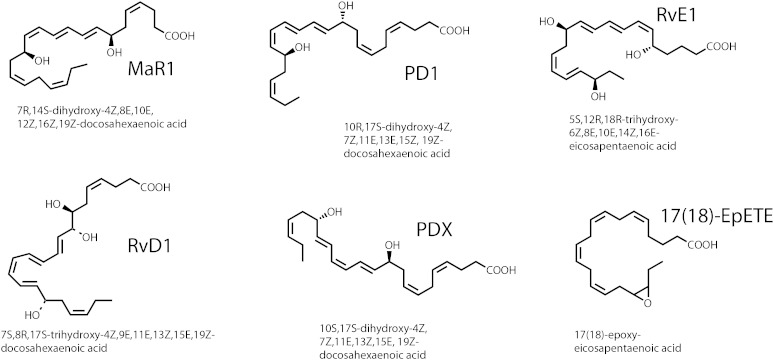
This issue of the Journal of Lipid Research reports a patient-oriented study by workers at the University of Pennsylvania (Penn group) (1) using n-3 PUFA ester supplementation in attempts to detect the appearance of oxidized DHA and EPA in plasma and urine. Fish oil and purified n-3 PUFA supplementations have certainly emerged on the conscious level of the Western consumer by intense advertising, both for prescription supplements (e.g., Lovaza fish oil) and over-the-counter fish oil products and related biological extracts. In large part, interest in these products stems from the promise of potential health benefits when the ratio of n-6 esters (linoleate and arachidonate) to n-3 esters (EPA and DHA) is lowered, which results in the reduction in the biosynthesis of pro-inflammatory, n-6 derived, prostaglandins and leukotrienes. Adding to the favorable reduction of pro-inflammatory lipid mediators has been the discovery of EPA and DHA metabolites with properties of enhancing the resolution of inflammation in an active fashion (2). These novel lipids have been termed “specialized pro-resolving mediators” (SPMs) and a large number of such biologically active products have now been chemically described, including protectin D1 (2), protectin DX (3), maresin 1 (2), resolvin D1 (2), and resolvin E1 (2) (Fig. 1). Studies of the biosynthesis, pharmacologic activity, and measurement of these products in cellular models has driven interest in this area, leading to the question being asked: Can the biosynthesis of one or more of these SPMs be enhanced in vivo as a result of n-3 PUFA dietary supplementation?
Fig. 1.
Chemical structures and stereochemistry of pro-resolution metabolites of EPA and DHA. These are some of the major SPMs that have been described.
Effects of n-3 fatty acid supplements on cardiovascular disease were first popularized by studies in Greenland Eskimos several decades ago, which launched hundreds of clinical studies of the effect of an enriched fish oil diet on various diseases, including cardiovascular disease, neurological diseases, dermatological disorders, and cancer, to name just a few (4). Today, this area remains somewhat cloudy, controversial, but economically healthy (http://www.washingtonpost.com/business/economy/claims-that-fish-oil-boosts-health-linger-despite-science-saying-the-opposite/2015/07/08/db7567d2-1848-11e5-bd7f-4611a60dd8e5_story.html).
It has been surprisingly difficult to demonstrate a clear and significant benefit of increasing n-3 fatty acids in the diet, including reduction of disease symptoms related to inflammation and postulated to involve n-6 inflammatory mediators. The Journal has been an active participant in reporting results from several human subject studies addressing this topic. Some of these reports are at odds with each other, including the point of whether or not SPMs can be detected in the plasma of subjects with enhanced dietary n-3 fatty acid esters intake. There have been reports in the Journal of the detection and quantitation of certain SPMs in blood as well as reports that SPMs cannot be detected in plasma. In a very important part of their study, the Penn group carried out the clinical maneuver of an inflammatory challenge and using doses of fish oil sufficiently high to influence blood pressure and platelet function (and much higher than usually consumed as a health supplement) but failed to find SPMs in plasma. Interestingly, measurements by both sides of this controversy have been made using sophisticated tandem mass spectrometry as the analytical platform to increase specificity and sensitivity for detection of SPMs (LC-MS/MS targeted strategies).
The analytical results and conclusions that have been reached by these studies are at odds with each other as illustrated by recent reports from the University of Western Australia (5, 6), where levels of these SPMs were detected close to the analytical detection limit and from the Penn group (1) that could not find specific SPMs. In general, these papers used very similar approaches of targeted LC-MS/MS, but there are sufficient differences between the two approaches to make it difficult to assess which one is valid. Perhaps both are correct? There are inherent challenges illustrated by these studies when measuring lipids, even with state-of-the-art mass spectrometric equipment at these very trace levels in a complex biological matrix such as plasma. A distinct advantage of the Penn group’s approach was the availability of deuterated standards for all SPMs to precisely indicate LC elution of each target molecule as well as the ability to calculate precise extraction efficiency for each of the target SPMs in plasma. The University of Western Australia only had one internal standard in their LC-MS/MS targeted assays and this internal standard was not an SPM. In any event, the controversy remains as to whether these SPMs are detectable in plasma and as a corollary, whether they do or do not change as a result of n-3 fatty acid dietary supplementation. Development of higher sensitivity as well as ancillary specificity in mass spectrometry should add considerably to clarify this important issue.
Another recent patient-oriented study of EPA and DHA supplementation published in the Journal (7), also failed to observe resolvins E or D in plasma. However, this group from Berlin did identify a family of metabolites in plasma derived from the cytochrome P450 pathway of metabolism of EPA and DHA. Arachidonate metabolites derived from the CYP-epoxygenase pathway are known to be bioactive, but little is known about the EPA- and DHA-derived epoxides or their diol products that result from epoxide hydrolysis. Interestingly, the majority of these EPA and DHA epoxides (90%) were found as esterified products in plasma that were released upon base hydrolysis (7). The Penn group also found significant elevation of the most abundant of these CYP-epoxygenase products identified by the Berlin group as 17(18)-EpETE (Fig. 1) and 19(20)-EpDoPE. The Berlin group used abbreviation 17,18-EEQ and 19,20-EDP for these same compounds. These products might turn out to be the best indicators of n-3 fatty acid supplementation in plasma and interestingly, anti-inflammatory activity has been described for these epoxides (8).
One positive lead presented in this report from the Penn group was that urinary metabolites of these SPMs might be detected and therefore serve as relevant markers to indicate in vivo biosynthesis. This strategy of using urinary metabolites was established many years ago in eicosanoid biochemistry and is an accepted method by which to address both leukotriene and prostaglandin synthesis in animals and human subjects. In fact, it was the measurement of a prostacyclin metabolite excreted into the urine that was used to assess the potential cardiovascular risk inherent in the COX-2 inhibitors (9). This discovery about COX-2 inhibitors was made by this Penn group. We will look forward to improvements in the analytical methodology and approach in order to address these important issues of the role of DHA and EPA in regulating the resolution of inflammation in various diseases, including cardiovascular diseases, by the formation of SPMs.
REFERENCES
- 1.Skarke C., Alamuddin N., Lawson J. A., Ferguson J. F., Reilly M. P., FitzGerald G. A. 2015. Bioactive products formed in humans from fish oils. J. Lipid Res. 56: 1808–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy B. D., Serhan C. N. 2014. Resolution of acute inflammation in the lung. Annu. Rev. Physiol. 76: 467–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu M., Chen P., Vericel E., Lelli M., Beguin L., Lagarde M., Guichardant M. 2013. Characterization and biological effects of di-hydroxylated compounds deriving from the lipoxygenation of ALA. J. Lipid Res. 54: 2083–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooper L., Thompson R. L., Harrison R. A., Summerbell C. D., Ness A. R., Moore H. J., Worthington H. V., Durrington P. N., Higgins J. P., Capps N. E., et al. 2006. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ. 332: 752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barden A., Mas E., Croft K. D., Phillips M., Mori T. A. 2014. Short-term n-3 fatty acid supplementation but not aspirin increases plasma proresolving mediators of inflammation. J. Lipid Res. 55: 2401–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mas E., Croft K. D., Zahra P., Barden A., Mori T. A. 2012. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin. Chem. 58: 1476–1484. [DOI] [PubMed] [Google Scholar]
- 7.Fischer R., Konkel A., Mehling H., Blossey K., Gapelyuk A., Wessel N., von Schacky C., Dechend R., Muller D. N., Rothe M., Luft F. C., Weylandt K., Schunck W. H. 2014. Dietary omega-3 fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway. J. Lipid Res. 55: 1150–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morin C., Sirois M., Echave V., Albadine R., Rousseau E. 2010. 17,18-epoxyeicosatetraenoic acid targets PPARgamma and p38 mitogen-activated protein kinase to mediate its anti-inflammatory effects in the lung: role of soluble epoxide hydrolase. Am. J. Respir. Cell Mol. Biol. 43: 564–575. [DOI] [PubMed] [Google Scholar]
- 9.McAdam B. F., Catella-Lawson F., Mardini I. A., Kapoor S., Lawson J. A., FitzGerald G. A. 1999. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc. Natl. Acad. Sci. USA. 96: 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]