Abstract
Background
Malignant ascites is a pathological condition caused by intra- or extra-abdominal disseminated cancer. The object of treatment is palliation. In search of an effective and minimally invasive palliative treatment of malignant ascites placement of a permanent intra peritoneal catheter has been suggested.
Purpose
To evaluate our experiences with treatment of malignant ascites by implantation of a permanent PleurX catheter.
Material and Methods
A retrospective study was conducted, comprising 20 consecutive patients with terminal cancer, who had a permanent PleurX catheter implanted because of malignant ascites in the period from February to November 2014. Using the patients’ medical records, we retrieved data on patients and procedures.
Results
The technical success rate was 100%. Catheter patency was 95.2%, one catheter was removed due to dislocation. Ten patients (50.0%) experienced minor adverse events. No procedural difficulties were reported and there was no need for additional treatment of malignant ascites after catheter implantation. Median residual survival after catheter implantation was 27 days.
Conclusion
Implantation of a permanent PleurX catheter is a minimally invasive and effective procedure with only minor adverse events and a high rate of catheter patency in patients with malignant ascites caused by terminal cancer disease.
Keywords: Abdomen/GI, interventional, drainage, peritoneum, adults, catheters
Introduction
Malignant ascites (MA) is a pathological condition caused by cancer. The pathophysiology is multifactorial and include reduction of lymphatic drainage from the peritoneal cavity and increased vascular permeability (1). Both intra- and extra-abdominal cancers can cause formation of MA, and in 80% of the cases breast, ovarian, endometrial, gastrointestinal, or pancreatic cancer is the primary source of malignancy (2).
MA has a major impact on the quality of life in patients with disseminated cancer, by contributing to both the symptom burden and hospitalizations (3). The object of treatment of MA is palliation. No guidelines exist for treatment of MA (1), but diuretics and paracentesis are the most used modalities (4). However, the efficacy of diuretics declines with tumor progression, whereas paracentesis only provides a temporary relief of symptoms, MA re-accumulates, and paracentesis has to be repeated (1,5,6).
Searching for an effective and minimally invasive palliative treatment of MA, placement of a permanent intra peritoneal catheter has been suggested (7–15).
The aim of this study is to evaluate our experiences when treating MA with implantation of a permanent PleurX catheter, with special emphasis on technical success, adverse events (AEs), and catheter patency.
Material and Methods
At our hospital we conducted a retrospective review of 20 terminal cancer patients, who had a permanent PleurX (CareFusion Catheter System, McGaw Park, IL, USA) catheter implanted because of MA in the period from February to November 2014. The study was approved by the Danish Data Protection Agency (record number 1-16-02-556-14).
Study population
Using the hospital’s patient administrative system, we identified 20 consecutive patients with terminal cancer who had a permanent PleurX catheter implanted because of MA. Data were acquired retrospectively. We did not identify the number of patients who declined such implantation.
Data on patients and procedures
From the medical records the following data were acquired: age, gender, primary cancer, cancer treatment, symptoms related to accumulation of ascites, number of paracenteses prior to permanent catheter implantation, preprocedural serum albumin, site of the catheter, technical success, volume drained during the first 24 h after implantation, AEs, length of hospital stay, re-admissions, catheter patency, and residual lifetime after catheter implantation.
The indication for implantation of a permanent PleurX catheter was recurrent MA due to terminal cancer disease. Contraindications for implantation were non-correctable coagulopathy and peritonitis. Technical success was defined as successful placement of the catheter with prompt drainage of ascites. The follow-up period after catheter implantation was defined from the date of procedure start and until end of data collection in December 2014 or until death of the patient. AEs were categorized into three groups: intra procedural AEs, early AEs occurring during post procedural days 1 to 7, and late AEs occurring more than 7 days after the procedure. Procedure specific AEs were graded according to the Common Terminology Criteria for Adverse Events version 4.0 (16).
Procedures
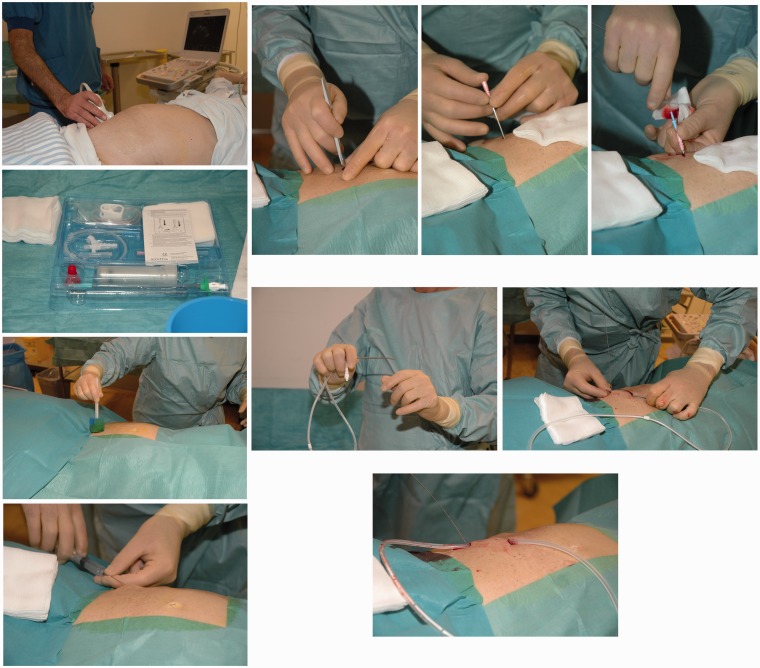
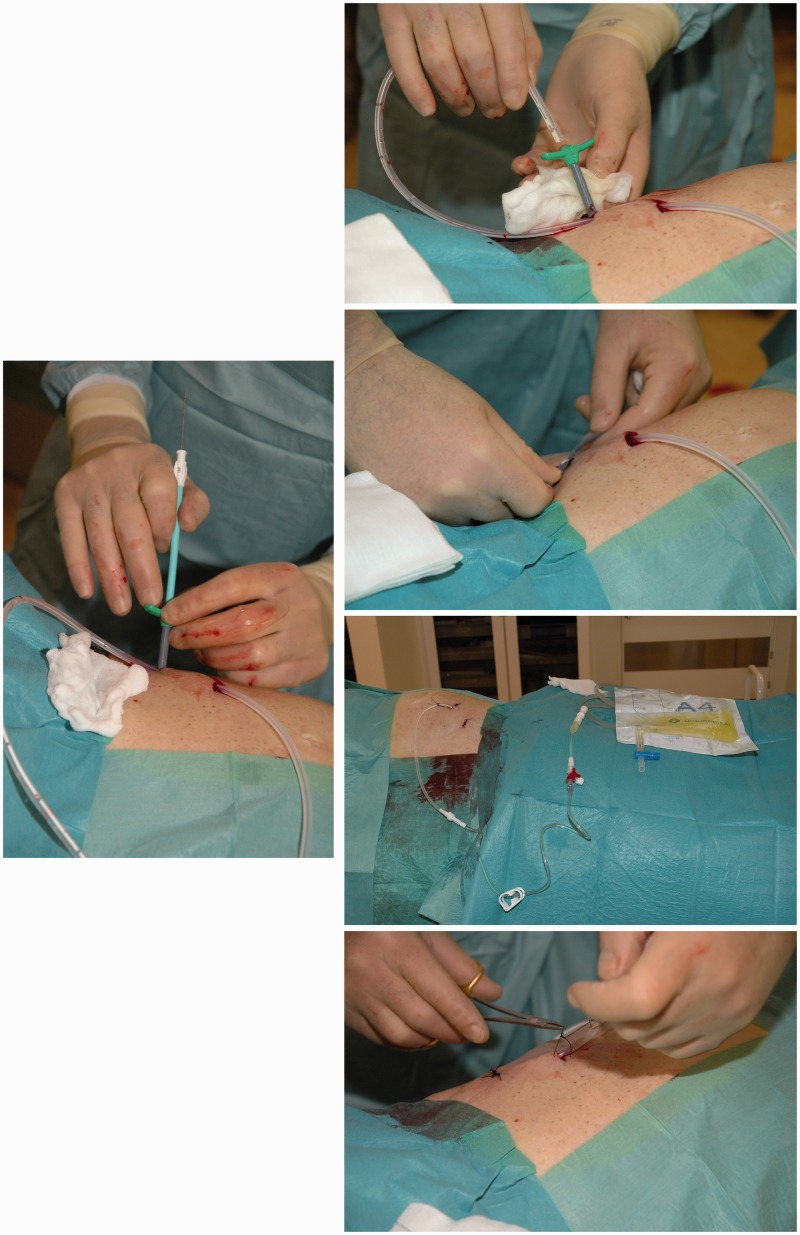
Prior to the procedure accumulation of MA was identified by computed tomography (CT) or ultrasonography (US). Contents from the PleurX catheter kit was used in the following: Lidocaine 1% was injected subcutaneously at the site of desired drain insertion and a skin incision was made. A second incision was made 5–8 cm superior and medial to the first. Under US guidance a J-Tip guide wire was inserted into the peritoneal cavity by an 18 Gauge (G) needle through the inferior incision. The needle was removed, leaving the guide wire in place. The 15.5 G fenestrated peritoneal catheter was tunneled subcutaneously from the superior to the inferior incision. A 16 G peel-away introducer was passed over the guide wire, and the guide wire was removed. The fenestrated end of the catheter was inserted into the peel-away introducer and further into the peritoneal cavity, and the peel-away introducer was subsequently removed. Both incisions were sutured, and the catheter was sutured to the skin, connected to the catheter bag, and opened to ensure flow of free fluid. No prophylactic antibiotics were given. The implantation of the PleurX catheter is illustrated step-by-step in Fig. 1.
Fig. 1.
A step-by-step illustration of the implantation of the PleurX catheter: 1. Identification of accumulated ascites and an appropriate insertion site by US. In this case the right lower part of the abdomen was chosen for catheter insertion. 2. The PleurX catheter kit (CareFusion Catheter System, McGaw Park, IL, USA) is opened. 3. Disinfection of the skin. The procedure is sterile. 4. Local anesthesia of the skin and peritoneum with Lidocain 1%. 5. Two skin incisions are made. The first incision is made for guide wire insertion. The second incision is made 5–8 cm superior and medial to the first incision. This incision will be the catheter exit site. 6. Through the inferior incision the needle for the guide wire is inserted. 7. The guide wire is inserted. 8. The fenestrated end of the catheter is attached to the tunneler. The tip of the tunneler is bended just a bit and kept in direction toward the skin to avoid contact with intra-abdominal cavity when tunneling. 9. The tunneler and catheter are passed subcutaneously from the second incision down to and out through the first incision. The catheter is drawn until the polyester cuff lies inside the tunnel 1 cm from the second incision. 10. The catheter is placed subcutaneously. 11. The peel-away introducer is positioned over the guide wire. 12. The fenestrated end of the catheter is inserted into the introducer and positioned in the peritoneal cavity. 13. The peel-away introducer is removed leaving only the catheter into the peritoneal cavity. 14. The catheter is connected to a catheter bag and opened to ensure free flow of fluid. 15. The skin incisions are sutured and the catheter is sutured to the skin. The stitches are removed 10–12 days later.
After the procedure and before discharge, nurses informed the patients how to handle the permanent catheter. Written information and a DVD with instructions were handed out. If needed, family members or caregivers in close relation to the patient were instructed as well. The patients were free to contact the hospital at any time.
Results
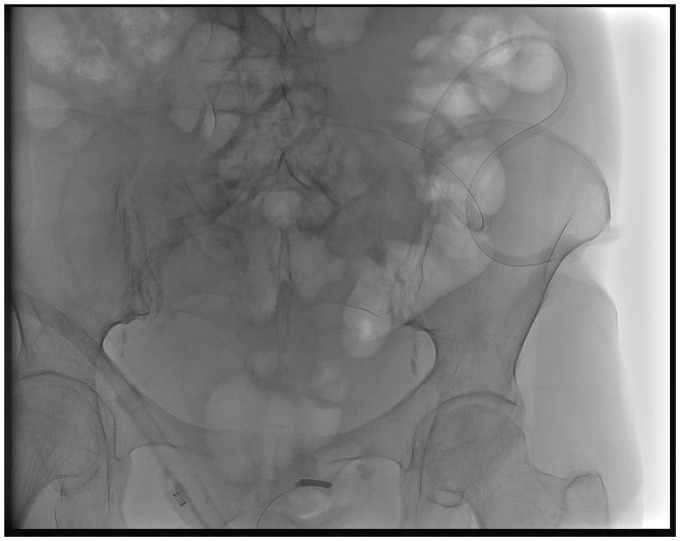
Twenty consecutive patients (14 women, 6 men) underwent permanent PleurX catheter implantation for MA (Fig. 2). Patient demographics are presented in Table 1.
Fig. 2.
Frontal X-ray showing the abdominal peritoneal PleurX catheter.
Table 1.
Patient demographics.
| Female:Male | ||
|---|---|---|
| 14:6 | ||
| Age (years) | ||
| Median | Range | |
| 62.5 | (35.0–91.0) | |
| Primary disease | ||
| n | % | |
| Ovarian cancer | 6 | 30.0 |
| Breast cancer | 4 | 20.0 |
| Pancreatic cancer | 3 | 15.0 |
| Bile duct cancer | 2 | 10.0 |
| Ventricular cancer | 1 | 5.0 |
| Liver cancer | 1 | 5.0 |
| Peritoneal mesothelioma + vesicae urinaria cancer | 1 | 5.0 |
Preprocedural symptoms of MA included abdominal discomfort (n = 12) (60.0%), dyspnea (n = 8) (40.0%), edema of lower extremities (n = 5) (25.0%), nausea (n = 5) (25.0%), constipation (n = 4) (20.0%), and vomiting (n = 2) (10.0%). Treatment of the primary disease and number of paracenteses are shown in Table 2.
Table 2.
Treatment of primary disease and number of paracenteses performed prior to implantation of permanent PleurX catheter.
| Treatment of primary disease | ||
|---|---|---|
| n | % | |
| Chemotherapy | 19 | 95.0 |
| Surgery | 7 | 35.0 |
| Radiation therapy | 6 | 30.0 |
| Number of paracenteses performed prior to permanent catheter implantation | ||
| n | % | |
| <5 | 9 | 45.0 |
| >5 | 11 | 55.0 |
| None | 0 | 0.0 |
In total, 21 PleurX catheters were placed with a technical success rate of 100%. All procedures were inpatient procedures with a median length of hospitalization of 1 day (range, 1–3 days). Median serum albumin level prior to the procedure was 24 g/L (range, 15–32 g/L). For 19 patients the median volume of MA drained during the first 24 h post procedure was 5000 mL (range, 1500–16,400 mL).
Out of the original 20 catheters implanted, 19 (95.0%) remained in situ and functioned until the patients’ death or until end of follow-up period. Ten patients (50.0%) experienced AEs. Nine patients experienced a single AE and two patients experienced two AEs. Data on AEs and patency are shown in Table 3. The catheter of one patient (5.0%) was removed 2 days after the procedure because of dislocation of the catheter tip to the area between the liver and diaphragm, causing severe abdominal pain and referred shoulder pain. The patient was re-admitted 8 days later to have a new catheter implanted, and apart from a transient soreness at the catheter access site this catheter functioned until the patient’s death.
Table 3.
Characterization of adverse events and catheter patency.
| Adverse events | |||
|---|---|---|---|
| Grade* | n | % | |
| Intra procedural | |||
| None | 21 | 100.0 | |
| Early | |||
| Soreness at the catheter access site | 1 | 5 | 23.8 |
| Catheter dislocation | 3 | 1 | 4.5 |
| Leakage at the catheter access site | 1 | 1 | 4.5 |
| Late | |||
| Soreness at the catheter access site | 1 | 1 | 4.5 |
| Hypotension | 1 | 1 | 4.5 |
| Leakage at catheter access site | 1 | 1 | 4.5 |
| Hypoalbuminemia | 3 | 1 | 4.5 |
| Catheter patency | |||
| n | % | ||
| Functional | 20 | 95.2 | |
| Non-functional | 1 | 4.8 | |
Graded according to the Common Terminology Criteria for Adverse Events version 4.0 (16): Grade 1, minor; Grade 2, moderate; Grade 3, severe; Grade 4, life-threatening; Grade 5, death.
The patients had in total 11 postprocedural admissions, out of which only one (9.1%) was related to the permanent catheter (dislocation with re-implantation of a new catheter). Hypoalbuminemia (9 g/L) was found incidentally in one patient (5.0%), who was admitted because of fever. The hypoalbuminemia was treated with albumin resuscitation. Also this patient experienced transient soreness at the catheter access site. Generally soreness at the catheter access site (n = 6) (30.0%) was transient, well treated with simple analgesics, and did not require admission to the hospital. Neither did two cases (10.0%) of transient leakage, which spontaneously stopped, nor one case (5.0%) of intermittent hypotension. No procedural difficulties due to drainage sessions were reported, and there was no need for additional treatment of MA after catheter implantation.
Three patients (15.0%) were alive at the end of the follow-up period, and their catheters were still functioning. In total the three catheters had been implanted for 205 days, with a median of 66 days (range, 18–101 days). Seventeen patients died during the follow-up period of the present study. After catheter implantation the median residual lifetime was 27 days (range, 9–164 days). All deaths were expected due to the terminal cancer, and no deaths were related to the permanent catheter.
Discussion
The present study showed that implantation of permanent PleurX in the treatment of MA, is a minimally invasive and effective procedure with only minor AEs and a high rate of catheter patency and symptom relief.
In total, 21 PleurX catheters were placed with a technical success rate of 100%. The same high technical success rate has been shown in other studies (11,12,14).
Twenty catheters (95.2%) were still functioning at the end of the follow-up period of the present study. One device (4.8%) had to be removed because of dislocation. Other studies report a patency of 85–86% (12,13).
We report no procedural difficulties due to the drainage sessions and no need for additional MA treatment after the implantation. In the prospective evaluation of permanent PleurX by Courtney et al. (13), procedural problems were reported in less than 1% of all drainage sessions, which is in accordance with our findings. Moreover, they showed a reduced degree of patient-described MA symptoms after implantation of the catheter. In the study of Saiz-Mendiguren et al. (10) patients reported no discomfort or inconvenience after implantation of the PleurX catheter.
Nine patients (45.0%) experienced minor AEs. The most common minor AE was soreness at catheter access site, which occurred in six patients (30.0%) and was treated with simple analgesics. In two patients (10.0%) severe AEs were noted; one (5.0%) because of catheter dislocation, the other (5.0%) because of hypoalbuminemia (9 g/L), incidentally found during hospitalization due to a non-catheter related admission. Prior to catheter implantation the serum albumin level of this patient was 27 g/L. Howard et al. (11) demonstrated reduced mean serum albumin after PleurX catheter placement. However, a prospective study by Courtney et al. (13) did not show significant changes in albumin due to permanent catheterization. In our study, systematic measurements of serum albumin after catheter implantations were not performed, and therefore we cannot conclude on post procedural protein loss. Courtney et al. (13) report an incidence of 41.4% minor AEs and 10.3% severe or life-threatening AEs which is consistent with our findings, though no life-threatening AEs were noted in our study. The fact that 45.0% of the patients in this study experienced minor AEs might appear as a high rate. However, it should be taken into consideration that one-third of these minor AEs were soreness at catheter access site, inevitably present after an invasive procedure as catheter implantation.
A feared AE following catheter implantation is peritonitis. In our study none of the patients suffered from peritonitis, and in the study by Courtney et al. (13) only one case (2.8%) of peritonitis was observed.
For the implantation of the PleurX procedure in this study an interventional suite was booked for 45 min, all-inclusive. A radiologist and two radiographs/nurses were required. Surgical preparation of the patient and drape is mandatory. All procedures were inpatient procedures with a median length of hospitalization of 1 day (range, 1–3 days), indicating that the procedure could be performed in an outpatient setting in the future. This is in agreement with a study by O’Neill et al. (7) and a study by Narayanan et al. (14). This approach is probably both cost-effective and might improve the patient’s quality of life, especially considering their short life expectancy due to their terminal cancer disease, by avoiding hospitalizations with overnight stays.
A major strength of this study is the use of the Danish National Patient Registry allowing complete follow-up of the 20 patients who were included in this study consecutively. No patients were excluded from the study population.
The findings of the present retrospective study should, of course, be interpreted with caution due to possible selection and information bias and the lack of prospectively evaluated measurements. We did not perform a cost-benefit analysis of the implantation of the PleurX catheter compared to repeated paracenteses. However, as Cambell states in his guidance relevant to surgical practice, a likely cost saving to the English national health system of £679 per patient can be expected (17). Additionally, we did not do a follow-up questionnaire of the patients’ quality of life as 17 out of 20 patients had died at the time of data collection. Instead the assessment of quality of life in this study was based on the quantitative data concerning catheter patency, number and severity of AEs, re-admissions due to catheter problems, and procedural difficulties due to drainage sessions.
Newer treatment modalities have been proposed, but these are still requiring further clinical evaluation. Among these are systemic or intra peritoneal chemotherapy, targeted therapy, immunotherapy, and radiotherapy (1,18).
Most patients with a terminal cancer disease have a very short life expectancy. Seventeen of the patients (85.0%) in the present study died during the follow-up period. The median residual lifetime after catheter implantation was 27 days (range, 9–164 days). Prior to implantation of the permanent catheter 11 patients (55.0%) had more than five paracenteses performed. With the present results in mind a change of clinical practice, with an even earlier implantation of a permanent catheter as treatment of MA in terminal cancer patients, could be relevant and seems feasible. This could lead to a relief in the burden of symptoms, fewer hospitalizations, and a higher quality of life.
In conclusion, implantation of a permanent PleurX catheter is a minimally invasive and effective procedure with only minor AEs and a high rate of catheter patency in patients with MA caused by terminal cancer disease.
Conflict of interest
None declared.
References
- 1.Cavazzoni E, Bugiantella W, Graziosi L, et al. Malignant ascites: Pathophysiology and treatment. Int J Clin Oncol 2013; 18: 1–9. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SM. Palliation of malignant ascites. Gastroenterol Clin North Am 2006; 35: 189–199. [DOI] [PubMed] [Google Scholar]
- 3.Fleming ND, Alvarez-Secord A, Von Gruenigen V, et al. Indwelling catheters for the management of refractory malignant ascites: A systematic literature overview and retrospective chart review. J Pain Symptom Manage 2009; 38: 341–349. [DOI] [PubMed] [Google Scholar]
- 4.Lee CW, Bociek G, Faught W. A survey of practice in management of malignant ascites. J Pain Symptom Manage 1998; 16: 96–101. [DOI] [PubMed] [Google Scholar]
- 5.Smith EM, Jayson GC. The current and future management of malignant ascites. Clin Oncol 2003; 15: 59–72. [DOI] [PubMed] [Google Scholar]
- 6.Stokes LS. Percoutaneous management of malignant fluid collections. Semin Intervent Radiol 2007; 24: 398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Neill MJ, Weissleder R, Gervais DA, et al. Tunneled peritoneal catheter placement under sonographic and fluoroscopic guidance in the palliative treatment of malignant ascites. Am J Roentgenol 2001; 177: 615–618. [DOI] [PubMed] [Google Scholar]
- 8.Barnett TD, Rubins J. Placement of a permanent tunneled peritoneal drainage catheter for palliation of malignant ascites: A simplified percutaneous approach. J Vasc Interv Radiol 2002; 13: 379–383. [DOI] [PubMed] [Google Scholar]
- 9.Mercadante S, Intravaia G, Ferrera P, et al. Peritoneal catheter for continuous drainage of ascites in advanced cancer patients. Support Care Cancer 2008; 16: 975–978. [DOI] [PubMed] [Google Scholar]
- 10.Saiz-Mendiguren R, Gómez-Ayechu M, Noguera JJ, et al. Permanent tunneled drainage for malignant ascites: Initial experience with the PleurX® catheter. Radiología (English Edition) 2010; 52: 541–545. [DOI] [PubMed] [Google Scholar]
- 11.Richard HM, Coldwell DM, Boyd-Kranis RL, et al. Pleurx tunneled catheter in the management of malignant ascites. J Vasc Interv Radiol 2001; 12: 373–375. [DOI] [PubMed] [Google Scholar]
- 12.Tapping CR, Ling L, Razack A. PleurX drain use in the management of malignant ascites: Safety, complications, long-term patency and factors predictive of success. Br J Radiol 2012; 85: 623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Courtney A, Nemcek AA, Rosenberg S, et al. Prospective evaluation of the PleurX catheter when used to treat recurrent ascites associated with malignancy. J Vasc Interv Radiol 2008; 19: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 14.Narayanan G, Pezeshkmehr A, Venkat S, et al. Safety and efficacy of the PleurX catheter for the treatment of malignant ascites. J Palliat Med 2014; 17: 906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg S, Courtney A, Nemcek AA, et al. Comparison of percutaneous management techniques for recurrent malignant ascites. J Vasc Interv Radiol 2004; 15: 1129–1131. [DOI] [PubMed] [Google Scholar]
- 16.Department of Health and Human Services, National Cancer Institute. Common terminology criteria for adverse events (CTCAE) version 4.0. 2009. Available at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 17.Cambell B. Recent medical technologies guidance relevant to surgeons. Ann R Coll Surg Engl 2012; 94: 525–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ammouri L, Prommer EE. Palliative treatment of malignant ascites: Profile of catumaxomab. Biologics 2010; 4: 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]