Abstract
Background
Renal angiomyolipoma is rare, but many of these patients may have an acute debut with severe bleeding. These patients need urgent treatment with interventional embolization as an attractive option.
Purpose
To investigate the technical and clinical effect of this treatment and to evaluate long-term clinical outcomes with clinical control and radiological imaging.
Material and Methods
Eight patients with angiomyolipoma were treated with embolization. Five patients were treated acutely. Five patients were followed-up for mean 4.5 years with clinical and radiological examinations.
Results
The renal angiomyolipoma decreased significantly from mean 7.2 cm to 2.9 cm after embolization (p = 0.04). Cortical infarctions of about one-third of the circumference of the embolized kidneys could be detected on follow-up examinations, but all patients had normal total kidney function. The bleeding was primarily stopped in all patients, however, in one patient bleeding from a lumbar artery was supplementary embolized within 24 h. In another case the interventional procedure ended up in embolization of the whole kidney as it was impossible to embolize all the feeding arteries selectively. One patient had a nephrectomy one month after embolization because of infection and re-bleeding and one patient after 2.5 years because of tumor size >4 cm. The technical success was 7/8 (88%) and clinical success was 6/8 patients (75%).
Conclusion
Selective embolization of renal angiomyolipoma is a minimally invasive and safe procedure with few complications. It is a nephron sparing alternative to renal resection. The reduction in tumor size after embolization is significant and long-lasting.
Keywords: Angiomyolipoma, kidney neoplasm, embolization, therapeutic, radiology, interventional
Introduction
Renal angiomyolipoma (AML) is a benign renal neoplasm composed of adipose tissue, abnormal blood vessels, and smooth muscle cells which often has characteristic imaging appearances on ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI) (1–6). It is rare and constitutes only 1–2% of all tumors in the kidney and has an incidence of about 0.3–3%; however, it is the most common benign mesenchymal tumor in the kidney. The majority of AMLs are isolated and occur sporadically (80%) and are typically identified in adults with mean age at symptomatic presentation of about 40 years with a strong female predilection (F:M 4:1) (7). Up to 20% of the AMLs occur in association with the tuberous sclerosis complex and occur in about 80% of these patients (8,9). Classically, AML can be diagnosed by identifying the negatively attenuating intra-tumor macroscopic fat component on non-enhanced CT or on MRI (2,3). Most AMLs are discovered incidentally in patients who are examined for other reasons. About 40% of the AMLs have an acute debut with severe hemorrhage and may cause hematuria, flank pain, acute abdomen, and shock, and be potentially life-threatening as a result of spontaneous bleeding in and outside the tumor. The risk of bleeding is said to be proportional to the size of the tumor, and increases significantly with size above 4 cm (7,10). Patients with spontaneous rupture of AML and hemodynamic instability need immediate treatment with interventional embolization as an attractive option (11). The aim of this study was to describe the immediate technical and clinical results with long-term clinical outcomes of selective embolization of AML.
Material and Methods
The study is a retrospective observational study from a single center and approved by the Regional Scientific Ethical Committees for Southern Denmark. Tracking down patients was performed through the central computerized patient register, through the manual registrations of all interventional procedures at the Department of Radiology, and the radiological information system (RIS) and picture archiving system (PACS) of the Radiologic Department at Odense University Hospital back till 2008. In all cases of interventional embolization of renal bleedings, all available case records and radiological imaginings were perused and the patients ending up with the diagnosis of AML entered the study. The diagnosis was based on clinical findings combined with CT and angiographic findings (Fig. 1a,b,c,d). Angiography was performed under local anesthesia via the femoral artery. Abdominal aortography was followed by selective right and left renal angiography. The bleeding vessels were selectively catheterized with 4 F or microcatheters and embolized with use of standard or micro coils according to the size of the vessel being embolized (Fig. 1e,f), and in one case with use of a supplementary vascular plug to occlude the main renal artery.
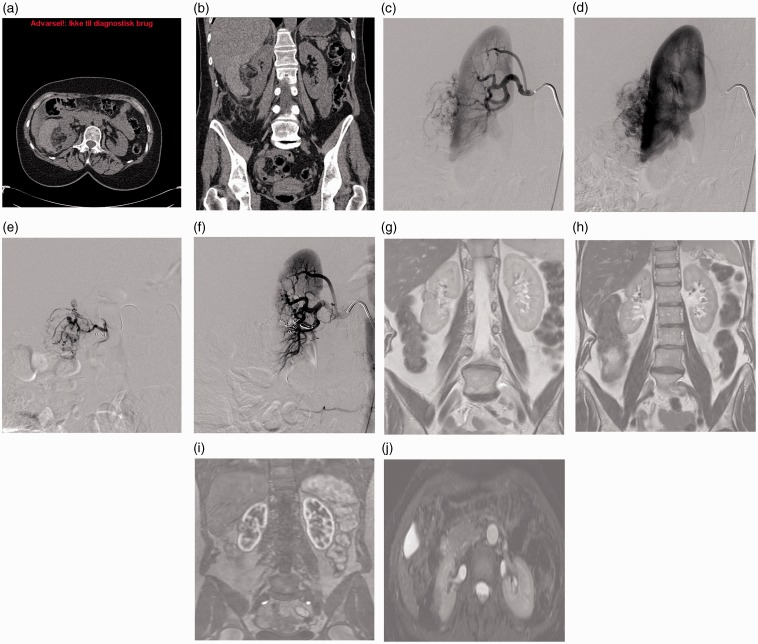
Fig. 1.
A 54-year-old woman (Patient 5) with spontaneous bleeding from a 4 cm angiomyolipoma laterally in right kidney. Hemodynamically stable (a,b). Angiography 5 days later. No ongoing bleeding. Angiomyolipoma vessels (c,d). Selective distal embolization of two segmental renal arteries with use of microcoils with following flow-stop to the angiomyolipoma (c,d). MRI at 2.5-year follow-up demonstrating a 1.5 cm residual angiomyolipoma and a small cortical infarction with reduced cortex of the right kidney laterally (g,h,i,j). The kidney function estimated by renography showed a left/right distribution of 63/37%. Total kidney function was normal (eGFR).
The location and size (longest axis) of the AML on primary CT scanning, the technical and clinical success of the interventional treatment and follow-up of the patients with clinical control and radiological imaging with MRI or CT until August 2014 (Fig. 1g,h,i,j) was investigated. The patients gave informed consent to the follow-up study. They were clinically investigated in the out-patient clinic, and, renal function (eGFR) was determined, blood pressure was measured and renography were performed at follow-up. The patients were offered MRI for follow-up and in one case where the patient refused, a CT was performed. The study’s primary outcome measures were: overall patient survival and nephrectomy-free survival. Secondary outcome measures were: (i) procedural/technical success, defined as selective embolization of the bleeding vessels as intended with following complete stop of the bleeding with no further need of transfusions and without procedure related complications within 24 h; (ii) clinical success, defined as no recurrence of bleeding episodes caused by the AML or complications that could be ascribed to the embolization procedure within 30 days; and (iii) re-interventions with embolization or surgery.
Results
In total, eight patients who underwent embolization during the study time period were diagnosed with AML (Table 1). There were two men and six women with a mean age of 59 years (age range, 34–81 years). Four AMLs were located in the right kidney and four in the left kidney. The mean size (longest axis) of the AMLs was 6.5 cm (range, 2–12 cm) with two <4 cm, three 4–9 cm, and three ≥10 cm at admission.
Table 1.
Data of the patients included in the study.
| Gender/ age (years) | Location/size longest axis (cm) | Embolization Materials | Embolization acute/Elective | Complications comments | Follow-up | Outcome | |
|---|---|---|---|---|---|---|---|
| 1 | F/54 | Left central/7 | Coils | Acute, hemodynamically instable; one-third of left kidney embolized | 10 years, MRI 2 cm, left/right 39/61% | Normal kidney function | |
| 2 | F/55 | Right/3 | Covered stent | Elective, 4 months; one-third of right kidney embolized | Perirenal hematoma – no primary intervention; aneurysm right renal artery; high BMI | 5.5 years, MRI 2.5 cm, left/right 62/38% | Normal kidney function |
| 3 | F/34 | Left upper pole/10 | Coils | Acute, hemodynamically instable; one-quarter of left kidney embolized | Spontaneous bleeding, transfusions during transportation | 2.5 years, CT 6 cm, left/right 48/52%, nephrectomy | One kidney lost – normal kidney function |
| 4 | F/44 | Right posterior/12 | Coils + plug | Elective, 3 months, embolization of whole right kidney | Discovered incidentally, asymptomatic, high BMI | 2.5 years MRI 2.5 cm, kidney size: left/right 11.4/5.2 cm, technical failure | One kidney lost – normal kidney function |
| 5 | F/54 | Right lateral/4 | Coils | Elective, 5 days, one-third of right kidney embolized | No ongoing bleeding during angiography, two renal arteries | 2.5 years, MRI 1.5, left/right 63/37% | Normal kidney function |
| 6 | M/81 | Left lower pole/4 | Coils | Acute, hemodynamically instable, one-third of left kidney embolized | Congenital single kidney, supplementary embolization of lumbar artery | 2.5 years, dead because of lymphoma, no relation to AML, clinical failure | Normal kidney function |
| 7 | M/75 | Left lower pole/2 | Coils | Acute, hemodynamically instable, one-third of left kidney embolized | After coronary angiography and PCI perirenal bleeding, BMI 34 | 2 weeks, dead after CABG for cardiac reasons, no relation to AML | Normal kidney function |
| 8 | F/74 | Right upper pole/10 | Coils | Acute, hemodynamically instable, one-third of right kidney embolized | Post embolization: hemodialysis and inflammation CRP >400, bladder pressure up to 22 mmHg; slow, but complete recovery | CT no bleeding after 3 days, acute nephrectomy after 1 month because of infected hematoma and re-bleeding, clinical failure | One kidney lost –normal kidney function |
Interventional treatment
Six patients had spontaneous bleedings and the AMLs were discovered following this (Figs. 2a,b,c and 3a,b). Five patients (patients 1, 3, 6, 7, and 8) were embolized acutely within 12 h of symptom debut because of severe bleeding (Fig. 3c,d,e) (Table 1). They were all hemodynamically instable with low Hb, low blood pressure, and with need of transfusions. Patient 5 was stabilized hemodynamically on conservative treatment, and was embolized electively after 5 days with tumor size of 4 cm because of supposed increased risk of re-bleeding. In Patient 4 the AML was discovered incidentally, and the interventional treatment was performed electively after 3 months because of the big size of the AML (12 cm). In Patient 7, the bleeding from the AML appeared after coronary angiography, and renal trauma by the catheter or guide wire could not be excluded. Lastly, Patient 2 was treated without embolization because of a small (3 cm) AML with self-limited bleeding. Four months later a covered stent was deployed in the renal artery to exclude a renal artery aneurysm on the same side. This stent at the same time occluded a segmental artery supplying the AML. In the remaining seven patients coils were deployed for embolization. In seven patients the embolization was selective with embolization of estimated one-quarter to one-third of the kidney. In one patient with technical failure the whole kidney was embolized (Patient 4). In this patient the intention was to treat with selective embolization, but ended up with total renal embolization with use of a supplementary vascular plug, because of numerous supplying arteries to the tumor, which were impossible to embolize selectively (Fig. 4a,b,c,d). This patient had a contralateral kidney with normal function detected at renography before the embolization. Thus, the technical success with stop of bleeding as intended, no re-intervention because of re-bleeding from AML, and no associated complications within 24 h was 7/8 (88%) patients.
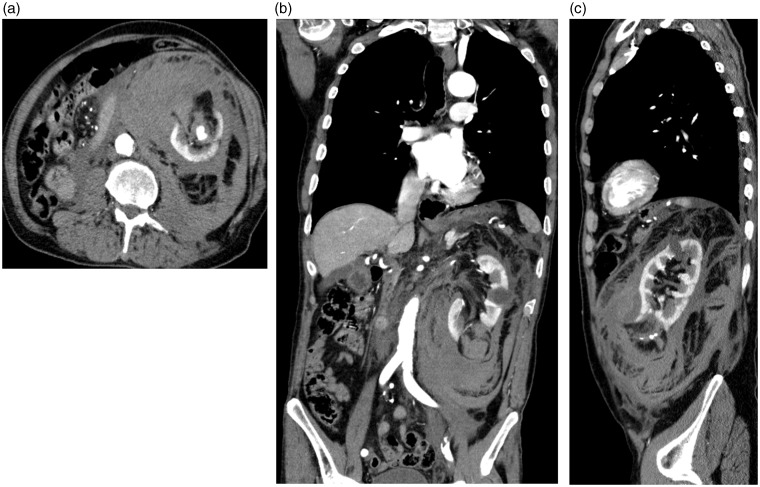
Fig. 2.
An 81-year-old man (Patient 6) with a congenital single kidney with a severe spontaneous retroperitoneal bleeding from a 4 cm angiomyolipoma in the lower pole of left kidney (a,b,c). He was hemodynamically unstable with low blood pressure and had several blood transfusions. About one-quarter of the kidney was selectively embolized and he recovered completely with normal kidney function (eGFR).
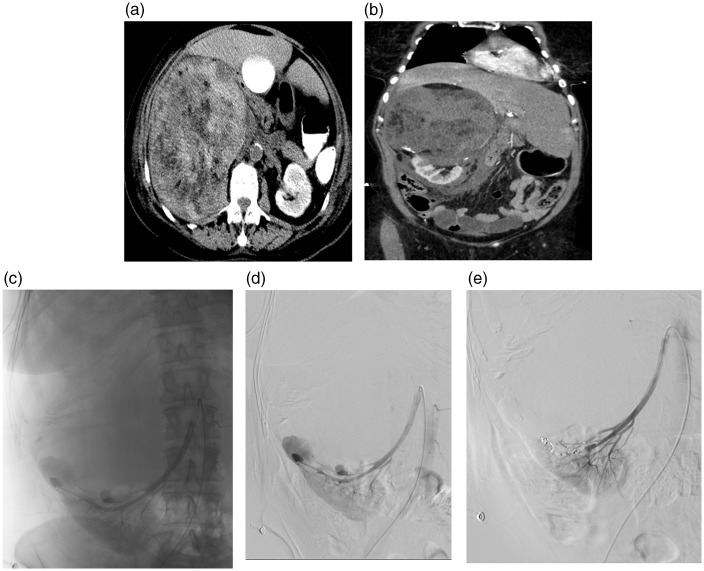
Fig. 3.
A 74-year-old woman (Patient 8) hemodynamically instable with massive bleeding from a 10 cm angiomyolipoma in the right upper pole (a,b). Angiography demonstrating several small aneurysms in relation to the angiomyolipoma (c,d). About one-third of the kidney was selectively embolized with use of microcoils (e). She had a compartment syndrome with bladder pressure up to 22 mmHg and inflammation after embolization and was on hemodialysis for a period, but recovered slowly and completely.
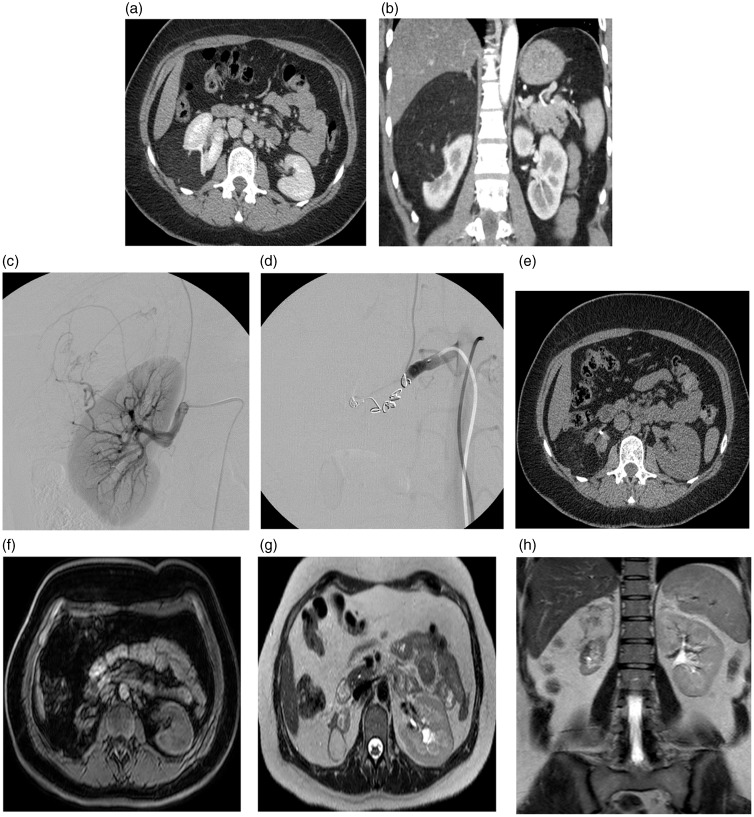
Fig. 4.
A 44-year-old woman (Patient 4) with an incidentally discovered 12 cm angiomyolipoma posterolaterally in the right kidney (a,b). Renography showed a distribution left/right kidney 59/41%. Selective embolization was intended, but was not possible and the procedure ended-up with embolization of the whole kidney with coils and a supplementary vascular plug (c,d). Follow-up after 7 months with CT (upper left, Hounsfields units [HU] of residual angiomyolipoma was –100) (e). After 2.5 years (f,g,h) MRI showed angiomyolipoma size 2.5 cm, right kidney size 5.2 cm, and left kidney size 11.4 cm. The total kidney function was normal (eGFR).
Patient 6, who was considered a clinical failure, had supplementary embolization within 24 h because of new bleeding from a lumbar artery. The other patient with clinical failure (Patient 8) had an acute nephrectomy 1 month after the embolization because of infected hematoma and re-bleeding from the former embolized kidney. She had a long hospital stay with abdominal compartment syndrome and was on dialysis for a period, but with complete recovery. Therefore, the clinical success was 6/8 (75%) patients. Patient 6 had pulmonary emboli a couple of days after the embolization but not related to the embolization per se and without clinical consequences.
Follow-up
The patients were followed up until July 2014 or until they died. The patient with nephrectomy after 1 month was not followed up with radiological imaging. Otherwise, no intercurrent renal bleeding episodes were registered in any patient. Disease-specific survival of the entire cohort was 100%. Two patients (Patients 6 and 7) died during the follow-up period (2.5 years and 2 weeks). Patient 7 died 2 weeks after sufficient AML embolization. He was a 75-year-old man with unstable angina and myocardial infarction. He died following coronary artery bypass surgery because of heart failure without relation to the AML. The other patient (Patient 6) died 2.5 years after the embolization because of lymphoma without relation to the AML. Three out of the eight patients (38%) lost a kidney: one during the embolization procedure (Patient 4) and two during follow-up by nephrectomy (Patient 8 4 weeks after embolization and Patient 3 2.5 years after the embolization). In the latter case, the AML had decreased in size from 10 cm before embolization to 6 cm after, but the nephrectomy was performed based on a clinical decision to prevent the supposed increased risk of bleeding because of AML size over 4 cm. Thus five patients were followed up for a mean of 4.5 years (range, 2.5–10 years) with clinical examinations, blood samplings and MRI in four cases, and CT in one case (Table 1). The size (longest axis) of the AML in these five patients had decreased significantly from median 7 cm to 2.5 cm (64%) and mean 7.2 cm to 2.9 cm (60%) (P = 0.04, paired samples t-test). In all patients cortical infarctions of about one-third of the circumference of the kidneys could be detected on follow-up examinations (Fig. 5). We have had no cases of re-growth of the AML, and no revascularizations or insufficient embolizations. All patients had normal total kidney function (eGFR) with a renographic distribution of about one-third on the embolized kidney and two-thirds on the non-embolized kidney (Table 1). All patients had normal blood pressure, one patient on treatment with three different antihypertensive drugs, two with one antihypertensive drugs, and the rest without medical treatment. All patients declared to be in good health.
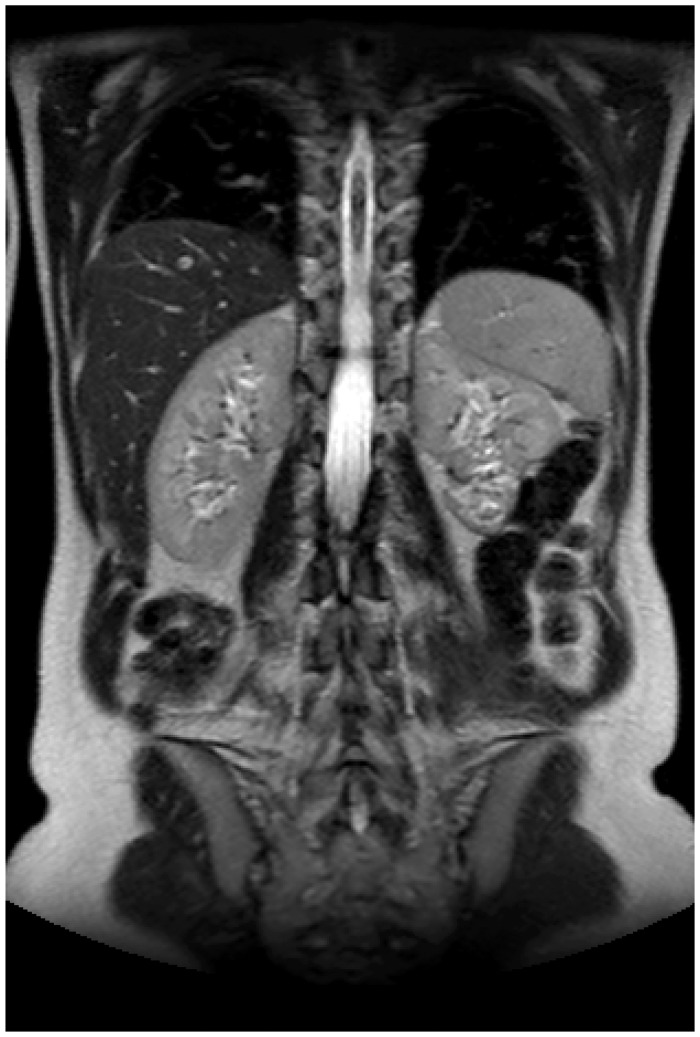
Fig. 5.
A 54-year-old woman (Patient 1) 10 years after acute embolizaton of spontaneous bleeding from a 7 cm angiomyolipoma in left kidney. About one-third of the kidney was selectively embolized. At follow-up the residual tumor measured 2 cm and the renographic functional distribution of left/right kidney was 39/61% and the total kidney function (eGFR) was normal.
Discussion
In the present study we have shown the efficacy of selective embolization of AML in stopping acute bleeding and in reducing the volume of the AMLs. There have been no cases with regrowth of the tumors. The procedure has been demonstrated to have few complications during a long follow-up period. Although our study is small the long-term results are comparable to others (12–14). We used coils in all cases but one for embolization. It has also been shown by others that embolization of AML produces durable long-term results regardless of the choice of embolic agent (12).
Small (<4 cm) AMLs found incidentally have traditionally been managed conservatively based on their lack of malignancy and decreased propensity for hemorrhage, but follow-up is recommended to assess for growth. Tumors >4 cm or those that have been symptomatic can be selectively embolized or resected with partial or radical nephrectomy. Resection, however, will often end up with total nephrectomy (13,15). There are no evidence-based recommendations whether to prophylactic embolize non-symptomatic >4 cm tumors when incidentally discovered. If the AML is predominantly composed by fat and poorly vascularized without aneurysms the bleeding risk is most probably low. The priority of treatment of AML is renal function preservation. Several other modalities besides selective embolization and surgical excision of the lesion have emerged during the last years for nephron-sparing approaches. These include laparoscopic and percutaneous ablative therapies such as radiofrequency ablation, cryo-ablation, microwave ablation, and robot-assisted laparoscopic partial nephrectomy (7). There is no evidence which modality is the best and local experience and expertise will often be decisive for which modality is preferred. There are no published comparative randomized trials between surgical excision and embolization, most probably because AML is a rare disease (16). Selective embolization of segmental arteries that supply the tumor is, however, by many considered the most effective minimally invasive approach in preventing hemorrhagic events and symptomatic manifestations. The procedure is well tolerated and is associated with few complications, but we have had three patients who lost a kidney by nephrectomy or total kidney embolization. The procedure has been said to be associated with more relapses compared with surgical alternatives (17). We had only one supplementary embolization (of a lumbar artery) within 24 h after primary AML embolization in contrary to 30–37% of recurrent symptoms in other publications, most of them in the group of tuberous sclerosis (17,18). Lesions that present with retroperitoneal hemorrhage often require emergency embolization as a life-saving measure. On angiography AMLs are hypervascular lesions, typically with micro or macro aneurysms (Fig. 3b), and they are sharply marginated with dense early arterial network and late whorled appearance.
The demonstration of fatty attenuation in renal tumor on CT, MRI, and MR- or sonoelastography studies (19) is virtually diagnostic of AML but not pathognomonic as renal cell carcinomas rarely may have fat components. Most lesions involve the cortex and demonstrate macroscopic fat (−20 Hounsfields Units on CT). When AML has typical appearance there is essentially no differential diagnosis, but if atypical, especially when fat-poor, retroperitoneal liposarcoma, adrenal myelolipoma, renal cell carcinoma, oncocytoma, and Wilms' tumor should be excluded. In up to 4.5% of AML cases the tumor may be composed mostly of smooth muscle cells and blood vessels with minimal adipose tissue, and in these cases intra tumor macroscopic fat may not be visible on CT, mimicking and difficult to distinguish from renal cell carcinoma (4,5). Distinguishing AML from renal cell carcinoma is critical in clinical management (20).
The major limitation of the present report is that it is a small cohort of patients, because AML is a rare disease. Further, the study is retrospective, and treatment was not given according to a prospective protocol, and therefore sample bias may be present. Even though, it has been possible to show that the embolization significantly has reduced the volume of the tumors, and no recurrence of tumor growth or re-bleeding has been demonstrated at long-term follow-up. Selective embolization is nephron-sparing and non-embolized areas of the kidney will be preserved and thus it is possible to preserve normal total kidney function, like demonstrated also in previous studies (13,14,21).
In conclusion, embolization of AMLs is an attractive option, as it is a minimally invasive and nephron sparing procedure. Selective arterial embolization is effective for AML devascularization and volume reduction which is long-lasting, and it has a long-term efficacy in preventing hemorrhagic complications of AML. It is fast to perform and with few complications but 3/8 of the patients have lost one kidney during the procedure or the follow-up period.
Conflict of interest
None declared.
References
- 1.Lienert AR, Nicol D. Renal angiomyolipoma. BJU Int 2012; 110(Suppl. 4): 25–27. [DOI] [PubMed] [Google Scholar]
- 2.Bosniak MA. Angiomyolipoma (hamartoma) of the kidney: a preoperative diagnosis is possible in virtually every case. Urol Radiol 1981; 3: 135–142. [DOI] [PubMed] [Google Scholar]
- 3.Schieda N, Avruch L, Flood TA. Small (<1 cm) incidental echogenic renal cortical nodules: chemical shift MRI outperforms CT for confirmatory diagnosis of angiomyolipoma (AML). Insights into Imaging 2014; 5: 295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hindman N, Ngo L, Genega EM, et al. Angiomyolipoma with minimal fat: can it be differentiated from clear cell renal cell carcinoma by using standard MR techniques? Radiology 2012; 265: 468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JK, Park SY, Shon JH, et al. Angiomyolipoma with minimal fat: differentiation from renal cell carcinoma at biphasic helical CT. Radiology 2004; 230: 677–684. [DOI] [PubMed] [Google Scholar]
- 6.Halpenny D, Snow A, McNeill G, et al. The radiological diagnosis and treatment of renal angiomyolipoma – current status. Clin Radiol 2010; 65: 99–108. [DOI] [PubMed] [Google Scholar]
- 7.Sivalingam S, Nakada SY. Contemporary minimally invasive treatment options for renal angiomyolipomas. Curr Urol Rep 2013; 14: 147–153. [DOI] [PubMed] [Google Scholar]
- 8.Rakowski SK, Winterkorn EB, Paul E, et al. Renal manifestations of tuberous sclerosis complex: Incidence, prognosis, and predictive factors. Kidney Int 2006; 70: 1777–1782. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MD. Genitourinary tumors. In: Cohen MD. (ed). Imaging of children with cancer, St Louis, MO: Mosby Year Book, 1992, pp. 552–588. [Google Scholar]
- 10.Oesterling JE, Fishman EK, Goldman SM, et al. The management of renal angiomyolipoma. J Urol 1986; 135: 1121–1124. [DOI] [PubMed] [Google Scholar]
- 11.Chang Y-H, Wang L-J, Chuang C-K, et al. The efficacy and outcomes of urgent superselective transcatheter arterial embolization of patients with ruptured renal angiomyolipomas. J Trauma 2007; 62: 1487–1490. [DOI] [PubMed] [Google Scholar]
- 12.Patatas K, Robinson GJ, Ettles DF, et al. Patterns of renal angiomyolipoma regression post embolization on medium- to long-term follow-up. Br J Radiol 2013; 86: 20120633–20120633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramon J, Rimon U, Garniek A, et al. Renal angiomyolipoma: long-term results following selective arterial embolization. Eur Urol 2009; 55: 1155–1161. [DOI] [PubMed] [Google Scholar]
- 14.Hocquelet A, Cornelis F, Le Bras Y, et al. Long-term results of preventive embolization of renal angiomyolipomas: evaluation of predictive factors of volume decrease. Eur Radiol 2014; 24: 1785–1793. [DOI] [PubMed] [Google Scholar]
- 15.Stephenson AJ, Hakimi AA, Snyder ME, et al. Complications of radical and partial nephrectomy in a large contemporary cohort. J Urol 2004; 171: 130–134. [DOI] [PubMed] [Google Scholar]
- 16.Faddegon S, So A. Treatment of angiomyolipoma at a tertiary care centre: the decision between surgery and angioembolization. Can Urol Assoc J 2011; 5: e138–e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urciuoli P, D’Orazi V, Livadoti G, et al. Treatment of renal angiomyolipoma: surgery versus angioembolization. G Chir 2013; 34: 326–331. [PMC free article] [PubMed] [Google Scholar]
- 18.Kothary N, Soulen MC, Clark TW, et al. Renal angiomyolipoma: long-term results after arterial embolization. J Vasc Interv Radiol 2005; 16: 45–50. [DOI] [PubMed] [Google Scholar]
- 19.Tan S, Özcan MF, Tezcan F, et al. Real-time elastography for distinguishing angiomyolipoma from renal cell carcinoma: preliminary observations. Am J Roentgenol 2013; 200: W369–375. [DOI] [PubMed] [Google Scholar]
- 20.Nelson CP, Sanda MG. Contemporary diagnosis and management of renal angiomyolipoma. J Urol 2002; 168: 1315–1325. [DOI] [PubMed] [Google Scholar]
- 21.Lee SY, Hsu HH, Chen YC, et al. Evaluation of renal function of angiomyolipoma patients after selective transcatheter arterial embolization. Am J Med Sci 2009; 337: 103–108. [DOI] [PubMed] [Google Scholar]