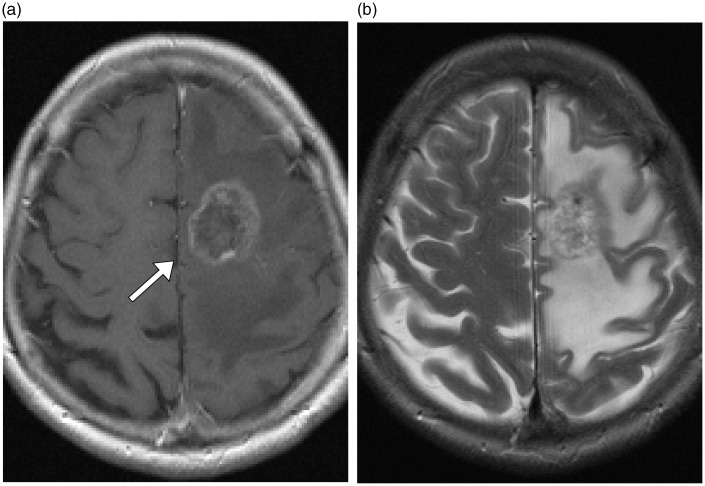
Fig. 2.
Follow-up MRI obtained 2 years after SRS. (a) A contrast material-enhanced T1W axial image showed a ring-like enhancing mass (arrow), indicating brain necrosis. (b) T2W axial MRI showed significant loss of abnormal vascular structures and absence of the ectatic draining vein. The region of high signal intensity in the left frontoparietal lobe surrounding the necrotic lesion was consistent with radiation-induced edema.
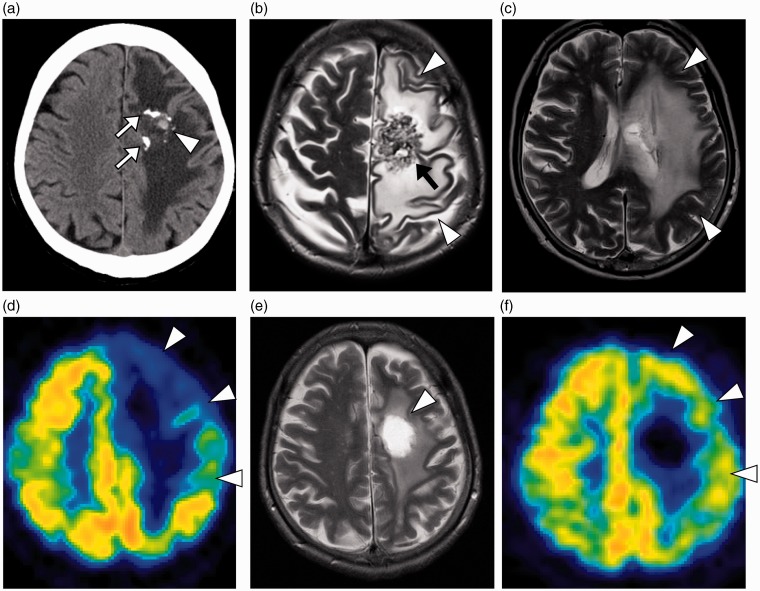
Fig. 3.
Radiologic studies 4 years after the SRS. (a) A non-contrasted axial CT showed partially calcified AVM in the left frontal lobe white matter (arrows). The small hyperattenuating lesion was compatible with microbleeding (arrowhead). (b, c) An axial T2W MRI showed a necrotic mass (arrow) and surrounding massive edematous lesion (arrowheads) in the subcortical white matter, from the left supra and middle frontal gyri to the middle temporal gyrus. (d) FDG PET demonstrated not only a defect of tracer accumulation in the necrotic core but also severe hypometabolism in the surrounding brain tissues (arrowheads). The lesion-to-contralateral ratio of standardized uptake value was 0.78 in the left precentral gyrus. (e) Three weeks after necrotomy, a T2W MRI showed high signal intensity (arrowhead) representing the resected site with brain edema significantly reduced. (f) A postoperative FDG PET examination showed remarkable recovery of metabolic activity in the left cortex (arrowheads). The lesion-to-contralateral ratio increased to 0.90 in the left precentral gyrus.
FDG PET was performed to rule out other epileptogenic foci (3). It demonstrated not only a defect of tracer accumulation in the necrotic core, but also severe hypometabolism in a broad area of the left cerebral cortex compared to the contralateral cortex (Fig. 3d). No other foci were detected in the rest of the brain. Because of the prolonged neurological deficit and microbleeding from necrotic tissue, a decision was made to remove the necrotic mass. Pathological inspection confirmed the diagnosis of radiation-induced tissue changes with necrosis. The right-sided hemiparesis gradually improved with less frequent seizures during the following three weeks. Subsequent MRI showed reduction of the brain edema around the resected site (Fig. 3e). In addition, FDG PET performed 3 weeks after the operation showed remarkable improvement of metabolic activity in the left cerebral cortex (Fig. 3f).
Discussion
For patients with AVM, SRS has been proven to occlude the dilated vessels and to reduce the risk of subarachnoidal hemorrhage from AVM rupture (4). Radiation necrosis is one of the severe complications of SRS; up to 7.6% of patients with radiation necrosis are symptomatic (5).
Radiation necrosis sometimes forms a solid mass lesion with enormous ipsilateral edema (6). SRS not only degenerates the nidus of AVM, but also affects other cerebral capillaries and small vessels near the nidus, thereby causing an inflammatory response involving larger vessels. Impairment of the blood brain barrier (BBB) by disruption of BBB components such as the endothelium, basal lamina, and astrocytes may also play a role (6).
FDG PET studies on patients with brain tumors frequently demonstrate areas of extensive hypometabolism in the cortex (7). Previous PET studies reported that brain tumors (e.g. glioma and meningioma) and intracerebral hemorrhage showed decrease in FDG uptake within the brain edema (7–9). Edema causes an additional mass effect and subsequent increase in intracranial pressure, leading to neurological disturbances by decreasing regional cerebral blood flow (6,7). The edematous white matter lesion impairs even the function of adjacent gray matter, as demonstrated by decreased uptake of FDG in the cortical area (7,8). This effect should be taken into consideration in interpreting brain PET studies.
We observed reduction of edema and improvement of neurological deficit after the neurosurgical intervention for necrosis induced by SRS treatment of AVM, which is consistent with previous reports (6,7,10,11). However, to the best of our knowledge, there has been no paper reporting an improvement of brain hypometabolism after resection of radiation-induced necrosis.
In conclusion, we observed hypometabolism caused by necrosis and its improvement after removal of the necrotic lesion. These findings suggested two important facts. First, necrotomy could be useful for patients suffering from symptoms caused by radiation-induced necrosis. Second, FDG PET could be a useful monitoring tool of therapeutic effect on neuronal functions. FDG PET may play an important role in the evaluation of pre- and postoperative functional activity in the cortex and provide objective information regarding the therapeutic effects.
Conflict of interest
None declared.
References
- 1.Aoyama H, Shirato H, Nishioka T, et al. Treatment outcome of single or hypofractionated single-isocentric stereotactic irradiation (STI) using a linear accelerator for intracranial arteriovenous malformation. Radiother Oncol 2001; 59: 323–328. [DOI] [PubMed] [Google Scholar]
- 2.Lee KE, Choi CG, Choi JW, et al. Detection of residual brain arteriovenous malformations after radiosurgery: diagnostic accuracy of contrast-enhanced three-dimensional time of flight MR angiography at 3.0 Tesla. Korean J Radiol 2009; 10: 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deshmukh A, Scott JA, Palmer EL, et al. Impact of fluorodeoxyglucose positron emission tomography on the clinical management of patients with glioma. Clin Nucl Med 1996; 21: 720–725. [DOI] [PubMed] [Google Scholar]
- 4.Lunsford LD, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for arteriovenous malformations of the brain. J Neurosurg 1991; 75: 512–524. [DOI] [PubMed] [Google Scholar]
- 5.Izawa M, Hayashi M, Chernov M, et al. Long-term complications after gamma knife surgery for arteriovenous malformations. J Neurosurg 2005; 102: 34–37. [DOI] [PubMed] [Google Scholar]
- 6.Schaller C, Liefner M, Ansari S, et al. Operation for delayed symptomatic brain oedema after treatment of an arteriovenous malformation by embolization and radiosurgery. Acta Neurochir (Wien) 2005; 147: 1103–1108. [DOI] [PubMed] [Google Scholar]
- 7.Pourdehnad M, Basu S, Duarte P, et al. Reduced grey matter metabolism due to white matter edema allows optimal assessment of brain tumors on 18 F-FDG-PET. Hell J Nucl Med 2011; 14: 219–223. [PubMed] [Google Scholar]
- 8.Kinoshita M, Goto T, Arita H, et al. Imaging 18F-fluorodeoxy glucose/11C-methionine uptake decoupling for identification of tumor cell infiltration in peritumoral brain edema. J Neurooncol 2012; 106: 417–425. [DOI] [PubMed] [Google Scholar]
- 9.Lin X, Tang Y, Sun B, et al. Cerebral glucose metabolism: Influence on perihematomal edema formation after intracerebral hemorrhage in cat models. Acta Radiol 2010; 51: 549–554. [DOI] [PubMed] [Google Scholar]
- 10.Massengale JL, Levy RP, Marcellus M, et al. Outcomes of surgery for resection of regions of symptomatic radiation injury after stereotactic radiosurgery for arteriovenous malformations. Neurosurgery 2006; 59: 553–560. [DOI] [PubMed] [Google Scholar]
- 11.Foroughi M, Kemeny AA, Lehecka M, et al. Operative intervention for delayed symptomatic radionecrotic masses developing following stereotactic radiosurgery for cerebral arteriovenous malformations—case analysis and literature review. Acta Neurochir (Wien) 2010; 152: 803–815. [DOI] [PubMed] [Google Scholar]