Abstract
Epidemiological studies have shown that vascular-related disorders are associated with high von Willebrand factor antigen (VWF:Ag) and VWF propeptide (VWFpp). VWFpp is secreted together with VWF:Ag upon endothelial cell activation, hence it could be a potential biomarker. This study was conducted to compare between VWF:Ag and VWFpp levels among 30 healthy individuals and 42 patients with high levels of VWF:Ag in different medical conditions and ABO blood groups. VWFpp levels were strongly correlated with VWF:Ag. VWF:Ag and VWFpp levels were significantly increased in patients compared to healthy individuals. VWFpp is not affected by ABO blood group in both healthy individual and patient groups unlike VWF:Ag. As expected, this study showed that VWFpp levels increased in parallel with VWF:Ag levels in patients with diseases associated with endothelial activation. VWFpp though nonspecific is a potential biomarker reflecting underlying pathophysiological changes in various medical conditions with an additional advantage of not being influenced by ABO blood groups.
Keywords: von Willebrand factor, biomarker, enzyme-linked immunosorbent assay
Introduction
Von Willebrand factor (VWF) is an adhesive protein synthesized in the endothelial cells and megakaryocytes, and it is found in the subendothelial matrix, blood plasma, platelets, and endothelium. Biosynthesis of VWF occurs when mRNA is translated as a pre-pro-VWF, a 2,813-residue polypeptide consisting of a 22-residue signal peptide, a 741-residue VWF propeptide (VWFpp), and the 2,050-residue mature subunit.1,2 Upon synthesis, VWF is cleaved from VWFpp that converts into a mature VWF. Both VWFpp and mature VWF are packaged into secretory granules in endothelial cells and megakaryocytes. VWF multimers and VWFpp are secreted together in 1:1 stoichiometric amounts.1–3 The plasma VWF levels vary widely and are determined by many factors and conditions such as older age, pregnancy, and diseases related to endothelial dysfunction.1 Genetic variation such as ABO blood group also affect the levels of VWF, of which individuals with blood group O showed a lower level of VWF than individuals with non-O blood groups.4–6
Previous studies showed that both VWF and VWFpp levels were elevated in acute vascular diseases that are associated with endothelial dysfunction.7–10 Recently, the measurement of plasma VWFpp has been proposed as a more sensitive marker of vascular endothelial cell dysfunction. Although both VWF and VWFpp are secreted from injured endothelial cells, the plasma VWFpp clearly reflects endothelial cell dysfunction as platelet aggregation does not consume the VWFpp unlike VWF antigen (VWF:Ag).9 Furthermore, previous studies have demonstrated that the plasma levels of VWFpp are not influenced by ABO blood group.4,11 In this present study, a correlation study was performed to determine the strength of the relationship between VWF:Ag and VWFpp levels in all the subjects recruited (healthy individuals and patients). The levels of VWF:Ag and VWFpp in healthy individuals (control group) and patients with various medical conditions were also compared according to their ABO blood groups to determine the effect of blood group types (O and non-O blood groups) on the levels of VWF:Ag and VWFpp.
Von Willebrand disease (VWD) is a bleeding disorder associated with VWF:Ag deficiency. Another interesting feature is the application of the ratio of VWFpp/VWF:Ag in the diagnosis of peculiar type I VWD.12,13 The VWFpp/VWF:Ag ratio is used to identify a subtype of VWD patients with VWF:Ag deficiency because of increased plasma VWF clearance.13,14 However, very limited studies on high VWF:Ag and VWFpp, which can be associated with thrombotic risk have been performed. Thus, the present study was carried out to provide more information on the subject of thrombotic risk. This study aimed to compare VWF:Ag levels, VWFpp levels, and VWFpp/VWF:Ag ratio in two groups of patients with vascular and nonvascular diseases associated with endothelial activation and thrombotic potential.
Methods
This study was approved by the Human Research Ethics Committee from Universiti Sains Malaysia (FWA Reg. No: 00007718; IRB Reg. No: 00004494), and all subjects signed an informed consent. The research was conducted in accordance with the principles of the Declaration of Helsinki.
Sample collection and platelet-poor plasma preparation
Thirty healthy individuals and 42 patients were included in this study. The healthy individuals (control group) were blood donors and volunteers who were nonsmokers, not on any medications (including aspirin and NSAIDs), and free from chronic diseases such as diabetes mellitus, hypertension, coronary artery, and renal diseases (inclusion criteria). Subjects who were pregnant and involved in the strenuous exercise were excluded. The patients included in this study were those who received treatment in Hospital Universiti Sains Malaysia with vascular-related diseases (n = 21) and other diseases (n = 21) (inclusion criteria). Pregnant and postsurgical patients were excluded. The clinical diagnosis of patients with cardiovascular- related event or vascular diseases was pulmonary embolism (n = 2), venous thrombosis (n = 1), acute coronary syndrome (n = 12), stable ischemic heart disease (n = 4), and congestive heart failure (n = 2). Patients with noncardiovascular diseases were patients diagnosed with malignant/hematological disorders (n = 6), liver disease (n = 3), bleeding related disorders (n = 4), sepsis/infectious diseases (n = 4), respiratory disorders (n = 2), and gastrointestinal diseases (n = 2). The details about these two different patient groups are listed in Supplementary Tables 2 and 3. Blood samples from the two patient groups were collected in September 2013 (n = 16), October 2013 (n = 8), November 2013 (n = 5), December 2013 (n = 8), January 2014 (n = 3), and February 2014 (n = 2). The dates of sample collection are listed in Supplementary Tables 2 and 3.
A blood sample from each subject was collected into collection tubes containing 3.2% sodium citrate (Becton, Dickinson and Company). The blood sample was centrifuged at 2,500 g at 24 °C for 15 minutes. After centrifugation, the top three quarters of plasma were transferred into a plastic tube using a plastic transfer pipette. The plasma in the plastic tube was centrifuged again at 2,500 g at 24 °C for 15 minutes. Following centrifugation, the plasma from the plastic tube was transferred into aliquot tubes, leaving about 0.5 mL in the bottom of the first tube. The plasma in the aliquot tubes was frozen at −70 °C until use. On the day of testing, plasma samples were thawed at 37 °C for ten minutes and mixed gently.
Test procedure
Determination of VWF:Ag and VWFpp levels in 30 healthy individuals and 42 patients were performed using the LIFECODES VWF and Propeptide Assay from Gen-Probe Incorporated (Cat. no.: TPN-VP). The LIFECODES VWF & Propeptide Assay is an enzyme-linked immunosorbent assay (ELISA) for the measurement of VWF and VWFpp in plasma. According to the manufacturer, intra-assay and inter-assay precisions of VWF:Ag assay were <6% and <12%, respectively. The intra-assay and inter-assay precisions of VWFpp assay were <10% and <25%, respectively. The ELISA method was conducted according to the manufacturer’s instructions.
Briefly, diluted calibrators, controls, and sample plasma were added to microwell strips to which monoclonal antibodies specific for VWF and VWFpp have been immobilized. The microwells were then incubated followed by a wash step. Subsequently, the microwell strips were incubated with a biotinylated monoclonal antibody specific for the VWF and VWFpp that has been captured in the wells. After that, a wash step was performed followed by incubation with streptavidin-labeled horseradish peroxidase. Following this incubation, a wash step was performed, and then, a fluorescent substrate was added. Finally, the microwells were incubated, and then, the reaction was stopped by the addition of a stopping solution.
The fluorescence of each calibrator, controls, and samples were measured using a spectral scanning multimode reader (Thermo Scientific Varioskan Flash) with excitation wavelengths between 315 and 340 nm and emission wavelengths between 370 and 470 nm. A standard curve was made by plotting the mean fluorescence reading (n = 2) for each calibrator versus its corresponding concentration of VWF:Ag and VWFpp using the analysis software provided in the assay kits. The standard curve was used to determine the levels of VWF:Ag and VWFpp in the controls and plasma samples.
Statistical analysis
Statistical analyses were conducted using the SPSS program (version 20.0, SPSS). Correlation analysis using the Spearman’s test was performed to determine the strength of the relationship between VWF:Ag and VWFpp levels that is showed by the correlation coefficient value (r). r ≥ 0.8 showed very strong relationship, r = 0.6–0.8 showed moderately strong relationship, r = 0.3–0.5 showed fair relationship, and r < 0.3 showed poor relationship.15 In a comparison study between VWF:Ag and VWFpp, data were given as median (25th–75th percentile) because the values were not normally distributed. Comparison of the median between groups was performed using Mann–Whitney U test. Reference ranges were estimated by analyzing 25th–75th percentile of VWF:Ag levels of 30 healthy individuals according to the blood groups. A P value of less than 0.05 was considered as statistically significant.
Results
Determination of VWF:Ag and VWFpp levels by ELISA method
Two sets of microwell plates coated with antibodies specific for VWF or VWFpp were used in the present study. VWF:Ag and VWFpp levels in the samples were determined using the standard curves of VWF:Ag (plate 1, R2 = 0.9996 and plate 2, R2 = 0.9976) and VWFpp (plate 1, R2 = 0.9996 and plate 2, R2 = 0.9993), respectively. The percentage differences between the duplicate results of VWF:Ag and VWFpp (ie, intra-assay precision) were less than 10%. The percentage difference between calibrators’ results of the two microwell plates (ie, intra-assay precision) were less than 20%.
Correlation analysis between VWF:Ag and VWFpp levels
A correlation study was performed by testing 72 plasma samples that were obtained from 30 healthy individuals, 21 patients with vascular-related diseases, and 21 patients with other diseases, including malignant and inflammatory disorders (Supplementary File). VWF:Ag and VWFpp levels were strongly correlated (Spearman’s rho = 0.849, P < 0.001) across a range of VWF:Ag from 56 to 546 IU/dL. Figure 1 shows a scatterplot of the relationship between VWF:Ag and VWFpp assays by the ELISA method. The scatterplot showed a linear, positive, and strong relationship between the two tests.
Figure 1.
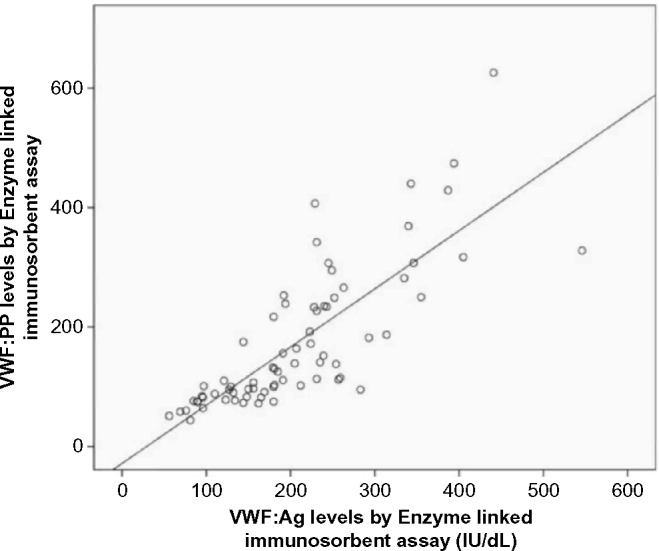
Scatterplot of the relationship between the levels of VWF:Ag and VWFpp determined by the ELISA method (Spearman’s rho = 0.849, P < 0.001).
Comparison between VWF:Ag and VWFpp levels in healthy individuals and patients
Subjects in the present study were divided into two groups: healthy individual and patient groups (Supplementary File). Demographic data of the groups are shown in Table 1. Median levels of VWF:Ag and VWFpp in the healthy individual and patient groups were compared according to all blood groups, O blood and non-O blood groups. Patients with various clinical conditions showed significantly higher levels of VWF:Ag and VWFpp (Table 2) compared to healthy individuals regardless of the blood group types.
Table 1.
Demographic characteristics of healthy individuals and patients.
| VARIABLES | HEALTHY INDIVIDUALS (n = 30) | PATIENTS (n = 42) |
|---|---|---|
| Age range (years) | 19–54 | 15–69 |
| Gender, n (%) | ||
| Male | 14 (46.7%) | 23 (54.8%) |
| Female | 16 (53.3%) | 19 (45.2%) |
| Blood group, n (%) | ||
| O | 13 (43.3%) | 11 (26.2%) |
| Non-O | 17 (56.7%) | 31 (73.8%) |
Table 2.
Median levels of VWF antigen, VWFpp, and VWFpp/VWF:Ag ratio in healthy individuals and patients.
| VARIABLES | MEDIAN (25TH-75TH PERCENTILE) | ||
|---|---|---|---|
| HEALTHY INDIVIDUALS | PATIENTS | aP VALUE | |
| VWF:Ag (IU/dL) | |||
| All blood group | 128 (94–163) | 242 (202–319) | <0.001 |
| O blood group | 95 (83–126) | 259 (239–293) | <0.001 |
| Non-O blood group | 148 (125–180) | 231 (194–340) | <0.001 |
| VWFpp (U/dL) | |||
| All blood group | 83 (74–98) | 230 (141–307) | <0.001 |
| O blood group | 76 (62–93) | 187 (115–266) | <0.001 |
| Non-O blood group | 83 (76–102) | 234 (141–317) | <0.001 |
| VWFpp/VWF:Ag ratio | |||
| All blood group | 0.7 (0.6–0.8) | 0.9 (0.6–1.2) | 0.006 |
| O blood group | 0.8 (0.7–0.9) | 0.6 (0.4–1.0) | 0.531 |
| Non-O blood group | 0.6 (0.5–0.8) | 0.9 (0.7–1.2) | <0.001 |
Note:
P < 0.05 is considered statistically significant.
Abbreviations: VWF, von Willebrand factor; VWF:Ag, von Willebrand factor antigen; VWFpp, von Willebrand factor propeptide.
Comparison within the subject group (ie, healthy individuals and patients) showed that healthy individuals with O blood group have significantly lower median levels of VWF:Ag compared to healthy individuals with non-O blood group (95 vs 148 IU/dL, P = 0.001). Nevertheless, the median VWF:Ag levels between patients with O blood group and patients with non-O blood group were not significantly different (259 vs 231 IU/dL, P = 0.165).
The differences in median levels of VWFpp between patients with O blood group and patients with non-O blood group were not significantly different (187 vs 234, P = 0.367). In contrast to VWF:Ag levels, the median levels of VWFpp in healthy individuals with O blood group and healthy individuals with non-O blood group were not significantly different (76 vs 83, P = 0.161).
Table 2 shows the median levels of VWFpp/VWF:Ag ratio in healthy individuals and patients according to all blood groups, O blood group and non-O blood group. As shown in the table, the median difference of VWFpp/VWF:Ag ratio between healthy individuals and patients was significantly different in all blood groups and non-O blood group. Comparison of the ratio between O blood group and non-O blood group within the subject group was statistically different in healthy individuals (0.8 vs 0.6, P = 0.028).
Comparison of the median was also made between patients with other diseases (n = 21) and patients with cardiovascular- and vascular-related diseases (n = 21). Median levels of VWF:Ag (231 vs 249, P = 0.715) and VWFpp (239 vs 236.5, P = 0.064) were comparable between the two groups of patients, and the difference was not statistically significant.
Discussion
High VWF:Ag and VWFpp levels were reported in vascular diseases associated with endothelial dysfunction.4–7,10 VWF:Ag has been listed as one of the hemostatic biomarkers although it is not widely used in clinical practice. Since VWFpp is secreted together with mature VWF upon stimulation, VWFpp is, therefore, a candidate marker of endothelial dysfunction. Hence, the measurement of VWFpp levels might be useful in the future clinical practice. It is important to understand the physiological and pathophysiological differences between VWF:Ag and VWFpp. At present, limited service is offered by the medical laboratories for the measurement of VWFpp. In addition, the application of VWFpp is only limited to the research field and specialized hemostasis center. Although the use of VWFpp in bleeding disorder (VWD) is established, its application is still uncertain in thrombotic diseases.
A strong correlation was found between VWF:Ag and VWFpp in this study that was supported by previous studies.4,16 In this study, VWF:Ag and VWFpp levels were significantly increased in patients compared to healthy individuals. Increase in VWF:Ag and VWFpp levels was also seen in the two groups of patients: vascular-related diseases and other disease groups (such as cancers and liver diseases). These findings were also reported by other investigators.7–10,16 Thus, VWFpp could serve as a potential biomarker reflecting pathophysiological changes reactive to the underlying diseases, similar to VWF:Ag as previously described.
The effect of blood group types (O and non-O blood groups) was only seen in VWF:Ag levels in healthy individuals in this present study. Low levels of VWF:Ag in O blood group healthy individuals is because of the rapid clearance of VWF that is related to the presence of ABO antigens in the N-linked oligosaccharide side chains on the VWF molecule.4 In contrast, VWFpp levels in healthy individuals are not affected by the ABO blood group types as found in this study and other studies.4,14 However, the effect of blood group types was not seen in this study among the patients with high levels of VWF:Ag. This finding is probably because of active endothelial cells damage, which could have masked the effect of the blood group types on VWF:Ag. Similarly, the levels of VWFpp in patients with high levels of VWF:Ag were not affected by the blood group types in this present study. However, these findings are only preliminary and need to be verified with more data on VWFpp in various medical conditions.
In the present study, there was a significant difference in VWFpp/VWF:Ag ratio between healthy individuals and patients with high levels of VWF:Ag. This finding was also reported in the other study.10 More investigations are required to confirm the role of VWFpp/VWF:Ag ratio in patients with specific medical condition especially in predicting the thrombotic risk. In this study, it was found that VWFpp/VWF:Ag ratio in the patient group was significantly higher than the ratio in the healthy individual group. The clinical usefulness of the VWFpp/VWF:Ag ratio in patients with normal or high VWF:Ag is uncertain. In contrast to the low baseline VWF:Ag levels, high ratio of VWFpp/VWF:Ag is associated with decreased half-life of VWF in circulation, and the ratio is used to identify VWD patients with a shorter VWF survival.11,12,14 An increase in VWFpp/VWF:Ag ratio (2–3) has been shown in some type 1 VWD, atypical type 2B, and type 2B VWD patients carrying the mutated VWF.13 In this present study, VWFpp/VWF:Ag ratio in healthy individuals with O blood group was significantly increased as compared to healthy individuals with non-O blood group. Previous studies have also reported similar findings.12,14 These findings might be because of the rapid clearance of VWF in O blood group individuals that showed a lower level of VWF:Ag compared to non-O blood group individuals. Interestingly, VWFpp is not affected by ABO blood groups probably because of the difference in VWF molecule and VWFpp structural organization.
There was no significant difference in the levels of VWF:Ag and VWFpp between the two groups of patients (cardiovascular- and vascular-related diseases and other diseases groups) in this study. Both VWF and VWFpp are acute phase proteins that are released into blood circulation upon synthesis or stimulation. High levels of VWF:Ag and VWFpp also indicate endothelial cells activation that might serve as markers of endothelial dysfunction. Hence, high levels of VWF:Ag and VWFpp might be useful as disease biomarker associated with endothelial injury that can be seen in various conditions, including infection, malignant disorders, and cardiovascular diseases. Many studies have shown VWF as a useful biomarker in mortality prediction,16 disease prediction,17 clinical event prediction,18 disease prognosis,19 and disease assessment.20 Although VWF:Ag and VWFpp are nonspecific markers, the clinical impact of endothelial dysfunction can be investigated by measuring their levels and may be useful in various clinical conditions particularly associated with thrombotic potentials (vascular-related disorders). VWFpp could be used for the monitoring of cardiovascular diseases by serial testing of the levels and probably have a role in the prediction of acute vascular event.
This present study is a preliminary study that investigated the potential role of VWFpp as a biomarker across diseases. Thus, there are some limitations that may affect the results of the present study. First, ELISA is the only assay used in this study. In future, other assays such as western blotting and mRNA determination could be considered to conclude the finding. Second, the sample size in this study was small and there was heterogeneity among the patient samples, which contained 21 patients with vascular-related diseases, including cardiovascular cases, and 21 patients with other diseases. Besides that, data from patients with cardiovascular diseases and other diseases were grouped together as patients. In future, the additional number of patients from each group needs to be studied. Besides that, the data from the two different patient groups should be analyzed separately using adequate number of samples. The age variation of the subjects and selection of the test method possibly had influenced the results of the present study and also previous studies. Moreover, the levels of VWF:Ag of some samples might be over- or underestimated because of the inter-assay variability. The subjects of the control group in the present study were not matched with the patients (in terms of gender and age) because of the constraint of recruiting healthy volunteers who are not on any medications or supplements. This limitation is also contributed by the differences in the sample populations in the present study and also in the previous studies. It is expected that age may influence the VWFpp levels as been reported for VWF:Ag levels. Although the data are not shown here, age was not a significant factor of VWF:Ag levels in this present study. Using simple linear regression analyses, age (P < 0.001) and subject group (P < 0.001) were significant factors of VWF:Ag levels. However, following multiple linear regression analysis, the subject group was the only significant factor of VWF:Ag levels (P < 0.001). Nevertheless, the finding should be interpreted cautiously as bigger sample size is required for confirmation.
Conclusion
This study demonstrated that the VWFpp is not affected by ABO blood group types unlike VWF:Ag. Although the ratio of VWFpp/VWF:Ag is important in VWD patients, the ratio might not be clinically useful to indicate increased clearance in patients with high levels of VWF:Ag and VWFpp. VWFpp levels increased in parallel with VWF:Ag levels in patients with various clinical conditions associated with endothelial injury. Hence, VWFpp level is recommended as one of the potential hemostatic biomarkers useful for many purposes requiring more studies to be conducted. VWFpp is a potential biomarker reflecting one of the pathophysiological changes because of endothelial injury and probably superior to VWF:Ag as it is not affected by the ABO blood group types.
Supplementary Materials
Supplementary Table 1. Data collection from healthy individuals.
Supplementary Table 2. Data collection from patients with cardiovascular disease.
Supplementary Table 3. Data collection from patients with other diseases.
Acknowledgments
The authors would like to thank the staff of Haematology Department and Blood Transfusion Service, Universiti Sains Malaysia, Kelantan, Malaysia, for their continuous support in this work.
Footnotes
ACADEMIC EDITOR: Karen Pulford, Editor in Chief
PEER REVIEW: Six peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1,019 words, excluding any confidential comments to the academic editor.
FUNDING: This study was supported by short-term grant 304/PPSP/61312085 from Universiti Sains Malaysia, Penang, Malaysia. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: MM, AWZ. Analyzed the data: MM. Wrote the first draft of the manuscript: MM. Contributed to the writing of the manuscript: MM, AWZ. Agreed with manuscript results and conclusions: MM, AWZ, CHCM. Jointly developed the structure and arguments for the paper: MM, AWZ. Made critical revisions and approved the final version: MM, AWZ, CHCM. All the authors reviewed and approved the final manuscript.
REFERENCES
- 1.Franchini M, Lippi G. von Willebrand factor and thrombosis. Ann Hematol. 2006;7:415–23. doi: 10.1007/s00277-006-0085-5. [DOI] [PubMed] [Google Scholar]
- 2.Reininger AJ. VWF attributes – impact on thrombus formation. Thromb Res. 2008;122(suppl 4):S9–13. doi: 10.1016/S0049-3848(08)70028-8. [DOI] [PubMed] [Google Scholar]
- 3.Peyvandi F, Garagiola I, Baronciani L. Role of von Willebrand factor in the haemostasis. Blood Transfus. 2011;9(suppl 2):s3–8. doi: 10.2450/2011.002S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nossent AY, Van Marion V, Van Tilburg NH, et al. von Willebrand factor and its propeptide: the influence of secretion and clearance on protein levels and the risk of venous thrombosis. J Thromb Haemost. 2006;4(12):2556–62. doi: 10.1111/j.1538-7836.2006.02273.x. [DOI] [PubMed] [Google Scholar]
- 5.Gallinaro L, Cattini MG, Sztukowska M, et al. A shorter von Willebrand factor survival in O blood group subjects explains how ABO determinants influence plasma von Willebrand factor. Blood. 2008;111(7):3540–5. doi: 10.1182/blood-2007-11-122945. [DOI] [PubMed] [Google Scholar]
- 6.Sonneveld MA, de Maat MP, Leebeek FW. Von Willebrand factor and ADAMTS13 in arterial thrombosis: a systematic review and meta-analysis. Blood Rev. 2014;28(4):167–78. doi: 10.1016/j.blre.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Verrotti A, Greco R, Basciani F, Morgese G, Chiarelli F. von Willebrand factor and its propeptide in children with diabetes. Relation between endothelial dysfunction and microalbuminuria. Pediatr Res. 2003;53(3):382–6. doi: 10.1203/01.PDR.0000049509.65496.BF. [DOI] [PubMed] [Google Scholar]
- 8.van Schie MC, MP DEM, Dippel DW, et al. von Willebrand factor propeptide and the occurrence of a first ischemic stroke. J Thromb Haemost. 2010;8(6):1424–6. doi: 10.1111/j.1538-7836.2010.03863.x. [DOI] [PubMed] [Google Scholar]
- 9.Habe K, Wada H, Ito-Habe N, et al. Plasma ADAMTS13, von Willebrand factor (VWF) and VWF propeptide profiles in patients with DIC and related diseases. Thromb Res. 2012;129(5):598–602. doi: 10.1016/j.thromres.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Stufano F, LA Marca S, Pontiggia S, Musallam KM, Peyvandi F. von Willebrand factor propeptide to antigen ratio in acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2012;10(4):728–30. doi: 10.1111/j.1538-7836.2012.04642.x. [DOI] [PubMed] [Google Scholar]
- 11.Eikenboom J, Federici AB, Dirven RJ, et al. MCMDM-1VWD Study Group VWF propeptide and ratios between VWF, VWF propeptide, and FVIII in the characterization of type 1 von Willebrand disease. Blood. 2013;121(12):2336–9. doi: 10.1182/blood-2012-09-455089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haberichter SL, Balistreri M, Christopherson P, et al. Assay of the von Willebrand factor (VWF) propeptide to identify patients with type 1 von Willebrand disease with decreased VWF survival. Blood. 2006;108(10):3344–51. doi: 10.1182/blood-2006-04-015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casonato A, Gallinaro L, Cattini MG, et al. Reduced survival of type 2B von Willebrand factor, irrespective of large multimer representation or thrombocytopenia. Haematologica. 2010;95(8):1366–72. doi: 10.3324/haematol.2009.019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sztukowska M, Gallinaro L, Cattini MG, et al. Von Willebrand factor propeptide makes it easy to identify the shorter Von Willebrand factor survival in patients with type 1 and type Vicenza von Willebrand disease. Br J Haematol. 2008;143(1):107–14. doi: 10.1111/j.1365-2141.2008.07311.x. [DOI] [PubMed] [Google Scholar]
- 15.Chan YH. Biostatistics 104: correlational analysis. Singapore Med J. 2003;44(12):614–9. [PubMed] [Google Scholar]
- 16.Hyseni A, Roest M, Braun SL, et al. Chronic dysfunction of the endothelium is associated with mortality in acute coronary syndrome patients. Thromb Res. 2013;131(3):198–203. doi: 10.1016/j.thromres.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Morange PE, Simon C, Alessi MC, et al. PRIME Study Group Endothelial cell markers and the risk of coronary heart disease: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) study. Circulation. 2004;109(11):1343–8. doi: 10.1161/01.CIR.0000120705.55512.EC. [DOI] [PubMed] [Google Scholar]
- 18.Wennberg P, Wensley F, Di Angelantonio E, et al. Haemostatic and inflammatory markers are independently associated with myocardial infarction in men and women. Thromb Res. 2012;129(1):68–73. doi: 10.1016/j.thromres.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 19.Lopes AA, Barreto AC, Maeda NY, et al. Plasma von Willebrand factor as a predictor of survival in pulmonary arterial hypertension associated with congenital heart disease. Brax J Med Biol Res. 2011;44(12):1269–75. doi: 10.1590/s0100-879x2011007500149. [DOI] [PubMed] [Google Scholar]
- 20.Maieron A, Salzl P, Peck-Radosavljevic M, et al. Von Willebrand Factor as a new marker for non-invasive assessment of liver fibrosis and cirrhosis in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2014;39(3):331–8. doi: 10.1111/apt.12564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Data collection from healthy individuals.
Supplementary Table 2. Data collection from patients with cardiovascular disease.
Supplementary Table 3. Data collection from patients with other diseases.


