Abstract
We previously reported that reducing the expression of cholesteryl ester transfer protein (CETP) disrupts cholesterol homeostasis in SW872 cells and causes an ∼50% reduction in TG. The causes of this reduced TG content, investigated here, could not be attributed to changes in the differentiation status of CETP-deficient cells, nor was there evidence of endoplasmic reticulum (ER) stress. In short-term studies, the total flux of oleate through the TG biosynthetic pathway was not altered in CETP-deficient cells, although mRNA levels of some pathway enzymes were different. However, the conversion of diglyceride (DG) to TG was impaired. In longer-term studies, newly synthesized TG was not effectively transported to lipid droplets, yet this lipid did not accumulate in the ER, apparently due to elevated lipase activity in this organelle. DG, shown to be a novel CETP substrate, was also inefficiently transferred to lipid droplets. This may reduce TG synthesis on droplets by resident diacylglycerol acyltransferase. Overall, these data suggest that the decreased TG content of CETP-deficient cells arises from the reduced conversion of DG to TG in the ER and/or on the lipid droplet surface, and enhanced TG degradation in the ER due to its ineffective transport from this organelle.
Keywords: cholesteryl ester transfer protein, lipid droplets, endoplasmic reticulum, diglyceride
Cholesteryl ester transfer protein (CETP) is a key mediator of plasma lipoprotein metabolism in humans (1–3). Through its capacity to facilitate net movement of cholesteryl ester (CE) and TG molecules between lipoprotein classes, CETP activity alters lipoprotein composition, and directly influences the catabolism of lipoproteins. CETP is highly expressed in several tissues including adipose, liver, and intestine (4, 5). Collectively, these tissues play crucial roles in lipid uptake, lipoprotein synthesis, and lipid storage. Plasma CETP concentrations are highly correlated with adipose tissue CETP mRNA levels in both man and hamster (6, 7), showing the importance of this tissue in both lipid storage and the modulation of intravascular lipid metabolism.
While most CETP is secreted by cells, a portion is retained (8–10). Much of the intracellular CETP pool is associated with the endoplasmic reticulum (ER), with increased concentrations near lipid droplets (8, 11). Both cytoplasmic and lipid-droplet pools of CETP have been observed as well (11). Although the mechanism for CETP release into the cytoplasm is unknown, the escape of other ER luminal proteins to the cytoplasm has been reported (12–14).
In addition to its role in plasma lipoprotein metabolism, multiple studies suggest that CETP has other functions. Cell-associated CETP promotes CE uptake from HDL, stimulates the cell’s ability to efflux cholesterol, and influences the storage of CE in cells (15–19). In addition to altering cellular cholesterol metabolism, both over-expression and under-expression of CETP in SW872 cells, a human cell line commonly used as an adipocyte model, reduce the capacity of these cells to store TG (10, 11). A role for CETP in adipocyte lipid storage is further supported by observations in transgenic mice. Adipose tissue-specific expression of human CETP in mice results in smaller adipocytes containing less TG and cholesterol, and significantly reduces the expression of key lipogenic genes (20). In hypertriglyceridemic mice, CETP expression normalizes subcutaneous adipose depots and visceral adipocyte size (21). And in humans, a CETP gene variant that affects the coding sequence of CETP is associated with increased adiposity following long-term overfeeding (22).
In this study, we have examined pathways involved in TG homeostasis in order to understand the metabolic basis for the decreased TG accumulation observed in CETP-deficient cells. The data show that decreased TG storage occurs because of lower TG synthesis and the ineffective transfer of TG and diglyceride (DG), a newly recognized CETP substrate, to the lipid droplet, which likely leads to their premature lipolysis in the ER.
METHODS
Materials
The [9,10(n)-3H]oleic acid was from Perkin-Elmer Life Sciences (Boston, MA). Stock 2.7 mM 3H-oleate/BSA and unlabeled oleate/BSA (7:1 oleate to BSA mole ratio) were prepared as previously described (23). LDL and HDL were isolated from human plasma by sequential ultracentrifugation (24), dialyzed extensively versus 0.9% NaCl and 0.02% EDTA, and then sterile filtered. Thimerosal, diethylumbelliferyl phosphate, penicillin, streptomycin, BSA, and sodium oleate were purchased from Sigma (St. Louis, MO). Mouse monoclonal antibody against human CETP (TP2) was purchased from the Ottawa Heart Institute (Ottawa, Ontario, Canada). Antibodies against ER stress proteins were from Cell Signaling Technology (Danvers, MA). Calnexin antibody was from Santa Cruz Biotechnology (Dallas, TX).
Cells
The human liposarcoma cell line, SW872 (HTB-92), was purchased from American Type Culture Collection (Manassas, VA). Cells were cultured in DMEM/Ham’s F-12 (DMEM/F-12) (1:1) containing 10% FBS (Atlas Biologicals, Fort Collins, CO) and 50 μg/ml penicillin/streptomycin in 5% CO2/95% air at 37°C. CETP-deficient cells were created as previously described (10). Briefly, SW872 cells were transfected with native pcDNA3 or this vector containing a 549 bp antisense human CETP cDNA fragment. Stable clones were selected by Geneticin resistance. Previously characterized clones 1 and 6 (10), which express 20–30% of control CETP activity, were used for these studies. Reduced CETP expression by these cells is evidenced by both the amount of CETP secreted and the size of the intracellular CETP pool (10). CETP-deficient and control (vector-transfected) cells were grown to ∼90% confluence before initiating the experimental protocols described below. Typically, these cells were incubated for an additional 48 h with or without oleate prior to use in an assay.
Perilipin protein analysis
To determine cellular perilipin (PLIN) protein levels, cell homogenates were spun at 1,000 g for 10 min. Equal amounts of supernatant protein were centrifuged at 100,000 g for 60 min. Equal volumes of the resulting supernatant (cytosol + lipid droplets) were acetone precipitated and the resuspended pellet fractionated on 4–20% SDS gels (Lonza, Rockland, ME). Western blots were performed using 1:2,000 dilutions of rabbit anti-human antibodies against PLIN1 (Sigma), PLIN2, and PLIN3 (ThermoFisher Scientific, Rockford, IL) followed by HRP-tagged secondary antibody. β-actin, detected by mouse anti-β-actin (8H10D10, Cell Signaling Technology) was used as a loading control.
Incorporation of oleate into TG and its precursors
Cells were washed with PBS and incubated in Opti-MEM (Life Technologies, Grand Island, NY) for 24 h before the addition of prewarmed 200 μM 3H-oleate/BSA in Opti-MEM. At the indicated time, the medium was removed and ice-cold PBS was added to the cells. Cells were kept on ice until released by trypsin. Cellular lipids were extracted (25) and separated by TLC. Chromatography was accomplished by two solvent systems. In the first, plates were developed half-way in chloroform:acetone:methanol:acetic acid:water (60:80:20:20:1, v/v). After plates were dried, chromatography in a second system of hexanes:diethyl ether:acetic acid (80:20:1, v/v) was performed. In some instances, lipids were fractionated by developing plates in only one of these solvent systems. Bands were identified based on comigration with authentic lipid standards (Nu-Chek Prep, Inc., Elysian, MN, and Avanti Polar Lipids, Inc., Alabaster, AL), scraped, and their radioactivity determined by scintillation counting.
TG synthesis and storage in lipid droplets
Cells in growth medium were switched to the same medium containing 200 μM unlabeled oleate/BSA for 48 h to initiate droplet formation. Cells were then washed with medium and incubated in the same medium containing 200 μM 3H-oleate/BSA. At the indicated time, the medium was removed and cold PBS was added to the cells. Cells were kept on ice until released by trypsin. Cells were incubated in 500 μl cold hypotonic medium [10 mM Tris (pH 7.4), 1 mM EDTA, 10 mM sodium fluoride, 300 μM diethylumbelliferyl phosphate, and EDTA-free protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN)] for 20 min at 4°C. Cells were lysed by 10 strokes in a Teflon-glass homogenizer and the cell homogenate centrifuged at 2,000 g for 10 min. The resultant supernatant was centrifuged at 100,000 g for 60 min to yield a lipid droplet-rich fraction (upper 1/2) and ER-enriched (bottom 1/2) fraction. Lipids in these fractions were extracted (26) and separated by TLC (hexanes:diethyl ether, 70:30, v/v). Radioactivity in separated lipids was determined by scintillation counting.
ER isolation
Cells were incubated with or without 200 μM 3H-oleate/BSA or unlabeled oleate/BSA for 48 h. Subsequently, cells were lysed by sonication and ER was purified from the 15,000 g supernatant by centrifugation on a discontinuous sucrose gradient (27). The discrete ER band was harvested and centrifuged at 100,000 g to pellet membranes. Based on Western blots for calnexin (ER) and cytochrome c oxidase subunit IV (mitochondria) (Cell Signaling Technology), there was high recovery of ER with very low mitochondrial contamination.
Diacylglycerol acyltransferase activity
Cells were lysed by sonication and centrifuged at 25,000 g, as previously described (28). Microsomes were isolated from the resultant supernatant as the 100,000 g pellet. Diacylglycerol acyltransferase (DGAT) activity was measured using [14C]oleoyl-CoA (Perkin-Elmer Life Sciences) and sn-1,2-dioleoylglycerol (Avanti Polar Lipids) as substrates. Assays were performed as described by Ganji et al. (28) using 20 μg microsomal protein and an incubation time of 30 min. DGAT assays were stopped by lipid extraction (26). TG in the extract was isolated by TLC in a developing system of hexanes:diethyl ether:acetic acid (70:30:1), and its 14C content determined by scintillation counting.
TG lipase activity
Cells were incubated with or without 200 μM oleate/BSA for 48 h, lysed by sonication, and centrifuged at 5,000 g. Microsomes were isolated from the resultant supernatant as the 100,000 g pellet. TG lipase activity was measured essentially as described by Hajjar, Minick, and Fowler (29) except that the taurocholate micellar substrate contained 3H-TG instead of labeled CE. Micelles typically contained ∼45,000 cpm 3H per nanomole TG.
Real-time quantitative PCR
Total RNA was extracted from control and CETP-deficient cells using RNeasy Mini kit (Qiagen, Germantown, MD) following the manufacturer’s protocol. cDNA was generated using a high capacity RNA-to-cDNA kit and real-time quantitative PCR (qPCR) analysis was performed using TaqMan gene expression primer/probe sets and TaqMan Universal Master Mix following the manufacturer’s instructions (Applied Biosystems, Grand Island, NY). Ribosomal 18S, β-2 microglobulin, hypoxanthine phosphoribosyltransferase 1, glyceraldehyde-3-phosphate dehydrogenase, and β-actin were evaluated for suitability as stable reference genes in both cell types with or without oleate treatment. 18S and β-2 microglobulin (B2M) were judged to be the best choices, as determined by GeNorm and NormFinder software; 18S was selected as the reference gene. Gene expression was calculated using the 2−ΔΔCT method (30) and reported relative to control cells.
Radiolabeled DG synthesis and lipid transfer assays
To prepare radiolabeled DG, liposomes containing egg phosphatidylcholine (Avanti Polar Lipids) and tracer quantities of [9,10-3H(n)]triolein (Perkin-Elmer) were prepared by sonication (31). Liposomes (1.25 μmol phospholipid) were incubated with 0.5 mg lipoprotein lipase (Pseudomonas sp., Sigma) for 20 min at room temperature followed by lipid extraction (25). Lipids were fractionated by TLC in a developing system of hexanes:diethyl ether:acetic acid (65:35:1, v/v). The area of the plate containing DG was scraped and eluted from the silica gel with chloroform. Unlabeled DG (dioleoylglycerol) was purchased from Avanti Polar Lipids.
For CETP assays, egg phosphatidylcholine-cholesterol liposomes containing 1 mol% radiolabeled DG, cholesteryl oleate, or triolein were prepared by cholate dialysis (32, 33). Human plasma CETP was partially purified by phenyl-Sepharose and carboxymethyl cellulose chromatographies (34). The CETP-mediated transfer of lipid from liposome donors (190 nmol phospholipid) to LDL (10 μg cholesterol) was determined, as previously described (33). Where indicated, CETP was preincubated with anti-CETP (TP2, 5.5 μg) or thimerosal (1.9 mM) for 1 h at room temperature before addition to the transfer assay.
Analytical
Protein was quantified by a modification of the Lowry method (35) with BSA as standard. Total cholesterol and TG were determined by enzymatic kits from ThermoFisher Scientific, and free cholesterol was measured by an enzymatic kit from Wako Chemical (Richmond, VA). Alternatively, total and free cholesterol were measured by the Amplex Red method (Life Technologies). Lipid phosphorus was quantified by the method of Bartlett (36). Data shown are the mean ± SD. Statistical significance was determined by Student’s t-test.
RESULTS
Lipid droplets in CETP-deficient cells
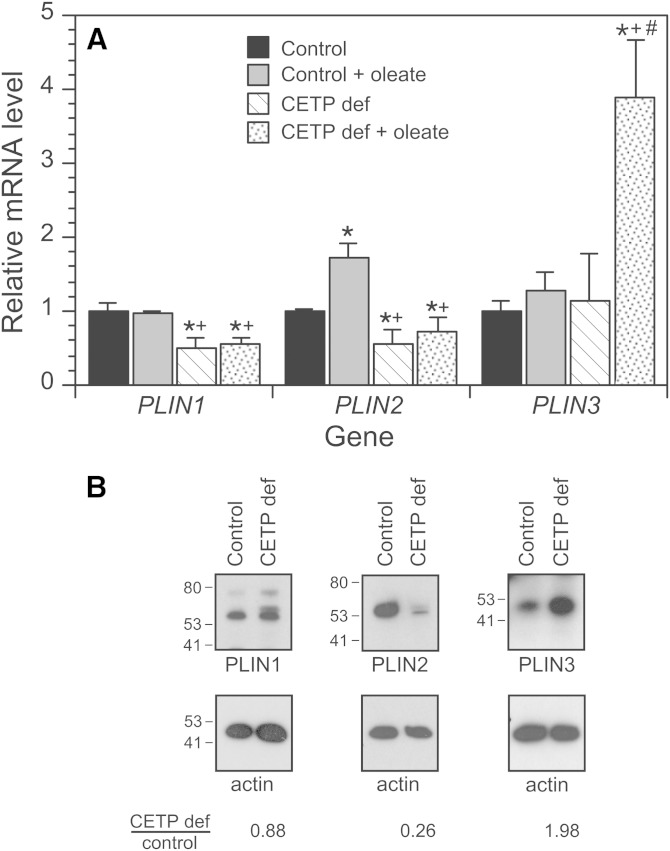
We previously reported that CETP-deficient cells accumulate about half as much TG as vector-transfected control cells, and that lipid droplets in these cells are smaller and fewer in number (10). To further our understanding, we examined the mRNA expression and protein levels of PLINs. In control cells, the addition of oleate increased the expression of PLIN2, but had little effect on PLIN1 or PLIN3 mRNA levels (Fig. 1A). In CETP-deficient cells, PLIN1 and PLIN2 expression was 40–50% of control levels and their expression was not induced by oleate. PLIN3 expression, on the other hand, was the same as control in the absence of oleate, but increased more than 3-fold in cells incubated with oleate. For the most part, PLIN protein levels followed a similar pattern. While PLIN1 levels were similar in oleate-treated control and CETP-deficient cells, PLIN2 levels were markedly lower and PLIN3 levels were elevated 2-fold in CETP-deficient cells compared with oleate-treated control cells (Fig. 1B). This pattern of PLIN expression is consistent with the preponderance of small lipid droplets in CETP-deficient cells (37).
Fig. 1.
PLIN family members in control and CETP-deficient (CETP def) cells. Cells were incubated with or without 200 μM unlabeled oleate/BSA for 48 h. A: PLIN mRNA levels were measured by qPCR. Values are the average of two experiments and representative of two additional experiments comparing wild-type SW872 cells with CETP-deficient cells. *P < 0.05 versus control without oleate; +P < 0.05 versus control with oleate; #P < 0.05 versus CETP-deficient cells incubated without oleate. PLIN1 (perilipin); PLIN2 (adipose differentiation-related protein); PLIN3 (TIP47). B: Western blot of PLINs in the 100,000 g supernatant of cells incubated with oleate/BSA as above. The positions of molecular mass standards, in kDa units, are shown. Results are typical of three separate studies.
CETP-deficient cell differentiation status
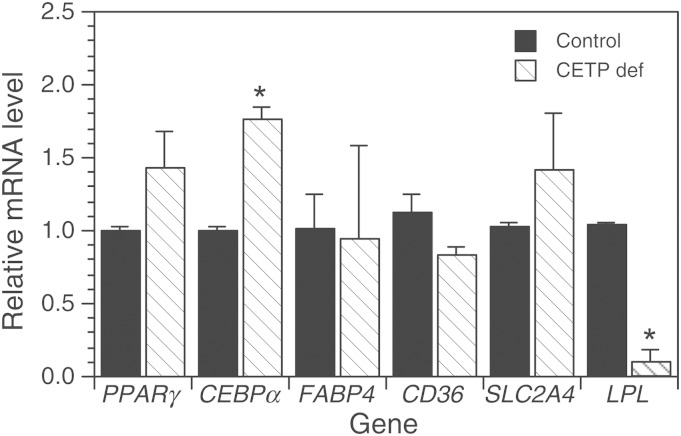
The reduced TG content of CETP-deficient cells is due, in part, to reduced TG biosynthesis (10). To understand the basis for this reduction, we initially considered that CETP suppression might alter the differentiation status of these cells. The adipocyte differentiation marker genes PPARγ and CCAAT/enhancer-binding protein α (CEBPα) are sequentially expressed during the conversion of preadipocytes to adipocytes (38). Comparing CETP-deficient cells to vector-transfected controls, PPARγ levels were not statistically different and CEBPα mRNA levels were modestly increased in CETP-deficient cells (Fig. 2A). Given that the expression of these genes varies many-fold during differentiation, we concluded that the differentiation status of CETP-deficient cells is very similar to control cells. Consistent with this interpretation, the expression levels of adipocyte-related genes, fatty acid binding protein 4 [FABP4 (aP2)], CD36, and SLC2A4 [glucose transporter type 4 (GLUT4)], were not different in CETP-deficient cells (Fig. 2A). Also, levels of the differentiation marker, FASN, were not different from control (1.13 ± 0.10 versus 0.82 ± 0.12), consistent with our previous observation that CETP-deficient cells have normal capacity to incorporate acetate into fatty acid (10). In contrast, LPL expression was markedly reduced regardless of whether cells were preincubated with oleate, as in Fig. 2, or in cells cultured without oleate (0.02 ± 0.01, n = 3).
Fig. 2.
Gene expression in control and CETP-deficient (CETP def) cells. Cells were grown as indicated and the expression level of the indicated genes determined by qPCR. Relative levels of adipocyte differentiation marker genes were measured in cells grown in DMEM/F12 media containing 10% FBS. For CD36, SLC2A4, and LPL expression, cells were grown in the same medium containing 200 μM oleate for 48 h prior to harvest. Values are the mean ± SD of two to three experiments. *P < 0.05 versus control. CD36, CD36 scavenger receptor.
ER stress
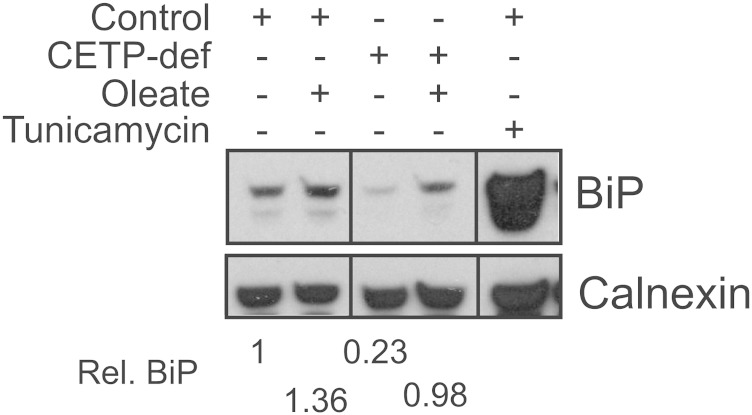
Given the key role of the ER in lipid metabolism, we also considered whether aberrant TG homeostasis in CETP-deficient cells might be symptomatic of ER stress (39, 40). By Western blot analysis, cellular levels of the ER stress protein, immunoglobulin binding protein (BiP/GRP78), were not elevated in CETP-deficient cells grown in serum-containing media (Fig. 3). While oleate treatment modestly increased BiP levels in both cell types, BiP levels in CETP-deficient cells were not elevated compared with control. Very similar results were observed for the ER stress markers, inositol requiring protein 1α and protein disulfide isomerase (data not shown). These data confirm the absence of elevated ER stress in CETP-deficient cells and show that 200 μM oleate does not induce an aberrant stress response in these cells, despite their defective incorporation of fatty acids into TG.
Fig. 3.
Evaluation of ER stress status. Cells were treated with 200 μM oleate/BSA or tunicamycin (5 μg/ml), as indicated, for 48 h. Cells were lysed and 30 μg protein aliquots fractionated by SDS-PAGE. Western blots for the ER stress marker, BiP, and calnexin (loading control) are shown. Results are typical of two experiments and representative of results with two other ER stress proteins, as noted in the text. CETP-def, CETP-deficient; Rel., relative.
Analysis of the TG biosynthetic pathway
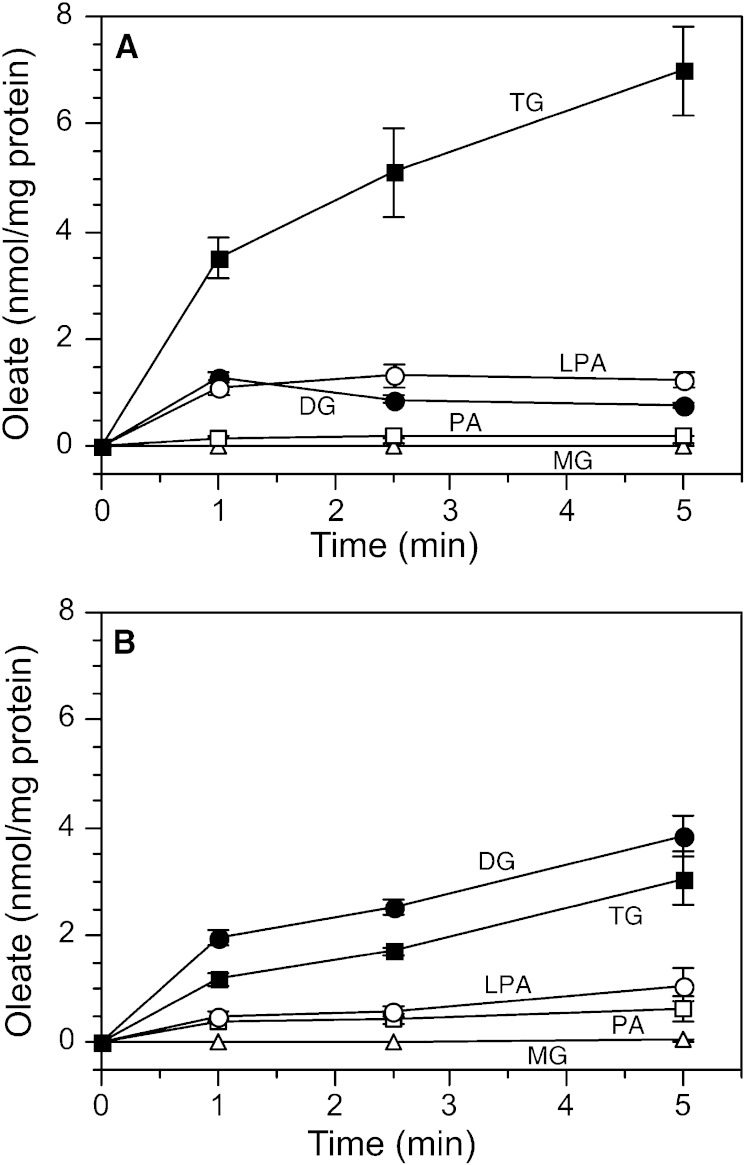
Cells were incubated with radiolabeled oleate and the initial transit of radiolabel through intermediates of the TG biosynthetic pathway was followed. In control cells, lysophosphatidic acid levels gradually increased, but there was no significant accumulation of radiolabeled monoglyceride or phosphatidic acid (Fig. 4A). A similar pattern for these three lipids was seen in CETP-deficient cells (Fig. 4B). In control cells, DG peaked at 1 min then declined, while TG, the major product, increased linearly over the 1 to 5 min assay interval. Although TG in CETP-deficient cells also increased linearly over this time, the kinetics of DG accumulation were markedly different from control cells. DG levels did not decline, but remained high and exceeded TG throughout the time course. At 5 min, the DG/(DG + TG) ratio was 0.20 ± 0.10 (n = 5) in control cells, which was very close to the 0.17 ± 0.07 (n = 4) value in wild-type SW872 cells, determined in separate studies. However, in CETP-deficient cells, this ratio was significantly higher [0.53 ± 0.14 (n = 4)]. DG is also a precursor for phosphatidylcholine synthesis. However, the conversion of DG to phosphatidylcholine was not different between cell types (0.18 ± 0.07 versus 0.17 ± 0.07 nmol/mg protein per 5 min). Overall these data show that the total amount of oleate flowing through the TG synthetic pathway (sum of oleate in TG and its precursors) is not lower in CETP-deficient cells versus control [8.70 ± 0.73 versus 9.28 ± 0.83 nmol, respectively, at 5 min (Fig. 4)]. These data suggest that the conversion of DG to TG, not the flux of lipid along the TG biosynthetic pathway, is impaired in CETP-deficient cells.
Fig. 4.
Initial incorporation of 3H-oleate into TG and its precursors. Control (A) or CETP-deficient (B) cells were incubated with 200 μM 3H-oleate/BSA for the indicated times. Lipids were extracted and fractionated by TLC and quantified by scintillation counting. PA, phosphatidic acid; LPA, lysophosphatidic acid; MG, monoglyceride. Values are mean ± SD, n = 3. Results are representative of three experiments for each cell type. Very similar results were observed for control (vector-transfected) cells (A) and wild-type SW872 cells (not shown).
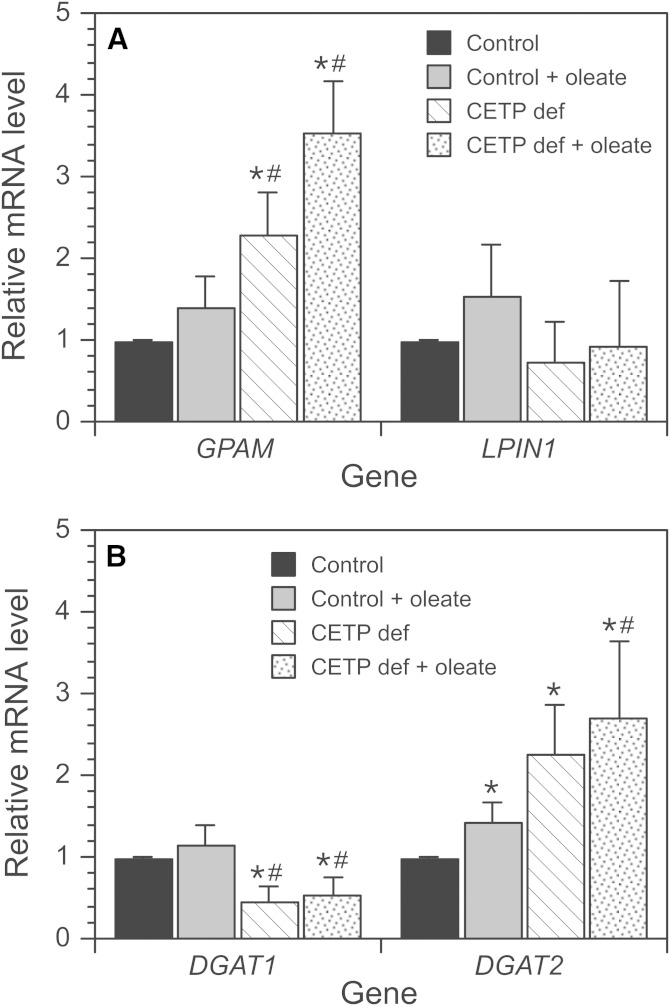
To understand the basis for the accumulation of the DG in CETP-deficient cells, mRNA levels of enzymes involved in TG assembly were measured by qPCR. Measurements were performed in cells incubated without oleate and with oleate to stimulate TG production and accumulation. In control cells, only DGAT2 expression was affected by oleate treatment (Fig. 5A, B). Levels of glycerol-3-phosphate acyltransferase 1 (GPAM) mRNA, whose product mediates the initial and rate-limiting step in TG biosynthesis, were elevated 2- to 3-fold in CETP-deficient cells compared with control cells, regardless of oleate pretreatment. mRNA levels of LPIN1, whose activity converts phosphatidic acid into DG, were not significantly different between cell types. DGAT1 and -2 convert DG into TG. In CETP-deficient cells, mRNA levels for DGAT1 were ∼50% of control, but DGAT2 mRNA levels were increased 2- to 2.5-fold independent of oleate treatment (Fig. 5). Importantly, however, total cellular DGAT activity measured in vitro was normal in CETP-deficient cells regardless of oleate treatment (Table 1). Overall, neither changes in DGAT1/2 expression nor DGAT activity explain the reduced conversion of DG into TG in CETP-deficient cells.
Fig. 5.
mRNA levels of TG biosynthesis pathway enzymes. A, B: Cells were incubated without or with 200 μM oleate for 48 h, and then mRNA levels of GPAM, LPIN1 (phosphatidate phosphatase), and DGAT1 and -2 were measured by qPCR. Data are expressed relative to the mRNA levels in control cells incubated without oleate. Values are the mean ± SD of results from two to three experiments and are representative of two to three additional experiments comparing wild-type SW872 cells with CETP-deficient (CETP def) cells. *P < 0.05 versus control without oleate. #P < 0.05 versus control incubated with oleate.
TABLE 1.
DGAT activity
| Cell Type | Without Oleate | With Oleate |
| Control | 1.76 ± 0.07 | 1.52 ± 0.09a |
| CETP-deficient | 1.85 ± 1.01 | 1.60 ± 0.36 |
Cells were incubated without or with 200 μM oleate/BSA for 48 h, lysed, and microsomes were isolated. DGAT activity was determined from the extent of 14C-oleoyl-CoA esterified to unlabeled DG. Shown are the mean ± SD of values determined on three separate microsomal preparations. Units are nanomoles of 14C-oleate per milligram of protein per 30 min.
P < 0.05 versus control without oleate.
In adipose tissue, glyceroneogenesis provides the major portion of glycerol 3-phosphate that forms the carbon backbone for TG assembly (41, 42). Even when plasma glucose levels are high, glyceroneogenesis accounts for ∼90% of the glycerol backbone in adipose tissue (41). Phosphoenolpyruvate carboxykinase (PCK)1 activity is the rate-limiting step in glyceroneogenesis. Because we did not observe a reduced flux of intermediates through the TG synthetic pathway in CETP-deficient cells, we were surprised to find that PCK1 mRNA levels were reduced by ∼90% in these cells compared with control (1.09 ± 0.05 versus 0.08 ± 0.07). Because RNAi-mediated suppression of PCK1 expression causes an equivalent decline in PCK protein (43), these data suggest that glycolysis, driven by glucose in the media, provides adequate glycerol 3-phosphate for this pathway under cell culture conditions.
Cellular TG synthesis and movement
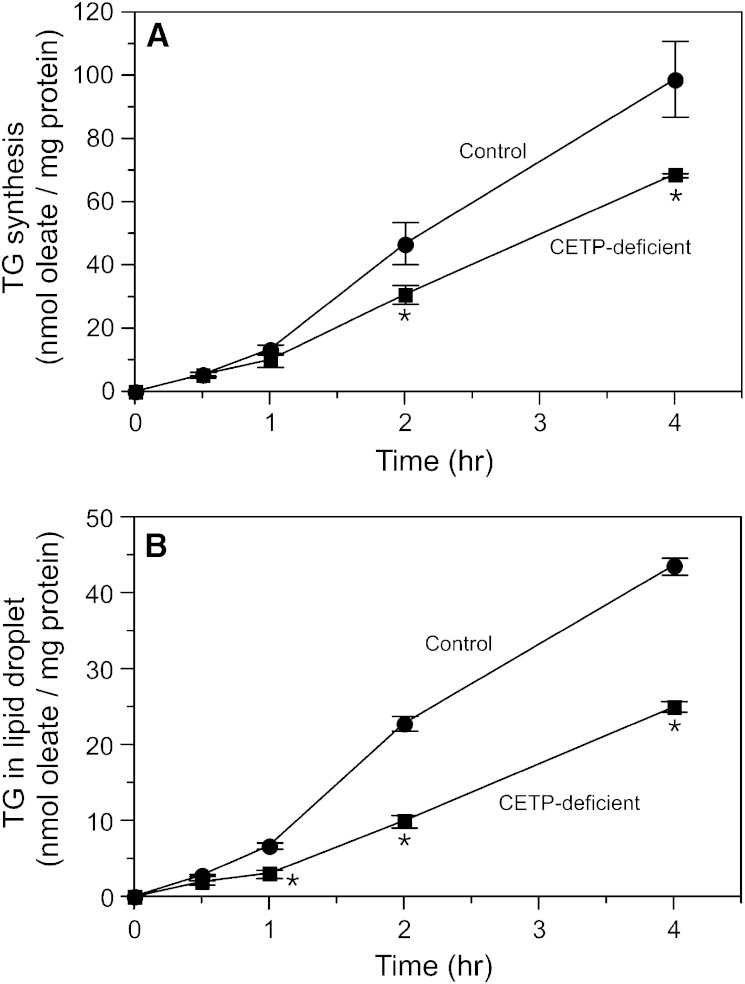
We previously suggested that intracellular CETP may transport CE and TG between cellular membranes, and demonstrated in vitro that CETP can mediate the movement of these lipids from the ER to lipid droplets in an organelle transfer assay (10). Whether CETP has this function in intact cells has not been assessed. To investigate this, we preincubated control and CETP-deficient cells with oleate/BSA for 48 h to induce lipid droplet formation. Subsequently, these cells received 3H-oleate/BSA to measure the kinetics of radiolabeled TG synthesis and its movement from its site of synthesis in the ER to lipid droplets. Like that seen in cells not preincubated with oleate (10), TG synthesis in oleate-treated CETP-deficient cells was also reduced (Fig. 6A). The TG synthetic rate in oleate-treated control and CETP-deficient cells averaged 24.6 ± 3.0 and 17.2 ± 0.2 nmol oleate incorporated per milligram of protein per hour, respectively (n = 3, P < 0.02). In both cell types, newly synthesized TG began to appear in the lipid droplet fraction after an initial lag of about 1 h (Fig. 6B). In keeping with our in vitro organelle transfer assay findings (10), the movement of this newly synthesized TG into lipid droplets in CETP-deficient cells was only 49% of that in control cells. This value is slightly higher than can be explained by the 30% residual CETP contained in these CETP-deficient cells (10).
Fig. 6.
Incorporation of 3H-oleate into cellular TG pools. Cells were preincubated with unlabeled oleate/BSA for 48 h, and then incubated with 3H-oleate/BSA for the indicated time. A: TG synthesis. B: Incorporation of newly synthesized TG into the lipid droplet fraction. Values are mean ± SD of triplicate determinations and are representative of two additional experiments comparing wild-type SW872 cells with CETP-deficient cells. *P < 0.05 versus control.
The decreased transfer of newly synthesized TG into lipid droplets in CETP-deficient cells suggests that TG might accumulate in the ER. Although we previously reported that a greater percentage of cellular TG is associated with the membrane fraction rather than the lipid droplet fraction (10), whether TG levels are actually higher in the ER of CETP-deficient cells has not been addressed. To investigate this, highly purified ER was isolated by sucrose-gradient ultracentrifugation from cells incubated with 3H-oleate/BSA for 48 h to uniformly label TG pools. Although the ER fraction from CETP-deficient cells contained a greater proportion of cellular 3H-TG compared with control cells, as previously observed, the absolute concentration of TG in the ER of both cell types was not different whether expressed relative to the protein or phospholipid content of the ER (Table 2). Similarly, DG was not elevated in the ER of CETP-deficient cells. These data suggest that CETP-deficient cells may have enhanced lipase activity in order to control TG and DG levels in the ER. To test this, we measured neutral TG hydrolase activity in microsomes isolated from control and CETP-deficient cells in the presence and absence of oleate. In the absence of oleate, microsome-associated neutral TG hydrolase activities were similar in control and CETP-deficient cells (Table 3). The addition of oleate to stimulate TG production caused TG lipase activity to decrease 60% in control cells. In CETP-deficient cells, the effect of oleate was much smaller, resulting in 2-fold higher microsome-associated lipase activity under conditions where TG biosynthesis was enhanced. While the identity of the lipase(s) responsible for this activity is not known, adipose TG lipase (PNPLA2) mRNA levels were increased in oleate-treated CETP-deficient cells (0.97 ± 0.22 versus 2.23 ± 0.14).
TABLE 2.
TG and DG content of ER
| Cell Type | TG (cpm/μg protein) | DG (cpm/μg protein) | TG (cpm/nmol phospholipid) | DG (cpm/nmol phospholipid) |
| Control | 7.69 ± 0.93 | 1.49 ± 0.12 | 5.39 ± 0.13 | 1.06 ± 0.17 |
| CETP-deficient | 6.90 ± 1.55 | 1.52 ± 0.73 | 4.71 ± 0.89 | 1.29 ± 0.43 |
Cells were incubated with 200 μM 3H-oleate/BSA for 48 h to label cellular lipid pools. Highly purified ER was isolated by gradient ultracentrifugation. Lipids were extracted and fractionated by TLC. Shown are the mean ± SD of values determined on three separate ER preparations.
TABLE 3.
TG hydrolase activity in microsomes
| Cell Type | Without Oleate | With Oleate |
| Control | 44.2 ± 1.6 | 17.3 ± 5.4a |
| CETP-deficient | 48.9 ± 0.2 | 37.5 ± 2.8a,b |
Control and CETP-deficient cells were grown to near confluence then treated with or without 200 µM oleate/BSA for 48 h. Microsomes were isolated and TG lipolytic activity was measured with micelles containing 3H-TG. Values are picomoles of TG hydrolyzed per 200 μg protein per 4.5 h. Data are the mean ± SD (n = 2) and are representative of two experiments on separate microsome preparations.
P < 0.05 versus same cell without oleate.
P < 0.05 versus control with oleate.
DG as a CETP substrate
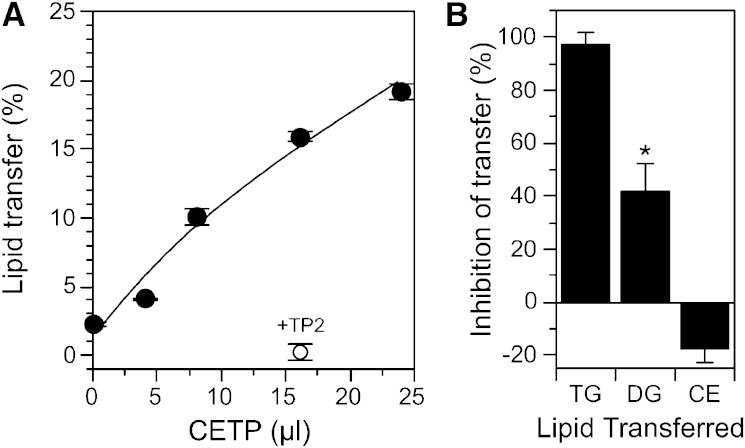
The studies above assume that newly synthesized TG recovered in the lipid droplet fraction is derived from TG synthesized in the ER. However, the cellular location of DGAT is controversial, with some studies indicating that a portion of DGAT2 may exist on the lipid droplet surface itself (44). This provides a mechanism by which TG can be synthesized directly on the storage droplet. Because we observed abnormalities in the conversion of DG to TG in CETP-deficient cells (Fig. 3B), we questioned whether DG is also a CETP substrate. If so, its ineffective transfer to the lipid droplet for conversion to TG could underlie some of the aberrations we observe in CETP-deficient cells. Because it is not possible to study this in intact cells because of the rapid metabolism of DG, we tested the ability of CETP to transfer DG in vitro. DG was incorporated into phosphatidylcholine/cholesterol liposomes and the effect of CETP on the transfer of DG to LDL was measured. CETP caused a dose-dependent transfer of DG between these phospholipid surfaces (Fig. 7A). This transfer was completely blocked by the anti-CETP antibody, TP2 (Fig. 7A). It is notable that DG movement in the absence of CETP was very low (∼2%), indicating that DG does not have sufficient solubility to move between these membranes in the absence of a carrier.
Fig. 7.
DG as a CETP substrate. A: The CETP-mediated transfer of 3H-DG from phosphatidylcholine-cholesterol liposomes to LDL was measured in the presence of the indicated amount of partially purified human plasma CETP. TP2, a blocking anti-CETP antibody, was combined with CETP and preincubated for 1 h at room temperature prior to addition to the lipid transfer assay. B: CETP was preincubated with or without 1.9 mM thimerosal for 1 h at room temperature before being added to the lipid transfer assay. Values are the mean ± SD (n = 2) and are representative of three similar experiments.
A distinguishing feature of CETP transfer activity is its selective sensitivity to the mercurial reagent, thimerosal. Thimerosal completely blocks TG transfer, but mildly stimulates CE transfer [(34) and Fig. 7B)]. However, we found that the effect of thimerosal on DG transfer was intermediate to these extremes. Thimerosal and other mercurial agents derivatize CETP cysteines. Because these cysteines are not necessary for CETP activity (45), the effect of these compounds on CETP activity likely results from alterations in its conformation. The partial inhibition of DG compared with TG is likely due to its smaller molecular size, making it less sensitive to small conformational changes in CETP structure. Overall, these data show that DG is a novel CETP substrate.
DISCUSSION
During cellular differentiation into adipocytes, the transcription factors CEBP and PPARγ play key roles in the transcriptional cascade that lead to marked induction of pathways involved in TG synthesis and storage (46, 47). In human adipocytes, CETP expression is also induced during this differentiation process (48, 49). CETP expression is highest in small lipid-poor adipocytes, and then decreases when fat cells become TG-filled (48, 50). The timing of these events suggests that CETP may have a role in lipid storage. Indeed, CETP is most highly expressed in tissues that store lipids and/or synthesize lipoproteins (4, 5). Consistent with this connection, CETP-deficient SW872 cells store only 45% as much TG as control cells, and accumulate fewer and smaller lipid droplets (10). Reduced TG synthesis appears to underlie this lipid deficiency because hydrolysis of lipid droplet TG is actually lower in CETP-deficient cells than in control cells (10).
Reduced TG synthesis in CETP-deficient cells is not due to an altered differentiation status, as assessed by the expression of the differentiation genes, CEBPα and PPARγ (38). Several other genes whose expression reflects cellular differentiation status, FABP4, CD36, FASN, and SLC2A4 (GLUT4) were also similar between control and CETP-deficient cells.
A key aberration in the TG biosynthetic pathway of CETP-deficient cells was the accumulation of DG. Although mRNA levels for GPAM, the rate limiting enzyme in the TG synthetic pathway, were elevated, the total lipid flux through this pathway was unchanged in CETP-deficient cells. This suggests that DG synthesis is not increased. Furthermore, given the kinetics observed, it seems unlikely that the hydrolysis of newly made 3H-TG contributes significantly to the 3H-DG pool. Thus, CETP-deficient cells have reduced capacity to convert DG into TG, despite having normal DGAT activity.
We also demonstrate for the first time that intact CETP-deficient cells have reduced capacity to transfer newly synthesized TG into lipid droplets, validating our previous in vitro studies (10). However, despite this defect, TG did not accumulate in the ER of CETP-deficient cells. This suggests that there are cellular protective mechanisms that prevent the accumulation of TG in this organelle. Consistent with this, we observed that microsome-associated TG lipase activity is 2-fold higher in CETP-deficient cells when TG biosynthesis is stimulated. This may be due to the 2-fold increase in ATGL mRNA. Human ATGL resides, in part, in the ER (51) and has significant TG hydrolysis activity independent of its translocation to lipid droplets where it interacts with its activator, CGI-58 (52). However, microsomes also contain other TG lipases, such as CES3/TGH (53). The lipase responsible for the increased lipolytic activity of microsomes from CETP-deficient cells remains to be determined. Because TG stored in lipid droplets in CETP-deficient cells has normal or slightly reduced turnover (10), the enhanced lipolytic activity observed in these cells seems to be directed toward the hydrolysis of a different pool, such as lipids associated with the ER.
In adipocytes, DGAT, specifically DGAT2, is associated with either the cytosolic side of the ER or the surface of lipid droplets (44, 54). The latter cellular location provides an alternative mechanism for the accumulation of TG in lipid droplets independent of its transport from the ER. Because DG is ineffectively converted to TG in CETP-deficient cells, we investigated whether CETP might be involved in the transfer of newly synthesized DG into lipid droplets, where it could be converted to TG by resident DGAT. Our data show, in an in vitro assay, that CETP can transfer DG, a previously unrecognized CETP substrate. With the donor liposome use in these lipid transfer assays, DG transfer was ∼11% of that for TG. However, CETP’s substrates reside in the surface phospholipids of membranes (33), and the relative availability of these substrates to CETP is remarkably sensitive to membrane composition (55). How well these liposomes model the relative availability of DG and TG to CETP in the ER membrane is not known. The finding that CETP can transfer DG is consistent with its broad substrate specificity, which includes structurally diverse molecules such as CE, retinyl ester, TG, and some hydrophobic drugs (34, 56, 57). Our finding that DG is a CETP substrate suggests that it may have a role in delivering substrate to DGAT2 on the lipid droplet surface. This route of DG delivery provides a regulatable mechanism that may supplement passive delivery pathways such as diffusion at points of ER, lipid droplet contact.
Much of the detailed understanding about the lipid droplet formation process has come from studies in rodent cells. Because these species do not express CETP (58), it can be argued that either CETP must not be relevant to lipid droplet formation because this process occurs robustly in cells lacking this protein or that there are fundamental differences in lipid droplet biogenesis between species. Because multiple studies in different human cell types show that disruption of CETP expression leads to significant alterations in cellular cholesterol homeostasis and in TG and CE storage (10, 15–20), we suggest that the evidence favors the latter conclusion. However, these species differences in lipid droplet genesis may lie in the details of the proteins involved in these processes rather than in the general steps of the process. For example, recent studies indicate that lipid droplet growth in 3T3-L1 mouse cells involves lipid transfer between organelles by an unknown factor (59).
While the expression of many lipid-related genes was unaffected by CETP deficiency, we observed an interesting pattern of aberrant gene expression for several genes involved in cellular TG deposition. Decreased LPL and PCK1 expression (<10% of control) and CD36 expression (∼60% of control) suggest that CETP deficiency may cause these cells to be less capable of hydrolyzing extracellular TG (LPL), internalizing the resultant fatty acids (CD36), and synthesizing the TG glycerol backbone through the glyceroneogenesis pathway (PCK1). Although we did not see a reduction in the flux of TG intermediates through the TG biosynthetic pathway in CETP-deficient cells, this may be due to the presence of glucose in the media, which may supply sufficient glycerol backbone via glycolysis. However, in vivo, glyceroneogenesis provides ∼90% of the glycerol backbone in adipocytes, even during periods of high sucrose feeding (41). The inadequate expression of these three genes may combine to exacerbate the reduced lipid storage phenotype of CETP-deficient cells in vivo.
It is striking that the effect of CETP deficiency on cellular TG accumulation and storage has many similarities to that observed for apoE deficiency. The expression of both CETP and apoE is induced during adipocyte development (48, 60). For both proteins, expression is closely linked to cellular TG and cholesterol content (50, 60). Blocking CETP expression reduces cellular TG levels (10), and adipocytes derived from apoE−/− mice have impaired capacity to accumulate TG (61, 62). Although both proteins are secreted, endogenous pools exist and the defects in lipid metabolism and storage caused by their low expression cannot be rescued by exogenous protein (19, 61). Interestingly, in preliminary studies, we observed that APOE mRNA levels in CETP-deficient cells are only 5% of control levels. In contrast, cells overexpressing CETP, which also have decreased TG storage capacity, have normal APOE expression (11). It remains to be determined how suppressing CETP expression leads to reduced APOE expression, and whether a portion of the low TG phenotype in CETP-deficient cells can be rescued by normalizing APOE expression.
In summary, these data suggest several contributing causes for the decreased accumulation of TG in CETP-deficient cells. In short-term studies, we found that the total flux of oleate through the TG biosynthetic pathway was not altered. However, the conversion of DG to TG was impaired. Coupled with our previous observation that the turnover of TG stored in lipid droplets is not increased (10), our overall findings are consistent with the following: CETP deficiency causes recently synthesized TG and the newly identified CETP substrate, DG, to be ineffectively transported to lipid droplets. The failure to deliver DG to lipid droplets may reduce TG synthesis on the lipid droplet by resident DGAT. The TG and DG retained inappropriately in the ER do not accumulate, but are hydrolyzed by homeostatic mechanisms responsible for maintaining the integrity of this organelle. While other interpretations of our findings may exist, this scenario fits with the recognized role of CETP as a lipid transfer protein. These data further enhance our understanding of how reduced intracellular CETP impacts cellular lipid metabolism. Because perturbation of lipid storage in adipocytes is associated with altered secretion of adipokines that control whole body metabolism (61–64), disruption of CETP expression in these cells may have far reaching consequences.
Footnotes
Abbreviations:
- CE
- cholesteryl ester
- CEBPα
- CCAAT/enhancer-binding protein α
- CETP
- cholesteryl ester transfer protein
- DG
- diglyceride
- DGAT
- diacylglycerol acyltransferase
- ER
- endoplasmic reticulum
- FABP4
- fatty acid binding protein 4
- GPAM
- glycerol-3-phosphate acyltransferase 1, mitochondrial
- PCK
- phosphoenolpyruvate carboxykinase
- PLIN
- perilipin
- qPCR
- real-time quantitative PCR
- SLC2A4
- glucose transporter type 4 (GLUT4)
This research was supported in part by Grant HL60934 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD.
REFERENCES
- 1.Morton R. E., Zilversmit D. B. 1983. Inter-relationship of lipids transferred by the lipid-transfer protein isolated from human lipoprotein-deficient plasma. J. Biol. Chem. 258: 11751–11757. [PubMed] [Google Scholar]
- 2.Tall A. 1995. Plasma lipid transfer proteins. Annu. Rev. Biochem. 64: 235–257. [DOI] [PubMed] [Google Scholar]
- 3.Barter P. J., Brewer H. B., Jr., Chapman M. J., Hennekens C. H., Rader D. J., Tall A. R. 2003. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 23: 160–167. [DOI] [PubMed] [Google Scholar]
- 4.Drayna D., Jarnagin A. S., McLean J., Henzel W., Kohr W., Fielding C., Lawn R. 1987. Cloning and sequencing of human cholesteryl ester transfer protein cDNA. Nature. 327: 632–634. [DOI] [PubMed] [Google Scholar]
- 5.Jiang X. C., Moulin P., Quinet E., Goldberg I. J., Yacoub L. K., Agellon L. B., Compton D., Schnitzer-Polokoff R., Tall A. R. 1991. Mammalian adipose tissue and muscle are major sources of lipid transfer protein mRNA. J. Biol. Chem. 266: 4631–4639. [PubMed] [Google Scholar]
- 6.Radeau T., Robb M., Lau P., Borthwick J., McPherson R. 1998. Relationship of adipose tissue cholesteryl ester transfer protein (CETP) mRNA to plasma concentrations of CETP in man. Atherosclerosis. 139: 369–376. [DOI] [PubMed] [Google Scholar]
- 7.Quinet E. M., Huerta P., Nancoo D., Tall A. R., Marcel Y. L., McPherson R. 1993. Adipose tissue cholesteryl ester transfer protein mRNA in response to probucol treatment: cholesterol and species dependence. J. Lipid Res. 34: 845–852. [PubMed] [Google Scholar]
- 8.Inazu A., Quinet E. M., Wang S., Brown M. L., Stevenson S., Barr M. L., Moulin P., Tall A. R. 1992. Alternative splicing of the mRNA encoding the human cholesteryl ester transfer protein. Biochemistry. 31: 2352–2358. [DOI] [PubMed] [Google Scholar]
- 9.Yang T. P., Agellon L. B., Walsh A., Breslow J. L., Tall A. R. 1996. Alternative splicing of the human cholesteryl ester transfer protein gene in transgenic mice. Exon exclusion modulates gene expression in response to dietary or developmental change. J. Biol. Chem. 271: 12603–12609. [DOI] [PubMed] [Google Scholar]
- 10.Izem L., Morton R. E. 2007. Possible role for intracellular cholesteryl ester transfer protein in adipocyte lipid metabolism and storage. J. Biol. Chem. 282: 21856–21865. [DOI] [PubMed] [Google Scholar]
- 11.Izem L., Greene D. G., Bialkowska K., Morton R. E. 2015. Overexpression of full-length cholesteryl ester transfer protein in SW872 cells reduces lipid accumulation. J. Lipid Res. 56: 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quinn C. M., Kagedal K., Terman A., Stroikin U., Brunk U. T., Jessup W., Garner B. 2004. Induction of fibroblast apolipoprotein E expression during apoptosis, starvation-induced growth arrest and mitosis. Biochem. J. 378: 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shu X., Chan J., Ryan R. O., Forte T. M. 2007. Apolipoprotein A-V association with intracellular lipid droplets. J. Lipid Res. 48: 1445–1450. [DOI] [PubMed] [Google Scholar]
- 14.Lamant M., Smih F., Harmancey R., Philip-Cauderc P., Pathak A., Roncalli J., Galinier M., Collet X., Massabuau P., Senard J-M., et al. 2006. ApoO, a novel apolipoprotein, is an original glycoprotein up-regulated by diabetes in human heart. J. Biol. Chem. 281: 36289–36302. [DOI] [PubMed] [Google Scholar]
- 15.Gauthier A., Lau P., Zha X., Milne R., McPherson R. 2005. Cholesteryl ester transfer protein directly mediates selective uptake of high density lipoprotein cholesteryl esters by the liver. Arterioscler. Thromb. Vasc. Biol. 25: 2177–2184. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z., Yamashita S., Hirano K., Nakagawa-Toyama Y., Matsuyama A., Nishida M., Sakai N., Fukasawa M., Arai H., Miyagawa J., et al. 2001. Expression of cholesteryl ester transfer protein in human atherosclerotic lesions and its implication in reverse cholesterol transport. Atherosclerosis. 159: 67–75. [DOI] [PubMed] [Google Scholar]
- 17.Sawada S., Sugano M., Makino N., Okamoto H., Tsuchida K. 1999. Secretion of preβ HDL increases with the suppression of cholesteryl ester transfer protein in Hep G2 cells. Atherosclerosis. 146: 291–298. [DOI] [PubMed] [Google Scholar]
- 18.Huang Z., Inazu A., Kawashiri M. A., Nohara A., Higashikata T., Mabuchi H. 2003. Dual effects on HDL metabolism by cholesteryl ester transfer protein inhibition in HepG2 cells. Am. J. Physiol. Endocrinol. Metab. 284: E1210–E1219. [DOI] [PubMed] [Google Scholar]
- 19.Izem L., Morton R. E. 2001. Cholesteryl ester transfer protein biosynthesis and cellular cholesterol homeostasis are tightly interconnected. J. Biol. Chem. 276: 26534–26541. [DOI] [PubMed] [Google Scholar]
- 20.Zhou H., Li Z., Hojjati M. R., Jang D., Beyer T. P., Cao G., Tall A. R., Jiang X. C. 2006. Adipose tissue-specific CETP expression in mice: impact on plasma lipoprotein metabolism. J. Lipid Res. 47: 2011–2019. [DOI] [PubMed] [Google Scholar]
- 21.Salerno A. G., Silva T. R., Amaral M. E., Alberici L. C., Bonfleur M. L., Patricio P. R., Francesconi E. P., Grassi-Kassisse D. M., Vercesi A. M., Boschero A. C., et al. 2007. Overexpression of apolipoprotein CIII increases and CETP reverses diet-induced obesity in transgenic mice. Int. J. Obes. (Lond). 31: 1586–1595. [DOI] [PubMed] [Google Scholar]
- 22.Terán-García M., Després J. P., Tremblay A., Bouchard C. 2008. Effects of cholesterol ester transfer protein (CETP) gene on adipocity in response to long-term overfeeding. Atherosclerosis. 196: 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clevidence B. A., Morton R. E., West G., Dusek D. M., Hoff H. F. 1984. Cholesterol esterification in macrophages: stimulation by lipoproteins containing apo B isolated from human aortas. Arteriosclerosis. 4: 196–207. [DOI] [PubMed] [Google Scholar]
- 24.Havel R. J., Eder H. A., Bragdon J. H. 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34: 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 26.Thompson J. N., Erdody P., Brien R., Murray T. K. 1971. Fluorometric determination of vitamin A in human blood and liver. Biochem. Med. 5: 67–89. [DOI] [PubMed] [Google Scholar]
- 27.Bozidis P., Williamson C. D., Colberg-Poley A. M. 2007. Isolation of endoplasmic reticulum, mitochondria, and mitochondia-associated membrane fractions from transfected cells and from human cytomagolovius-infected primary fibroblasts. Curr. Protoc. Cell Biol. Chapter 3: 3.27. [DOI] [PubMed] [Google Scholar]
- 28.Ganji S. H., Tavintharan S., Zhu D., Xing Y., Kamanna V. S., Kashyap M. L. 2004. Niacin noncompetitively inhibits DGAT2 but not DGAT1 activity in HepG2 cells. J. Lipid Res. 45: 1835–1845. [DOI] [PubMed] [Google Scholar]
- 29.Hajjar D. P., Minick C. R., Fowler S. 1983. Arterial neutral cholesteryl esterase: A hormone-sensitive enzyme distinct from lysosomal cholesteryl esterase. J. Biol. Chem. 258: 192–198. [PubMed] [Google Scholar]
- 30.Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta deltaC(T)) method. Methods. 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 31.Morton R. E., Zilversmit D. B. 1981. A plasma inhibitor of triglyceride and cholesteryl ester transfer activities. J. Biol. Chem. 256: 11992–11995. [PubMed] [Google Scholar]
- 32.Brunner J., Skrabal P., Hauser H. 1976. Single bilayer vesicles prepared without sonication. Physico-chemical properties. Biochim. Biophys. Acta. 455: 322–331. [DOI] [PubMed] [Google Scholar]
- 33.Morton R. E., Steinbrunner J. V. 1990. Concentration of neutral lipids in the phospholipid surface of substrate particles determines lipid transfer protein activity. J. Lipid Res. 31: 1559–1567. [PubMed] [Google Scholar]
- 34.Morton R. E., Zilversmit D. B. 1982. Purification and characterization of lipid transfer protein(s) from human lipoprotein-deficient plasma. J. Lipid Res. 23: 1058–1067. [PubMed] [Google Scholar]
- 35.Peterson G. L. 1977. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 83: 346–356. [DOI] [PubMed] [Google Scholar]
- 36.Bartlett G. R. 1959. Phosphorus assay in column chromatography. J. Biol. Chem. 234: 466–468. [PubMed] [Google Scholar]
- 37.Wolins N. E., Quaynor B. K., Skinner J. R., Schoenfish M. J., Tzekov A., Bickel P. E. 2005. S3-12, adipophilin, and TIP47 package lipid in adipocytes. J. Biol. Chem. 280: 19146–19155. [DOI] [PubMed] [Google Scholar]
- 38.Wu Z., Xie Y., Morrison R. F., Bucher N. L. R., Farmer S. R. 1998. PPARgamma induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPalpha during the conversion of 3T3 fibroblasts into adipocytes. J. Clin. Invest. 101: 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schröder M. 2008. Endoplasmic reticulum stress responses. Cell. Mol. Life Sci. 65: 862–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basseri S., Austin R. C. 2012. Endoplasmic reticulum stress and lipid metabolism: mechanisms and therapeutic potential. Biochem. Res. Int. 2012: 841362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nye C. K., Hanson R. W., Kalhan S. C. 2008. Glyceroneogenesis is the dominant pathway for triglyceride glycerol synthesis in vivo in the rat. J. Biol. Chem. 283: 27565–27574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nye C., Kim J., Kalhan S. C., Hanson R. W. 2008. Reassessing triglyceride synthesis in adipose tissue. Trends Endocrinol. Metab. 19: 356–361. [DOI] [PubMed] [Google Scholar]
- 43.Gómez-Valadés A. G., Méndez-Lucas A., Vidal-Alabró A., Blasco F. X., Chillon M., Bartrons R., Bermúdez J., Perales J. C. 2008. Pck1 gene silencing in the liver improves glycemia control, insulin sensitivity, and dyslipidemia in db/db mice. Diabetes. 57: 2199–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuerschner L., Moessinger C., Thiel C. 2008. Imaging of lipid biosynthesis: how a neutral lipid enters lipid droplets. Traffic. 9: 338–352. [DOI] [PubMed] [Google Scholar]
- 45.Kotake H., Agellon L. B., Yokoyama S. 1997. Modification of the N-terminal cysteine of plasma cholesteryl ester transfer protein selectively inhibits triglyceride transfer activity. Biochim. Biophys. Acta. 1347: 69–74. [DOI] [PubMed] [Google Scholar]
- 46.Gregoire F. M., Smas C. M., Sul H. S. 1998. Understanding adipocyte differentiation. Physiol. Rev. 78: 783–809. [DOI] [PubMed] [Google Scholar]
- 47.Gregoire F. M. 2001. Adipocyte differentiation: From fibroblast to endocrine cell. Exp. Biol. Med. (Maywood). 226: 997–1002. [DOI] [PubMed] [Google Scholar]
- 48.Radeau T., Robb M., McDonnell M., McPherson R. 1998. Preferential expression of cholesteryl ester transfer protein mRNA by stromal-vascular cells of human adipose tissue. Biochim. Biophys. Acta. 1392: 245–253. [DOI] [PubMed] [Google Scholar]
- 49.Gauthier B., Robb M., McPherson R. 1999. Cholesteryl ester transfer protein gene expression during differentiation of human preadipocytes to adipocytes in primary culture. Atherosclerosis. 142: 301–307. [DOI] [PubMed] [Google Scholar]
- 50.Radeau T., Lau P., Robb M., McDonnell M., Ailhaud G., McPherson R. 1995. Cholesteryl ester transfer protein (CETP) mRNA abundance in human adipose tissue: Relationship to cell size and membrane cholesterol content. J. Lipid Res. 36: 2552–2561. [PubMed] [Google Scholar]
- 51.Ellong E. N., Soni K. G., Bui Q-T., Sougrat R., Golinelli-Cohen M-P., Jackson C. L. 2011. Interaction between the triglyceride lipase ATGL and the Arf1 activator GBF1. PLoS One. 6: e21889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lass A., Zimmerman R., Haemmerle G., Riederer M., Schoiswohl G., Schweiger M., Kienesberger P., Strauss J. G., Gorkiewicz G., Zechner R. 2006. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman syndrome. Cell Metab. 3: 309–319. [DOI] [PubMed] [Google Scholar]
- 53.Wei E., Gao W., Lehner R. 2007. Attenuation of adipocyte triacylglycerol hydrolase activity decreases basal fatty acid efflux. J. Biol. Chem. 282: 8027–8035. [DOI] [PubMed] [Google Scholar]
- 54.McFie P. J., Banman S. L., Kary S., Stone S. J. 2011. Murine diacylglycerol acyltransferase-2 (DGAT2) can catalyze triacylglycerol synthesis and promote lipid droplet formation independent of its localization to the endoplasmic reticulum. J. Biol. Chem. 286: 28235–28246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morton R. E. 1988. Free cholesterol is a potent regulator of lipid transfer protein function. J. Biol. Chem. 263: 12235–12241. [PubMed] [Google Scholar]
- 56.Zilversmit D. B., Morton R. E., Hughes L. B., Thompson K. H. 1982. Exchange of retinyl and cholesteryl esters between lipoproteins of rabbit plasma. Biochim. Biophys. Acta. 712: 88–93. [DOI] [PubMed] [Google Scholar]
- 57.Kwong M., Wasan K. M. 2002. Cholesteryl ester transfer protein facilitates the movement of water-insoluble drugs between lipoproteins: a novel biological function for a well-characterized lipid transfer protein. Biochem. Pharmacol. 64: 1669–1675. [DOI] [PubMed] [Google Scholar]
- 58.Hogarth C. A., Roy A., Ebert D. L. 2003. Genomic evidence for the absence of a functional cholesteryl ester transfer protein gene in mice and rats. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 135: 219–229. [DOI] [PubMed] [Google Scholar]
- 59.Paar M., Jüngst C., Steiner N. A., Magnes C., Sinner F., Kolb D., Lass A., Zimmerman R., Zumbusch A., Kohlwein S. D., et al. 2012. Remodeling of lipid droplets during lipolysis and growth in adipocytes. J. Biol. Chem. 287: 11164–11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carmel J-F., Tarnus E., Cohn J. S., Bourdon E., Davignon J., Bernier L. 2009. High expression of apolipoprotein E impairs lipid storage and promotes cell proliferation in human adipocytes. J. Cell. Biochem. 106: 608–617. [DOI] [PubMed] [Google Scholar]
- 61.Huang Z. H., Reardon C. A., Mazzone T. 2006. Endogenous apoE expression modulates adipocyte triglyceride content and turnover. Diabetes. 55: 3394–3402. [DOI] [PubMed] [Google Scholar]
- 62.Huang Z. H., Gu D., Mazzone T. 2009. Role of adipocyte-derived apoE for modulating adipocyte size, lipid metabolism, and gene expression in vivo. Am. J. Physiol. Endocrinol. Metab. 296: E1110–E1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mora S., Pessin J. E. 2002. An adipocentric view of signaling and intracellular trafficking. Diabetes Metab. Res. Rev. 18: 345–356. [DOI] [PubMed] [Google Scholar]
- 64.Walczak R., Tontonoz P. 2002. PPARadimes and PPARadoxes: expanding roles for PPARγ in the control of lipid metabolism. J. Lipid Res. 43: 177–186. [PubMed] [Google Scholar]