Abstract
Background
The risk for contrast-induced nephropathy (CIN) after intra-arterial application of an iodine-based contrast material is unknown for patients with chronic kidney disease (CKD) and peripheral arterial disease (PAD).
Purpose
To investigate the incidence of CIN in patients with CKD and PAD.
Material and Methods
This retrospective study was approved by the local ethics committee. One hundred and twenty patients with 128 procedures (73 with baseline eGFR in the range of 45–60 mL/min/1.73m2, 55 with eGFR < 45 mL/min/1.73m2) were evaluated. All patients received intra-arterially an iodine-based low-osmolar contrast material (CM) after adequate intravenous hydration with isotonic NaCl 0.9% solution. CIN was defined as an increase in serum creatinine of more than 44 μmol/L within 4 days. The influence of patient-related risk factors (age, weight, body mass index, eGFR, serum creatinine, hypertension, diabetes mellitus, coronary heart disease, heart failure) and therapy-related risk factors (amount of CM, nephrotoxic drugs, number of CM applications) on CIN were examined.
Results
CIN developed in 0% (0/73) of procedures in patients with PAD and an eGFR in the range of 45–60 mL/min/1.73m2 and in 10.9% (6/55) of procedures in patients with an eGFR <45 mL/min/1.73m2. No risk factor significantly influenced the development of CIN, although baseline serum creatinine (P = 0.06) and baseline eGFR (P = 0.10) showed a considerable dependency.
Conclusion
Patients with an eGFR in the range of 45–60 mL/min/1.73m2 and PAD seem not at risk for CIN after intra-arterial CM application and adequate hydration. Whereas, an eGFR < 45 mL/min/1.73m2 correlated with a risk of 10.9% for a CIN.
Keywords: Contrast-induced nephropathy (CIN), peripheral arterial disease (PAD), chronic kidney disease (CDK), estimated glomerular filtration rate (eGFR)
Introduction
Contrast-induced nephropathy (CIN) is defined as the impairment of renal function, measured through an increase of the serum creatinine (SCr) of more than 44 μmol/L or 25%, following the intravascular administration of a contrast medium (CM) (1). In particular, patients with chronic kidney disease (CKD), being defined as a decreased estimated glomerular filtration rate (eGFR) of under 60 mL/min/1.73m2, have a higher risk of developing CIN (1). Further, patient-related risk factors are diabetes mellitus, coronary heart disease, congestive heart failure, advanced age, dehydration, and the intake of nephrotoxic drugs. Peripheral arterial diseases (PAD) also influence the development of CIN (2–4). CKD as well as PAD show a relatively high prevalence in the population aged ≥70 years (5,6) with a rate of 37.8% and 14.5%, respectively. The overall 6-year mortality rate of patients with CKD amounts to 28% and for patients with PAD amounts to 26% (7). In 20–24% of cases, patients suffer from both CKD and PAD (7,8). In those cases, the overall 6-year mortality rate even reaches 45% (7). To diagnose and treat PAD, an intra-arterial iodine-based CM is often applied. It is known that CIN in patients with CKD is accompanied with a significant higher mortality rate compared to patients without CKD (9). Therefore, CM should strictly be administered only after a careful risk-benefit evaluation. A preventive treatment to reduce the risk of CIN is hydration with normal saline (0.9%) (1,10). Since there are no data for risk evaluation after CM applications in patients with CKD and PAD, the objective of the study was to define the frequency of CIN after an intra-arterial application of iodine-based CM and adequate intravenous hydration with isotonic saline (NaCl 0.9%) in patients with an eGFR <60 mL/min/1.73m2 and PAD. Under these conditions, the known risks for CIN were additionally examined.
Material and Methods
This retrospective study was approved by the local ethics committee.
All inpatients who underwent an interventional-radiological CM application for diagnosis or PAD treatment between November 2010 and May 2012 in our department and who met the following criteria were included in the study:
– Intra-arterial injection of iodine, low-osmolar iodine-based contrast media;
– Estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73m2, measured either shortly before intervention or up to 2 days before. If more parameters were available, the latest value was taken into account and defined as baseline eGFR. The outcome value of serum creatinine and eGFR, measured 2–4 days after intervention, was documented;
– Application of a weight-based dose of isotonic saline (NaCl 0.9%) (1 mL/kg/h) over a minimum period of 6 h before intervention.
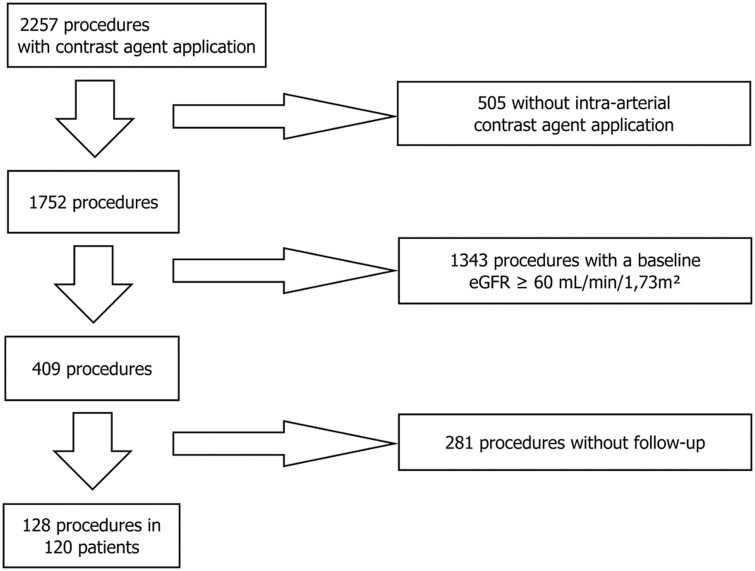
Patients were excluded if they did not correspond to the above mentioned criteria, or if insufficient data were available concerning the SCr/eGFR development before and after intervention (Fig. 1).
Fig. 1.
Patient inclusion.
Patient characteristics
The mean age of patients was 74.3 years ± 8.8. Sixty female subjects (46.9%) and 68 male subjects (53.1%) underwent this procedure. The mean age was 76.6 years ± 10.1 in female subjects and 72.4 years ± 7.0 in male subjects. A second intra-arterial CM was given to eight patients 6–21 days after the first application. In these patients both interventions were recorded separately, described as one with and one without a repeated dosage of CM. Forty-three percent of the procedures (55/128) were performed in patients with type 2 diabetes mellitus and 86% (110/128) in patients with an arterial hypertension. Sixty-four percent (50/128) of the procedures were undertaken in patients with coronary heart disease and 44% (56/128) in patients with cerebrovascular disease, including ischemic stroke, hemorrhagic stroke, and transient ischemic attack.
The patients received either a diagnostic angiography or a percutaneous transluminal angioplasty with or without stent implantation. All procedures access was gained through the common femoral artery and the CM was administered via a pigtail catheter which was placed in the distal aorta below offspring of the renal arteries. All patients received in mean 70 ml (range, 4–250 mL) of an iodine-containing low-osmolar CM (Iomeprol; 300 mg iodine per mL; Bracco Imaging, Konstanz, Germany).
Measurements
The eGFR was calculated by the abbreviated MDRD equation for SCr measured in μmol/L: eGFR [mL/min/1.73m2] = 186 × [SCr/88.4]−1.154 × [age in years]−0.203 × 0.742 (female patients)
According to the above-mentioned equation, 57% (73/128) of the procedures were performed in patients with an initial eGFR in the range of 45–60 mL/min/1.73m2 and 43% (55/128) in patients with an initial eGFR <45 mL/min/1.73m2.
CIN was defined as an increase of SCr of more than 44 μmol/L (1). It was furthermore verified whether an increase of SCr by more than 25% or a decrease of eGFR of 25% would classify patients differently (11,12). From the patient records the highest SCr value and the lowest available eGFR value from days 2–4 after intervention were determined. In addition, the patient-related risk factors for CIN such as age, quantity of CM, numbers of CM administrations, weight, BMI, hypertension, type 2 diabetes mellitus, coronary heart disease, heart failure, and cerebrovascular disease were recorded as predisposing factors. Nephrotoxic drugs such as non-steroidal anti-inflammatory drugs and diuretics taken on the intervention day were documented as well. Since the preventive and adverse effects of angiotensin II type 1 receptor antagonists and calcium-antagonists are discussed in literature (13,14), these medications were also considered. Prophylactic injected volume of isotonic saline (NaCl 0.9%) within 24 h before intervention was registered.
Statistical analysis
A statistical evaluation was performed with SPSS Statistics Version 20, (IBM, Armonk, NY, USA). The frequency distribution of dichotomous categorical variables was calculated in percent. The mean value and the standard deviation were calculated for quantitative data. The Mann-Whitney U test was applied for unpaired samples whereas the Wilcoxon test was applied for paired samples. P values under 0.05 were considered as significant.
Results
A CIN was observed in six of 128 procedures. In patients with an initial eGFR lower than 60 mL/min/1.73m2 the incidence of CIN was therefore 4.7% (6/128) with a 95% confidence interval in the range of 2.0–7.4%. Dividing the patients into two groups (patients with an initial eGFR in the range of 45–60 mL/min/1.73m2 and patients with an initial eGFR of under 45 mL/min/1.73m2) showed that patients with an initial eGFR in the range of 45–60 mL/min/1.73m2 developed a CIN in none of the procedures (0%; 0/73) whereas patients with an initial eGFR of under 45 mL/min/1.73m2 developed a CIN in 10.9% (6/55) of the cases (Table 1). Both conventional definitions of CIN (absolute SCr increase of a minimum of 44 μmol/L or relative SCr increase of a minimum of 25%) led to the same results. These identical results also apply if CIN was defined as increase of eGFR to a minimum of 25% (Table 2). Respectively, 5/6 patients with CIN showed arterial hypertonus and were treated with diuretics (Table 3). All patients who developed CIN after contrast administration, had in addition to CKD and PAD at least three further risk factors (range, 3–4). However, patients with CIN did not have more statistically significant risk factors than patients without CIN. All six affected patients developed CIN already after the first CM application, and none of the patients with repeated contrast material applications developed CIN. The case-by-case assessment could not identify any risk factors with a statistically significant difference between patients with and without CIN, even though the level of the initial SCr and the baseline eGFR showed a considerable trend to relevance. The analysis of the absolute SCr values revealed that in three of 128 procedures, SCr decreased more than 44 μmol/L. Looking on the relative changes, the SCr dropped by more than 25% after 10 of 128 procedures while the eGFR increased more than 25% after 19 from 128 procedures.
Table 1.
Incidence of CIN, according to baseline eGFR.
| eGFR group (mL/min/1.73m2) | Portion of all procedures (%) | Incidence of CIN (%, 95% confidence level) [95% CI] |
|---|---|---|
| 45 ≤ eGFR < 60 | 57% (73/128) | 0% (0/73) |
| eGFR < 45 | 43% (55/128) | 10.9% (6/55) [2.4–19.4%] |
Table 2.
Incidence of CIN according to various definitions.
| eGFR group (mL/min/1.73m2) | SCr increase > 44 μmol/L (range) | SCr increase > 25% (range) | eGFR decrease > 25% (range) |
|---|---|---|---|
| 45 ≤ eGFR < 60 | 0/73 | 0/73 | 0/73 |
| eGFR < 45 | 6/55 (46.9–116.7 μmol/L) | 6/55 (31.9–68.1%) | 6/55 (26.2–45.0%) |
Table 3.
Risk factors of the cohort study, data in %, unless otherwise stated.
| Parameter | Total procedures (n = 128) | No CIN (n = 122) | CIN (n = 6) | P value |
|---|---|---|---|---|
| Age (years) | 74.3 ± 8.8 | 74.3 ± 8.9 | 75.8 ± 8.1 | 0.58 |
| Sex (women) | 47% (60/128) | 48% (58/122) | 33% (2/6) | 0.50 |
| Contrast medium dose (mL) | 80.8 ± 50.4 | 81.3 ± 50.7 | 71.7 ± 47.2 | 0.78 |
| Body height* (m) | 1.70 ± 0.10 | 1.70 ± 0.10 | 1.68 ± 0.15 | 0.91 |
| weight (kg) | 75.6 ± 16.8 | 75.5 ± 17.2 | 76.7 ± 8.1 | 0.60 |
| Contrast administrations (n) | 1.23 ± 0.60 | 1.25 ± 0.61 | 1 | 0.25 |
| Repeated contrast administrations <21 days | 13% (16/128) | 13% (16/122) | 0% (0/6) | 0.34 |
| BMI*† (kg/m2) | 26.6 ± 5.0 | 26.5 ± 5.0 | 27.4 ± 6.0 | 0.97 |
| baseline eGFR (mL/min/1.73m2) | 45.3 ± 10.5 | 45.6 ± 10.4 | 38.5 ± 12.4 | 0.10 |
| SCr before therapy (μmol/L) | 136 ± 61 | 134 ± 60 | 171 ± 75 | 0.06 |
| Volume of isotonic saline (NaCl 0.9%) iv (mL) | 1199 ± 749 | 1200 ± 749 | 1167 ± 816 | 0.96 |
| Arterial hypertension | 86% (110/128) | 86% (105/122) | 83% (5/6) | 0.85 |
| Type 2 diabetes mellitus | 43% (55/128) | 44% (54/122) | 17% (1/6) | 0.18 |
| Coronary heart disease | 50% (64/128) | 50% (61/122) | 50% (3/6) | >0.99 |
| Congestive heart failure | 29% (37/128) | 28% (34/122) | 50% (3/6) | 0.24 |
| Cerebrovascular disease | 34% (44/128) | 35% (42/122) | 33% (2/6) | 0.96 |
| AT1-antagonist | 20% (25/128) | 19% (23/122) | 33% (2/6) | 0.38 |
| Ca2+-antagonist | 26% (33/128) | 26% (32/122) | 17% (1/6) | 0.61 |
| Diuretics | 58% (74/128) | 57% (69/122) | 83% (5/6) | 0.20 |
| Non-steroidal anti-inflammatory drugs | 70% (90/128) | 71% (86/122) | 67% (4/6) | 0.84 |
| Paracetamol | 2% (3/128) | 3% (3/122) | 0% (0/6) | 0.70 |
| Total number of risk factors | 6.3 ± 2.0 | 7.0 ± 1.5 | 6.3 ± 1.5 | 0.982 |
Average value ± standard deviation.
Numbers in parentheses are absolute number of cases.
Body height and BMI could be determined in only 94 cases.
†Body mass index = body weight [kg] / (body height [m])2.
Discussion
CIN emerged after intra-arterial administration of iodine-based low-osmolar CM and previous adequate intravenous hydration with isotonic NaCl 0.9% solution in patients with CKD and PAD only if eGFR was <45 mL/min/1.73m2. In contrast, the injection of CM in patients with an eGFR in the range of 45–60 mL/min/1.73m2 caused CIN in none of the patients. In addition, none of the evaluated risk factors were significant for the occurrence of CIN prior to adequate hydration. Nevertheless, baseline eGFR (P = 0.10) and SCr (P = 0.06) before intervention showed a strong relation to the development of CIN.
Our results support the recommendations of the European Society of Urogenital Radiology (ESUR) regarding the administration of an iodine-based CM. Following the ESUR recommendations, an eGFR <45 mL/min/1.73m2 before an intravenous CM application and an eGFR <60 mL/min/1.73m2 before an intra-arterial CM administration represent a risk factor for CIN. The fact that in our study the CIN patients exclusively had an eGFR <45 mL/min/1.73m2 is probably due to the infrarenal, intra-arterial injection of CM that reaches the kidneys only after passing through the cardiopulmonary circulation. Compared to the suprarenal administration, the concentration of the CM reaching the kidneys is therefore much lower. Furthermore, infrarenal administration do not show any risk for renal atheroembolism which could resemble a CIN. A comparison with the published data about CIN is therefore limited. We are not aware of recent data regarding CIN after infrarenal, intra-arterial CM application with a baseline eGFR <45 mL/min/1.73m2. Only some studies about percutaneous coronary intervention were available, which however did not differentiate patients with a baseline eGFR <45 mL/min/1.73m2. The most comparable study about patients with a mean baseline serum creatinine value of 246 ± 44 μmol/L (eGFR data were not published) showed in 21.3 % (40/188) of the cases after hydration with isotonic saline (NaCl 0.9%) CIN equivalent changes of the SCr values (3). Studies about intravenous CM administrations in patients with a baseline eGFR <45 mL/min/1.73m2 stated a much lower incidence of CIN with 3.1% (7/227) or 5.1% (13/254), respectively (15,16). A possible explanation is that those studies analyzed outpatients with less co-morbidity and more hemodynamical stability than inpatients. It has been already shown that an inpatient status represents a significant risk for CIN (2). For this reason, a comparison of our results with the incidence of CIN after intravenous contrast administration in inpatients with baseline eGFR <45 mL/min/1.73m2 makes sense. The only known actual data for such a patient collective showed that 13.3% (2/15) of the cases developed CIN (2). It should be furthermore noted that not all patients in this study were hydrated with isotonic saline, so that 13.3% is a quite similar percentage to our 10.9% incidence for CIN in patients with an eGFR <45 mL/min/1.73m2. Despite these similar results, it should not be forgotten that intra-arterial contrast agents administered within clinical angiographic studies are repeatedly given in small doses and over a longer time than intravenous CM applications in CT examinations. This in turn could require important pathophysiological mechanistic differences in the development of CIN after intravenous or intra-arterial contrast agent administration (17).
The result that none of our patients with a baseline eGFR in the range of 45–60 mL/min/1.73m2 developed a CIN coincides with the literature. Admittedly, this is only if it is taken into account that the intra-arterial CM administration was carried out infrarenally and therefore a comparison with studies applying CM intravenously can be performed. In such a study with adult inpatients, it was demonstrated that a low osmolar iodine-based CM was not a risk factor for CIN in patients with a baseline eGFR >45 mL/min/1.73m2 (18). A very similar result with a CIN incidence of 0.6% (1/170) was achieved by a study with patients having an eGFR >40 mL/min/1.73m2 (19). Nevertheless, information about hydration and patient status (in- or outpatient) were missing. Differently to this, a study with an inpatient collective and a baseline eGFR >45 mL/min/1.73m2 showed an incidence of CIN of 5.7% (6/104) (2). However, only 20% of those patients were hydrated. Since hydration reduces the risk for CIN in renally-impaired patients, this could explain the differing incidence in patients with a baseline eGFR in the range of 45–60 mL/min/1.73m2 (1,3,10).
The detailed analysis of the SCr values showed that they decreased after contrast agent administration in a significant number of patients. Depending on the approach, this rate was 2.3% (3/128, critical value: increase of > 44 μmol/L), 7.8% (10/128, critical value: increase of > 25%) or even 15.2% (19/128, critical value: increase of eGFR > 25%). For this at first sight unusual result, similar observations can be found in literature. The change in SCr values in 32,161 patients without CM showed on day 2 an increase of > 25% in 18% of the patients and a decrease of >25% in 24% of the patients (20). This large deviation range of the serum creatinine values without CM even increased on day 4 to 25% (increase > 25%) and 35% (decrease > 25%) (20). The direct comparison of patients with intravenous and without intravenous contrast agent administration led to similar results (21,22). Thus, independently from the basal renal function the risk for an acute renal failure did not differ significantly between both patients groups (22). Counterfactual analysis of patients who underwent both contrast-enhanced and unenhanced CT scans suggested a coincidental rather than a causal relationship between intravenous CM exposure and acute kidney injury (22). It can therefore be postulated that singular CIN cases are probably not the consequence of contrast agent administration, but obscured by other causes of renal injury (21,22).
For clinical routine, the results of this study imply that after intra-arterial application of CM and previous adequate hydration, CIN in patients with a baseline eGFR in the range of 45–60 mL/min/1.73m2 is very unlikely. There is however a higher risk for CIN in patients with a baseline eGFR <45 mL/min/1.73m2.
A limitation of this study is the missing control group of patients without intra-arterial CM administration. A retrospective comparison is somewhat error-prone since there are medical reasons for the missing applications under otherwise same diseases and risk factors. An ideal control group would therefore be a prospective randomized one. Thus, according to our experiences, even using CO2 as alternative negative contrast medium would require in many interventions at least one intra-arterial administration of iodine-based contrast agent. A further limitation is the exclusion of 281 procedures because of insufficient follow-up data. It is therefore possible that such a selection bias or a lost-to-follow-up bias leads to an incorrect incidence of CIN. Since all post-interventional SCr increases of >44 μmol/L or > 25% were interpreted as CIN, but the values showed a considerable fluctuations with a decrease of >44 μmol/L in 2.3% or, respectively, a decrease of >25% in 7.8% of the cases, it can be assumed that the incidence of CIN was rather overestimated than underestimated in this study.
In conclusion, the results of our study showed that after prior adequate intravenous hydration of patients with CDK and PAD, the risk of CIN after intra-arterial iodine-based CM application was higher only in patients with a baseline eGFR <45 mL/min/1.73m2. Patients with a baseline eGFR in the range of 45–60 mL/min/1.73m2 and a PAD seemed in contrast with adequate hydration and intra-arterial iodine-based CM application not in a higher risk of CIN.
Acknowledgements
We thank Laura Graziani, BA, for translating the manuscript.
Conflict of interest
None declared.
References
- 1.Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol 2011; 21: 2527–2541. [DOI] [PubMed] [Google Scholar]
- 2.Weisbord SD, Mor MK, Resnick AL, et al. Incidence and outcomes of contrast-induced AKI following computed tomography. Clin J Am Soc Nephrol 2008; 3: 1274–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen SL, Zhang J, Yei F, et al. Clinical outcomes of contrast-induced nephropathy in patients undergoing percutaneous coronary intervention: a prospective, multicenter, randomized study to analyze the effect of hydration and acetylcysteine. Int J Cardiol 2008; 126: 407–413. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell AM, Jones AE, Tumlin JA, et al. Incidence of contrast-induced nephropathy after contrast-enhanced computed tomography in the outpatient setting. Clin J Am Soc Nephrol 2010; 5: 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation 2004; 110: 738–743. [DOI] [PubMed] [Google Scholar]
- 6.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298: 2038–2047. [DOI] [PubMed] [Google Scholar]
- 7.Liew YP, Bartholomew JR, Demirjian S, et al. Combined effect of chronic kidney disease and peripheral arterial disease on all-cause mortality in a high-risk population. Clin J Am Soc Nephrol 2008; 3: 1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Hare AM, Glidden DV, Fox CS, et al. High prevalence of peripheral arterial disease in persons with renal insufficiency: results from the National Health and Nutrition Examination Survey 1999-2000. Circulation 2004; 109: 320–323. [DOI] [PubMed] [Google Scholar]
- 9.Abe M, Morimoto T, Akao M, et al. Relation of contrast-induced nephropathy to long-term mortality after percutaneous coronary intervention. Am J Cardiol 2014; 114: 362–368. [DOI] [PubMed] [Google Scholar]
- 10.Trivedi HS, Moore H, Nasr S, et al. A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron Clin Pract 2003; 93: C29–34. [DOI] [PubMed] [Google Scholar]
- 11.Herts BR, Schneider E, Poggio ED, et al. Identifying outpatients with renal insufficiency before contrast-enhanced CT by using estimated glomerular filtration rates versus serum creatinine levels. Radiology 2008; 248: 106–113. [DOI] [PubMed] [Google Scholar]
- 12.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 2009; 20: 672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta RK, Kapoor A, Tewari S, et al. Captopril for prevention of contrast-induced nephropathy in diabetic patients: a randomised study. Indian Heart J. 1999; 51: 521–526. [PubMed] [Google Scholar]
- 14.Oguzhan N, Cilan H, Sipahioglu M, et al. The lack of benefit of a combination of an angiotensin receptor blocker and calcium channel blocker on contrast-induced nephropathy in patients with chronic kidney disease. Ren Fail 2013; 35: 434–439. [DOI] [PubMed] [Google Scholar]
- 15.Balemans CE, Reichert LJ, van Schelven BI, et al. Epidemiology of contrast material-induced nephropathy in the era of hydration. Radiology 2012; 263: 706–713. [DOI] [PubMed] [Google Scholar]
- 16.Kim SM, Cha RH, Lee JP, et al. Incidence and outcomes of contrast-induced nephropathy after computed tomography in patients with CKD: a quality improvement report. Am J Kidney Dis 2010; 55: 1018–1025. [DOI] [PubMed] [Google Scholar]
- 17.Rao QA, Newhouse JH. Risk of nephropathy after intravenous administration of contrast material: a critical literature analysis. Radiology 2006; 239: 392–397. [DOI] [PubMed] [Google Scholar]
- 18.Davenport MS, Khalatbari S, Cohan RH, et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology 2013; 268: 719–728. [DOI] [PubMed] [Google Scholar]
- 19.Thomson VS, Narayanan K, Singh JC. Contrast induced nephropathy in urology. Indian J Urol 2009; 25: 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newhouse JH, Kho D, Rao QA, et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: implications for studies of contrast nephrotoxicity. Am J Roentgenol 2008; 191: 376–382. [DOI] [PubMed] [Google Scholar]
- 21.Azzouz M, Romsing J, Thomsen HS. Fluctuations in eGFR in relation to unenhanced and enhanced MRI and CT outpatients. Eur J Radiol 2014; 83: 886–892. [DOI] [PubMed] [Google Scholar]
- 22.McDonald RJ, McDonald JS, Bida JP, et al. Intravenous contrast material-induced nephropathy: causal or coincident phenomenon? Radiology 2013; 267: 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]