Abstract
Background
Radiofrequency ablation (RFA) is widely used for treatment of colorectal liver metastases (CRLM).
Purpose
To evaluate the effect of increased experience in RFA of CRLM on morbidity and survival, and the trends in patient management and outcomes during the last decade.
Material and Methods
Hospital records of the initial 52 consecutive patients who underwent RFA (56 procedures/70 lesions) were retrospectively reviewed. The patients were divided into two groups according to time period of treatment, period I (2001–2006: n = 26) and period II (2007–2011: n = 26).
Results
Concomitant liver resection was performed in 15 patients in each period. Operative morbidity decreased from 47% to 19% (P = 0.047). Most complications were found in patients who underwent a concomitant liver resection and not related to the ablation per se. Local recurrence rate decreased from 19.4% to 12.9% (P = 0.526). At least one risk factor for recurrence was found in patients with local recurrence (n = 11): subcapsular localization (n = 4), tumor size >3 cm and subcapsular localization (n = 2), and perivascular localization (portal veins/hepatic veins) (n = 5). Median overall survival was 32 months in period I and 49 months in period II, whereas estimated 5-year survival was 19% and 36%, respectively (P = 0.09). Adjuvant chemotherapy was given to four patients (15.4%) in period I and 13 patients (50%) in period II (P = 0.017).
Conclusion
RFA alone or in combination with liver resection is a potentially curative treatment to selected patients with CRLM. Over time, the morbidity and survival have improved in RFA of CRLM. Although a possible effect of a learning curve should be taken into consideration in the appraisal of this improvement, it is more likely to be attributable to optimization of indication, development in surgical techniques, and increased use of perioperative chemotherapy.
Keywords: Abdomen/GI, interventional, ablation procedures, liver, adults, metastases
Introduction
Colorectal cancer is the most common gastrointestinal malignancy, and approximately one-half of the patients will develop liver metastasis during the course of their disease (1–3). Only surgical resection of colorectal liver metastases (CRLM) can provide cure of disease, and is associated with 5-year survival rates in the range of 32–58% (3–6). During the last 10–15 years, implementation of new surgical techniques and principles including parenchyma-sparing techniques, repeated liver resections, and two-stage hepatectomy with portal vein embolization have increased the number of patients eligible for resection (4,7–9). In addition, more effective chemotherapy agents capable of converting inoperable cases to become resectable by tumor downsizing have been introduced (7). However, 70–80% of patients with CRLM are not candidates for resection because of associated co-morbidity, advanced age, non-resectable extrahepatic disease, or multiple liver lesion with inadequate residual functioning liver parenchyma (3,7).
Patients unsuitable for liver resection may be considered for ablative therapies, either alone or in combination with liver resection. There are several techniques for ablation: radiofrequency, laser, microwave, cryotherapy, high intensity focused ultrasound, and irreversible electroporation. Currently, the most widely used tumor ablative technique for treatment of CRLM is radiofrequency ablation (RFA) (10). RFA uses high-frequency alternating current to produce heat that destroys tumors by denaturing proteins and destroying cell membranes through dissolution and melting of lipid bilayers (11). RFA of liver tumors was first described in patients with liver cirrhosis and hepatocellular carcinoma in 1992, and in the treatment of CRLM in 1996 (12,13).
Several studies have evaluated RFA in the treatment of malignant liver tumors, mainly hepatocellular carcinoma and CRLM (6,14–16). Several studies report 5-year survival in the range of 20–30% in patients undergoing RFA of CRLM (4–6,15,17). RFA has been widely adopted during the last 10–15 years (10). A significant learning curve in RFA for liver tumors has been reported, and hospital volume has been considered as an important factor associated with treatment outcome (18,19). However, other factors than experience may also influence the outcome over time when applying ablative therapies in the treatment of CRLM. Our hospital has used RFA in the treatment of CRLM since 2001. The aim of this study is to evaluate the effect of experience in RFA of CRLM and the trends in patient management and outcomes during the last decade.
Material and Methods
A consecutive series of 52 patients with unresectable CRLM treated with RFA with or without concomitant liver resection between January 2001 and September 2011 were investigated in this retrospective, non-randomized study. The patients were divided into groups according to time period of treatment, period I (2001–2006: n = 26) and period II (2007–2011: n = 26). The study was approved by the Data Protection Officer for Research. Patient management was decided at multidisciplinary team meetings at Oslo University Hospital, Ullevaal, with experience in the treatment of CRLM. All patients were managed by the same team of hepatobiliary surgeons, interventional radiologists and oncologists. All patients underwent a baseline evaluation, including a medical history, physical examination and laboratory tests including carcinoembryonic antigen (CEA). Computed tomography (CT) of the chest, abdomen, and pelvis were performed in all patients. In selected patients magnetic resonance imaging (MRI) or intraoperative contrast enhanced ultrasound (CEUS) of the liver were performed. Patients with unresectable CRLM <3.5 cm without extrahepatic metastasis were considered for RFA. Patients were deemed unresectable on the basis of: advanced age, co-morbidity, prior liver resection with small liver remnant, inadequate functional hepatic reserve, or residual tumor in the liver remnant when RFA was performed in combination with liver resection. One patient with a solitary CRLM with diameter 4.5 cm also underwent RFA.
RFA was performed either percutaneously or during laparotomy under ultrasound guidance. In one patient a percutaneous CT guided RFA procedure was performed due to difficulties in identifying the lesion by ultrasound guidance. All RFA procedures were performed by a radiologist, and a hepatobiliary surgeon participated during all the procedures. The first percutaneous RFA was performed in October 2004. Laparoscopic liver resections have been performed routinely in our hospital from 2006. However, laparoscopic contrast-enhanced ultrasound equipment was not available. Accordingly, no laparoscopic RFA procedures were performed. Postoperative complications were classified according to the Clavien classification system (20). Risk factors associated with local recurrence as tumor size, multiple tumors, perivascular or subcapsular localization of the tumor or the percutaneous route were evaluated in all patients with local recurrence (21).
In period I, neoadjuvant chemotherapy was primarily given to non-resectable patients in our institution, with the aim of converting the disease to become resectable. In period II, the indications for perioperative chemotherapy evolved, and perioperative chemotherapy was more likely given to resectable patients with high tumor load (≥3 metastases or >3.0 cm in diameter) or patients with primary tumor and synchronous metastases (22,23). Later, CEA level and performance status became part of the consideration. Oxaliplatin-based combination chemotherapy was most commonly used, however irinotecan-based regimens and targeted antibodies were also used.
Two different commercially available radiofrequency generators were used. The first 34 patients were treated using the RF 2000 generator system (Boston Scientific Corporation, Natick, MA, USA). From April 2008, 18 patients were treated with RITA model 1500X (RITA Medical Systems, Mountain View, CA, USA). Specific RFA protocols designed by each of the two manufacturers were used for each system according to manufacturers’ recommendations. During treatment, the area of tissue ablation was monitored with ultrasound to measure the zone of increased echogenicity corresponding to coagulation of the tissue. The ablation was controlled directly after the procedure by CEUS. Any sign of residual tumor was ablated in the same procedure. CT of the liver was performed the first day after the procedure.
All patients were followed routinely after treatment. The first year, follow-up included CT of the chest and abdomen and CEA every 4 months. Thereafter, CT of the abdomen and chest and CEA measurement were obtained every 6 months. Local tumor recurrence at the RFA site and other intra- or extrahepatic recurrences were registered. Patients with recurrence that could not be treated by surgery or RFA were considered for palliative chemotherapy or best supportive care. All patients were followed until 31 October 2014 or death.
The clinical data and treatment outcomes of the patients were recorded retrospectively. Patient, tumor, and treatment characteristics were compared using the Mann-Whitney test for continuous variables and the chi-squared test for categorical variables. Median disease-free survival (DFS) and overall survival (OS) were estimated from the time of the first RFA procedure using the Kaplan-Meier method and compared using the log-rank test. Statistical analyses were performed in SPSS Version 18.0 for Windows (SPSS, Inc, Chicago, IL, USA). All tests were two-tailed, and statistical significance was defined as P < 0.05.
Results
Patient and treatment characteristics
A total of 52 consecutive patients were treated with RFA from January 2001 to September 2011, with RFA of a total of 70 liver metastases during 56 procedures. Table 1 summarizes the differences in patient, tumor, and treatment characteristics between each era. There were no significant differences in age, gender, or median tumor size. RFA was performed percutaneously (period I, n = 7; period II, n = 9) or by open surgical approach (period I, n = 23; period II, n = 17). Ten patients had more than one metastasis ablated; one patient had four ablations, one patient had three ablations and eight patients had two ablations. Two procedures were combined with resection of a renal cancer and an abdominal wall metastasis, respectively. Thirty procedures (53.6%) were performed in combination with liver resection (period I, n = 15; period II, n = 15). Re-ablation of local recurrence was performed in three patients in period I.
Table 1.
Patient, tumor, and treatment characteristics.
| Period I (26 patients, 30 procedures) | Period II (26 patients, 26 procedures) | P value | |
|---|---|---|---|
| Age (years, median (range)) | 69 (34–82) | 70 (40–85) | ns |
| Gender, n (%) | |||
| Male | 15 (57.7 %) | 12 (46.2 %) | ns |
| Female | 11 | 14 | |
| Site of primary tumor (n) | |||
| Colon | 15 | 16 | ns |
| Rectum | 11 | 10 | |
| Number of mucinous primary tumors | 0 | 3 | ns |
| Tumor grade of primary tumor | |||
| High | 4 | 9 | ns |
| Medium | 22 | 14 | |
| Low | 0 | 3 | |
| Synchronous metastases | 13 | 20 | ns |
| Metachronous metastases | 13 | 6 | |
| Indication for RFA | |||
| Patient: Advanced age/co-morbidity | 8 | 5 | ns |
| Tumor: Inadequate residual functioning liver parenchyma | 18 | 21 | |
| Number of ablated lesions | 39* | 31 | ns |
| Tumor size of ablated lesion (cm, median (range)) | 1.5 (1.0–3.2) | 1.4 (0.5–4.5) | ns |
| RFA approach | |||
| Percutaneous | 7 | 9 | ns |
| Open surgical | 23 | 17 | |
| Time from primary resection to RFA (months, median (range)) | 17 (2–80) | 9 (2–76) | ns |
| Prior liver resection | 6 | 12 | ns |
| Concomitant liver resection | 15 | 15 | ns |
| Bilobar disease | 16 | 14 | ns |
| Number of lesions (ablated and resected lesions), median | 2 | 2 | ns |
| Size of largest lesion (cm, ablated or resected lesions), median | 2.95 | 2.20 | ns |
| CEA (ng/mL, median (range)) | 9.9 (1.7–3414) | 7.7 (0.5–145) | ns |
| ASA III | 6 | 9 | ns |
| Median follow-up (months (range)) | 34 (1–137) | 33 (2–78) | ns |
| Neoadjuvant chemotherapy | 6 | 13 | ns |
| Number of lines of chemotherapy before RFA, median (range) | 13 (4–22) | 4 (4–12) | |
| Adjuvant chemotherapy | 4 | 13 | P = 0.017 |
Including three reablations.
Complications
Operative morbidity decreased from 46.7% in period I to 19% in period II (P = 0.047) (Table 2). Clavien ≥ grade 3 complications occurred in 33% of patients in period I and 11.5% in period II (P = 0.065). No complications were experienced after percutaneous RFA. Most complications were as a result of the open liver resection and not of the RFA procedure. Three patients who underwent resection in combination with RFA developed hepatic abscess arising within a RFA lesion, which resolved with percutaneous ultrasound-guided drainage and intravenous antibiotics. In 2001, one patient died in hospital of acute liver failure 21 days after RFA of a lesion in segment 2/3 performed in combination with a right hemihepatectomy.
Table 2.
Morbidity, site, and treatment of first recurrence.
| Period I (26 patients, 30 procedures) | Period II (26 patients, 26 procedures) | P value | |
|---|---|---|---|
| Morbidity | |||
| Overall | 14/30 | 5/26 | P = 0.047 |
| Clavien ≥ grade 3 | 10/30 | 3/26 | ns |
| 30-day mortality | 1 | 0 | |
| 90-day mortality | 2 | 1 | |
| Complications* | |||
| Symptomatic pleural effusion | 4 | 1 | |
| Pneumonia | 3 | 1 | |
| Bleeding | – | 1 | |
| Pulmonary embolism | 2 | – | |
| Liver abscess | 1 | 2 | |
| Pneumothorax | 1 | – | |
| Bile leak | 2 | 1 | |
| Small bowel perforation | 1 | – | |
| Wound infection | 1 | – | |
| Acute liver failure | 1 | – | |
| Urinary tract infection | 1 | – | |
| Median time from RFA to first recurrence | 10 | 5 | ns |
| Total number of lesions with local recurrence | 7/36 | 4/31 | ns |
| Site of first recurrence | |||
| Local only | 3 | 0 | ns |
| Local + Intra or Extrahepatic | 0 | 3 | |
| Intrahepatic only | 10 | 4 | |
| Intra + Extrahepatic | 7 | 5 | |
| Extrahepatic only | 3 | 4 | |
| Treatment of first recurrence | |||
| Surgery | 5 | 2 | ns |
| Reablation | 2 | 0 | |
| Palliative chemotherapy | 9 | 12 | |
| Best supportive care | 7 | 2 | |
n > 19, >1 complication registered in some patients.
Recurrence and survival
At a median follow-up of 34 (period I) and 41 months (period II), a total of 41 patients (78.8 %) (period I, n = 23; period II, n = 16) had developed recurrence (Table 2). Local recurrence at the RFA site occurred in seven of 36 lesions in period I (19.4%) and four of 31 lesions in period II (12.9%) (P = 0.526). At least one risk factor for recurrence was found in all 11 patients with recurrence: subcapsular localization of the tumors (n = 4), tumor size >3 cm and subcapsular localization of the tumor (n = 2), and perivascular localization (portal veins/hepatic veins) of the tumor (n = 5) (21).
Treatment of first recurrence is presented in Table 2. In seven patients with recurrence surgery was performed; in six patients wedge resection of a liver metastasis, and in one patient pulmonary resection of a lung metastasis. In three of six patients reresected for liver metastases a subsequent resection of a lymph node metastasis in the hepatoduodenal ligament, a solitary lung metastasis, and psoas muscle recurrence were performed. In addition one patient reresected for a liver metastasis ultimately underwent a liver transplantation for hepatic recurrence. In period II, two patients did not complete the planned surgical strategy, i.e. one patient did not proceed to the planned second stage hepatic resection due to development of non-resectable extrahepatic metastases, and one patient did not proceed to rectal surgery due to progressive disease three months following a combined hepatic resection and RFA. These two patients were not included in survival analysis.
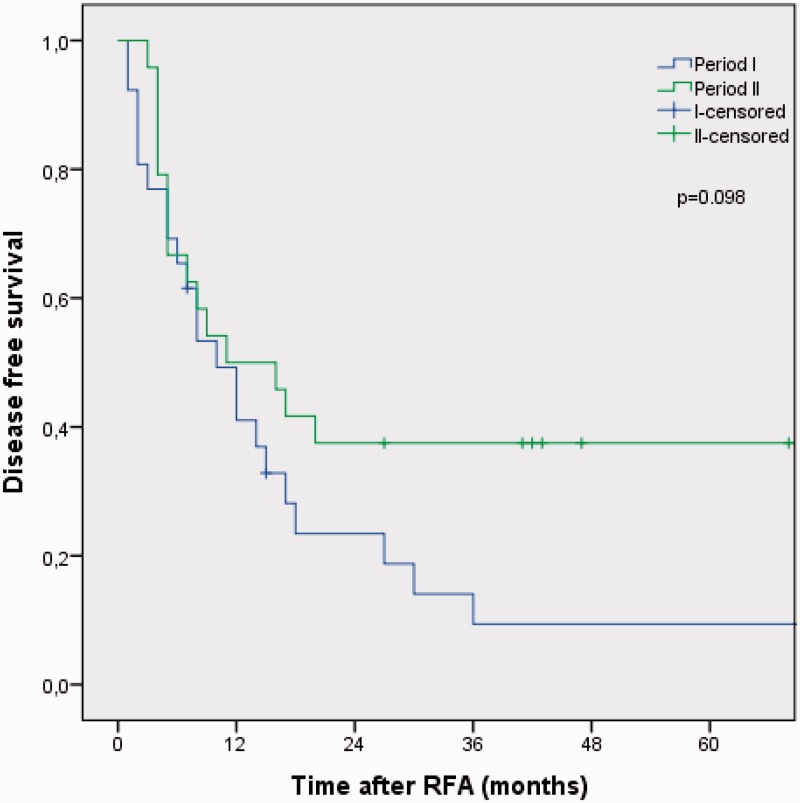
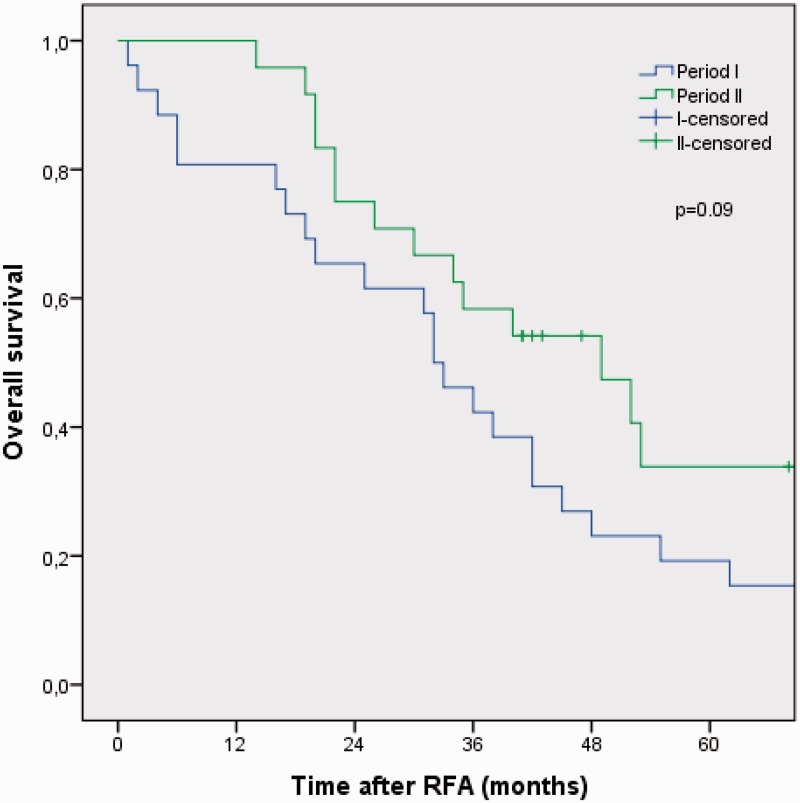
Median disease-free survival (DFS), overall survival (OS), and 5-year OS rates for the whole period were 11 months (95% CI, 4.3–17.8), 36 months (95% CI, 27.3–44.7), and 27%, respectively. Median DFS was 10 months (95 % CI, 5.2–14.7) in period I and 11 months (95% CI, 0.2–21.8) in period II (P = 0.098) (Fig. 1). Median OS was 32.0 months (95% CI, 25.8–38.2) in period I and 49.0 months (95% CI, 28.4–69.6) in period II, whereas estimated 5-year OS was 19% in period I and 36% in period II (P = 0.09) (Fig. 2).
Fig. 1.
Kaplan-Meier analysis of disease-free survival in 50 consecutive patients treated with RFA of unresectable CRLM stratified into period I (2001–2006) and period II (2007–2011).
Fig. 2.
Kaplan-Meier analysis of overall survival in 50 consecutive patients treated with RFA of unresectable CRLM stratified into period I (2001–2006) and period II (2007–2011).
Discussion
The current study presents a single-institution experience with RFA in a consecutive series of 52 patients with CRLM not amenable for resection. During the study period of 11 years new surgical techniques and principles were implemented and more effective chemotherapeutic and targeted agents were introduced. Importantly, our study demonstrates that RFA is a potentially curative treatment to selected patients with CRLM. Specifically, we found an estimated 5-year survival of 27% and median overall survival of 36 months, with a tendency toward improved survival in period II (P = 0.09). Several studies have shown 5-year survival in the range of 20–30% for patients treated with RFA for CRLM (4,5,15,17). The use of modern chemotherapy regimens for palliative treatment now results in median survival of up to 20–22 months in patients with unresectable CRLM (7). However, chemotherapy is rarely associated with durable resolution of disease or long-term survival beyond 5 years. Ruers showed that RFA plus systemic treatment resulted in significant longer progression-free survival than systemic treatment alone, demonstrating the survival benefit of RFA (24).
Several studies demonstrate that there is a significant learning curve in RFA for liver tumors (18,19,21). In our study, complications were experienced in 35% of the procedures. However, operative morbidity decreased from 47% in period I to 19% in period II. Most complications were found in patients who underwent RFA in combination with liver resection, whereas no complications were registered in patients who underwent percutaneous RFA. This is in accordance with the findings in a comprehensive review of 3670 patients from 82 different studies showing a complication rate of 7.2%, 9.5%, 9.9%, and 31.8% after a percutaneous, laparoscopic, simple open, and combined open approach, respectively (25).
In a meta-analysis of 5224 liver tumors treated with RFA, local recurrence rate was found to vary widely between 2% and 60% (21). Because local recurrence rates are higher than after surgical resection, surgical resection is considered the gold standard treatment for patients with CRLM who are candidates for resection (26). In two well-designed randomized studies the local recurrence rate per lesion in patients treated with RFA or surgical resection did not appear to be greatly different for lesions up to 3 cm (24,27). However, local recurrences of lesions treated by RFA were more frequent when lesion size exceeded 3 cm. In the current study, local recurrence at a prior RFA site occurred in 20.5% of lesions in period I and 12.9% in period II. A low complication rate and a high complete ablation rate has shown to be achieved with the accumulated experience from the first 50 cases of RFA for liver tumors by a specialized team (18,19). The current study present our experience with the first 52 cases. Thus, we expect continued improvement in complete ablation rates in the technical aspects of RFA. Importantly, in our analysis, subcapsular or perivascular localization of the tumor, or tumor size >3 cm, were risk factors present in all the 11 lesions that recurred at the same site. It is likely that this experience has had an impact on patient selection in period II. Tumor size >3 cm, multiple tumors, perivascular or subcapsular localization of the tumor, and the percutaneous route are independent predictors of local recurrence after RFA (21). It must be noted that in both periods ablation of tumors with subcapsular or perivascular location, which rarely seem to be amenable to effective ablation, were performed in patients with advanced age or associated co-morbidities.
Perioperative chemotherapy and multimodal approaches such as portal vein embolization, two-stage hepatectomy, vascular reconstructions, and autotransplantation have increased the proportion of patients with resectable CRLM (4,7–9). These new treatment strategies were introduced in our center during the study period (23). Although the yearly number of patients with CRLM undergoing resection increased significantly from 2001 to 2011, the yearly number of patients undergoing RFA in our center has not increased throughout the period (23). This is in contrast to other series showing that the use of ablation either alone or combination with resection has increased significantly (28). RFA was used as an adjunct to resection in 30 patients, and 58% of our total patients had high tumor load with bilobar disease that precluded a complete surgical resection. A resection of the dominant tumors in the liver was performed with RFA of the remaining, smaller lesion(s). Two-stage hepatectomy was introduced in our hospital in 2008, and performed in 25 of 239 (10 %) resected patients (23). High volume centers performing RFA have shown that one-third of patients who earlier were treated by RFA in combination with liver resection are now considered for two-stage hepatectomy (6,9). The introduction of this technique in period II in our center has likely influenced the indication for RFA, meaning that fewer patients with bilobar disease underwent RFA. Five patients in period II received RFA in combination with liver resection due to additional metastases detected by intraoperative CEUS, routinely performed since 2007 in our institution (29). This strategy permits discovery of and treatment with a curative intent of preoperatively undected intrahepatic metastases. This has shown to alter the surgical management in 10–30% of patients with classically resectable CRLM (29). RFA is an important treatment modality in this setting and can easily be performed in combination with liver resection by a team experienced in intraoperative CEUS and RFA.
Clinically important advances in chemotherapy for treating patients with CRLM have been made over the last decades. Population based studies have shown an increase in chemotherapy utilization over time (28). We found a significant increase in use of perioperative chemotherapy over the time periods examined. The increased utilization of chemotherapy in period II may partly explain the improved OS in this last period (24). However, older patients with more co-morbidities are often less likely to receive chemotherapy, and this group constituted 25% of the patients in our study (30).
The main limitation of this study must be acknowledged. This was a retrospective analysis of patients treated at a single institution with all the inherent biases associated with single-institution studies. Analysis of data to assess the treatment effect of a given therapeutic modality that is based on patients who have been non-randomly assigned a specific treatment is fraught with difficulty (5). A recent Cochrane review emphasize that studies on RFA of CRLM are vulnerable to different type of bias, and the main concern are the imbalance between characteristics of patients in the allocated groups (14). Thus, even though our study shows that RFA is a potentially curative treatment to selected patients with CRLM, the ultimate effect of RFA on overall survival remains uncertain (14,24).
In conclusion, RFA alone or in combination with liver resection is a potentially curative treatment to selected patients with CRLM who are not amenable for surgical resection. RFA appears to confer a survival benefit over systemic chemotherapy alone. RFA requires a strict patient selection in a multidisciplinary team and must be performed only by clinicians with adequate knowledge and experience in interventional therapies for liver tumors. Over time, the morbidity and survival have improved in RFA of CRLM. Although a possible effect of a learning curve should be taken into consideration in the appraisal of this improvement, it is more likely to be attributable to optimization of indication, development in surgical techniques, and increased use of perioperative chemotherapy.
Conflict of interest
None declared.
Funding
The sources of support behind this study came from Oslo University Hospital. There were no other sources of support and no other funding.
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics 2014. CA Cancer J Clin 2014; 64: 9–29. [DOI] [PubMed] [Google Scholar]
- 2.Malvezzi M, Arfe A, Bertuccio P, et al. European cancer mortality predictions for the year 2011. Ann Oncol 2011; 22: 947–956. [DOI] [PubMed] [Google Scholar]
- 3.Manfredi S, Lepage C, Hatem C, et al. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006; 244: 254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 2004; 240: 644–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gleisner AL, Choti MA, Assumpcao L, et al. Colorectal liver metastases: recurrence and survival following hepatic resection, radiofrequency ablation, and combined resection-radiofrequency ablation. Arch Surg 2008; 143: 1204–1212. [DOI] [PubMed] [Google Scholar]
- 6.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004; 239: 818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmoll HJ, Van Cutsem E, Stein A, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol 2012; 23: 2479–2516. [DOI] [PubMed] [Google Scholar]
- 8.Hagness M, Foss A, Line PD, et al. Liver transplantation for nonresectable liver metastases from colorectal cancer. Ann Surg 2013; 257: 800–806. [DOI] [PubMed] [Google Scholar]
- 9.Chun YS, Vauthey JN, Ribero D, et al. Systemic chemotherapy and two-stage hepatectomy for extensive bilateral colorectal liver metastases: perioperative safety and survival. J Gastrointest Surg 2007; 11: 1498–1504. [DOI] [PubMed] [Google Scholar]
- 10.Mayo SC, Pawlik TM. Thermal ablative therapies for secondary hepatic malignancies. Cancer J 2010; 16: 111–117. [DOI] [PubMed] [Google Scholar]
- 11.Gillams AR. Liver ablation therapy. Br J Radiol 2004; 77: 713–723. [DOI] [PubMed] [Google Scholar]
- 12.Buscarini L, F. F, Rossi S. Interstitial radiofrequency hyperthermia in the treatment of small hepatocellular carcinoma: percutaneous sonography-guidance of electrode needle. In: Anderegg A, Despland PA, Otto R, Henner H. (eds). Ultraschall-Diagnostik 91, Berlin: Springer, 1992. :218–222. [Google Scholar]
- 13.Rossi S, Di SM, Buscarini E, et al. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. Am J Roentgenol 1996; 167: 759–768. [DOI] [PubMed] [Google Scholar]
- 14.Cirocchi R, Trastulli S, Boselli C, et al. Radiofrequency ablation in the treatment of liver metastases from colorectal cancer. Cochrane Database Syst Rev 2012; 6: CD006317–CD006317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorensen SM, Mortensen FV, Nielsen DT. Radiofrequency ablation of colorectal liver metastases: long-term survival. Acta Radiol 2007; 48: 253–258. [DOI] [PubMed] [Google Scholar]
- 16.Tateishi R, Shiina S, Teratani T, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer 2005; 103: 1201–1209. [DOI] [PubMed] [Google Scholar]
- 17.Siperstein AE, Berber E, Ballem N, et al. Survival after radiofrequency ablation of colorectal liver metastases: 10-year experience. Ann Surg 2007; 246: 559–565. [DOI] [PubMed] [Google Scholar]
- 18.Poon RT, Ng KK, Lam CM, et al. Learning curve for radiofrequency ablation of liver tumors: prospective analysis of initial 100 patients in a tertiary institution. Ann Surg 2004; 239: 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hildebrand P, Leibecke T, Kleemann M, et al. Influence of operator experience in radiofrequency ablation of malignant liver tumours on treatment outcome. Eur J Surg Oncol 2006; 32: 430–434. [DOI] [PubMed] [Google Scholar]
- 20.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulier S, Ni Y, Jamart J, et al. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg 2005; 242: 158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008; 371: 1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brudvik KW, Bains SJ, Seeberg LT, et al. Aggressive treatment of patients with metastatic colorectal cancer increases survival: a Scandinavian single-center experience. HPB Surg 2013; 2013: 727095–727095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruers T, Punt C, van CF, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004). Ann Oncol 2012; 23: 2619–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulier S, Mulier P, Ni Y, et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg 2002; 89: 1206–1222. [DOI] [PubMed] [Google Scholar]
- 26.White RR, Avital I, Sofocleous CT, et al. Rates and patterns of recurrence for percutaneous radiofrequency ablation and open wedge resection for solitary colorectal liver metastasis. J Gastrointest Surg 2007; 11: 256–263. [DOI] [PubMed] [Google Scholar]
- 27.Tanis E, Nordlinger B, Mauer M, et al. Local recurrence rates after radiofrequency ablation or resection of colorectal liver metastases. Analysis of the European Organisation for Research and Treatment of Cancer #40004 and #40983. Eur J Cancer 2014; 50: 912–919. [DOI] [PubMed] [Google Scholar]
- 28.Mayo SC, Heckman JE, Shore AD, et al. Shifting trends in liver-directed management of patients with colorectal liver metastasis: a population-based analysis. Surgery 2011; 150: 204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schulz A, Dormagen JB, Drolsum A, et al. Impact of contrast-enhanced intraoperative ultrasound on operation strategy in case of colorectal liver metastasis. Acta Radiol 2012; 53: 1081–1087. [DOI] [PubMed] [Google Scholar]
- 30.Wildes TM, Kallogjeri D, Powers B, et al. The benefit of adjuvant chemotherapy in elderly patients with stage III colorectal cancer is independent of age and comorbidity. J Geriatr Oncol 2010; 1: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]