Abstract
We previously reported that cholesterol-enriched macrophages excrete cholesterol into the extracellular matrix. A monoclonal antibody that detects cholesterol microdomains labels the deposited extracellular particles. Macrophage deposition of extracellular cholesterol depends, in part, on ABCG1, and this cholesterol can be mobilized by HDL components of the reverse cholesterol transport process. The objective of the current study was to determine whether ABCA1 also contributes to macrophage deposition of extracellular cholesterol. ABCA1 functioned in extracellular cholesterol deposition. The liver X receptor agonist, TO901317 (TO9), an ABCA1-inducing factor, restored cholesterol deposition that was absent in cholesterol-enriched ABCG1−/− mouse macrophages. In addition, the ABCA1 inhibitor, probucol, blocked the increment in cholesterol deposited by TO9-treated wild-type macrophages, and completely inhibited deposition from TO9-treated ABCG1−/− macrophages. Lastly, ABCA1−/− macrophages deposited much less extracellular cholesterol than wild-type macrophages. These findings demonstrate a novel function of ABCA1 in contributing to macrophage export of cholesterol into the extracellular matrix.
Keywords: atherosclerosis, apolipoprotein A-I, high density lipoprotein, probucol, TO901317, ATP binding cassette transporter A1, ATP binding cassette transporter G1
Reverse cholesterol transport defines the processes by which cholesterol deposited in tissues is mobilized from these tissues and returned to the liver for either excretion or reutilization (1, 2). Key to this process within atherosclerotic plaques is the scavenging of excess cholesterol by macrophages that take up and store this cholesterol until it can be mobilized from these cells by components of the HDL system. In this process, either preformed plasma-derived HDL or HDL generated by cells such as macrophages within atherosclerotic plaques, function as acceptors of excess cellular cholesterol. Excess cellular cholesterol is believed to be transferred to HDL from either intracellular or plasma membrane pools of cholesterol.
ABCA1 and ABCG1 both function in mediating cellular cholesterol efflux and contributing to reverse cholesterol transport (3–5). ABCA1 mediates phospholipidation of ApoA-I, which then functions as an HDL cholesterol acceptor, stimulating cholesterol efflux. In contrast, ABCG1 mediates cellular cholesterol efflux to preformed HDL. HDL binds poorly to ABCA1 and ABCG1; nevertheless, both proteins mediate transfer of cholesterol from cells to HDL (6–10). While ABCA1 can mediate cholesterol efflux by generating nascent HDL from the simultaneous efflux of phospholipid and cholesterol, ABCA1 can also efflux cholesterol to preformed mature HDL (11). The possible molecular mechanisms for these cholesterol transfers have been recently reviewed (4). Hypothetical cholesterol microdomains are featured in some of the models of cellular cholesterol efflux.
Besides cellular cholesterol efflux to HDL, under certain conditions, cells release cholesterol in the form of spherical microparticles. Microparticles containing cholesterol and phospholipid are released into culture medium by cAMP-treated J774 mouse macrophages (12, 13), cholesterol-enriched human monocyte-derived macrophages (14), and BHK cells following increased expression of ABCA1 (12). All these microparticles lack HDL-associated apolipoproteins and do not appear to be precursors to HDL.
Previously, we described an extracellular matrix-associated pool of cholesterol that functions in delivering cholesterol to HDL for potential reverse cholesterol transport (15–17). In those studies, we employed a unique monoclonal antibody (MAb 58B1) that labels cholesterol microdomains formed when cholesterol reaches high concentrations within membranes. While the MAb labels cholesterol crystals and cholesterol monolayers, it does not label individual cholesterol molecules (18–22). Thus, the antibody recognizes a structural motif presented by an ordered array of cholesterol molecules. Such ordered arrays of cholesterol, in the form of cholesterol crystalline microdomains, have been demonstrated with small-angle X-ray diffraction in biological membranes, including the membranes of cells isolated from atherosclerotic plaques (23, 24).
With this anti-cholesterol microdomain antibody, we have shown that cultured cholesterol-enriched macrophages excrete spherical particles containing cholesterol microdomains into the extracellular matrix (17). Similar extracellular spherical particles labeled by the anti-cholesterol microdomain antibody are found in human atherosclerotic lesions (17). These extracellular particles showing cholesterol microdomains may function as an extracellular storage form of cholesterol. Macrophage deposition of cholesterol into the extracellular matrix could help maintain cholesterol homeostasis when macrophages accumulate excess cholesterol beyond that which can be stored in intracellular lipid droplets and cell membranes.
We previously reported that ABCG1 contributes to macrophage generation of these extracellular particles showing cholesterol microdomains (15). In the current work, we show that ABCA1 also contributes to generation of these extracellular deposited cholesterol microdomains. The generation of cholesterol microdomains may facilitate ABCA1- and ABCG1-mediated transport of cholesterol to HDL.
MATERIALS AND METHODS
Materials
RPMI-1640 was obtained from Mediatech (Herndon, VA); FBS and Dulbecco’s phosphate-buffered saline (DPBS) with Ca2+ and Mg2+ were obtained from Invitrogen (Carlsbad, CA); 12-well CellBIND plates were obtained from Corning (Corning, NY); T-75 CELLSTAR® tissue culture flasks were obtained from Greiner Bio One (Monroe, NC); mouse macrophage colony-stimulating factor was obtained from PeproTech (Rocky Hill, NJ); acetylated LDL (AcLDL) was obtained from Intracel (Frederick, MD); TO901317 (TO9) was obtained from Cayman Chemical (La Jolla, CA); glycerol-gelatin mounting media, probucol, penicillin-streptomycin, and L-glutamine were obtained from Sigma (St. Louis, MO); mouse anti-cholesterol microdomain MAb 58B1 IgM in ascites was produced as previously described (18); mouse anti-Clavibacter michiganense MAb (clone 9A1) IgM in ascites was obtained from Agdia (Elkhart, IN); paraformaldehyde was obtained from Polysciences (Warrington, PA); biotinylated goat anti-mouse IgM and Vectashield hard set mounting medium with 4′-6-diamidino-2-phenylindole (DAPI) were obtained from Vector Laboratories (Burlingame, CA); and streptavidin Alexa Fluor 488 conjugate and 0.25% trypsin-EDTA solution were obtained from Invitrogen (Grand Island, NY).
Culture of mouse bone marrow-derived macrophages
Male ABCG1−/− mice on a C57BL/6J background were generated as described previously (25). Female ABCA1−/− mice were generated from DBA/1-Abca1tm1Jdm/J mice (#003897) obtained from Jackson Laboratory (Bar Harbor, ME). These mice were of mixed genetic background. The ABCA1 mutation was transferred to a C57BL/6N background by 10 consecutive crossings with C57BL/6N. Wild-type C57BL/6 control mice were substrain-, sex- and age-matched to ABCG1−/− and ABCA1−/− mice. Animal studies were conducted in conformity with the Public Health Service Policy on Humane Care and Use of Laboratory Animals, and were approved by the National Heart, Lung, and Blood Institute Institutional Animal Care and Use Committee.
For culture of bone marrow-derived macrophages, femurs and tibias were isolated from mice and muscle was removed. Both ends of the bones were cut with scissors and then flushed with 5 ml of RMPI-1640 with a 25 gauge needle. Bone marrow cells were centrifuged and resuspended at a concentration of 4–6 × 106 cells/ml in 1 ml of freezing medium containing 90% FBS and 10% DMSO (26). Cells were stored in liquid nitrogen until use.
On the day of use, cells were thawed and suspended in 30 ml RPMI-1640 medium containing 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 2 mM L-glutamine before centrifugation to remove DMSO. Then, cells were resuspended at a concentration of 1 × 105 cells/ml RPMI-1640 medium containing 100 U/ml penicillin, 0.1 mg/ml streptomycin, 2 mM L-glutamine, 10% FBS, and 50 ng/ml macrophage colony-stimulating factor (complete medium). Cells were seeded in a 75 cm2 culture flask and incubated in a 37°C cell culture incubator with 5% CO2/95% air. On day 3, cultures were rinsed three times with RPMI-1640 medium containing 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 2 mM L-glutamine, and then cultured in fresh complete medium. Medium was changed every 2 days until sufficient macrophages had grown in the flask, which usually occurred by the seventh day. Next, experiments were initiated by harvesting macrophages at room temperature with 10 ml 0.25% trypsin-EDTA solution. After about 20–30 min, macrophages rounded, but mostly remained attached. Trypsinization was stopped by addition of 10 ml RMPI-1640 containing 100 U/ml penicillin, 0.1 mg/ml streptomycin, 2 mM L-glutamine, and 10% FBS. A cell lifter was used to retrieve macrophages from the culture surface. The cell suspension was centrifuged at 300 g for 5 min and the resulting cell pellet was resuspended in 1 ml complete medium. Macrophages were counted with a hemocytometer. 0.6 × 105 macrophages per milliliter were cultured in 12-well CellBIND culture plates containing 1.5 ml of complete medium per well. Macrophages were incubated overnight before experiments were initiated with complete medium and the indicated additions, but without FBS. Experimental incubations were carried out for 4 days with the medium and additions refreshed after 2 days.
Immunostaining of macrophages
Fixation, immunostaining, and microscopy were all performed with macrophages in their original CellBIND culture plates, and all steps were carried out at room temperature. Macrophage cultures were rinsed three times (5 min each rinse this and all subsequent times) in DPBS, fixed for 10 min with 4% paraformaldehyde in DPBS, and then rinsed an additional three times in DPBS. Macrophages were then incubated 60 min with 5 μg/ml purified mouse anti-cholesterol microdomain MAb 58B1 IgM diluted in DPBS containing 0.1% BSA. Control staining was performed with 5 μg/ml of an irrelevant purified mouse anti-Clavibacter michiganense MAb (clone 9A1) IgM diluted in DPBS containing 0.1% BSA. MAb IgM fractions were purified as previously described (16). Cultures were then rinsed three times in DPBS, followed by a 30 min incubation in 5 μg/ml biotinylated goat anti-mouse IgM diluted in DPBS containing 0.1% BSA. After three rinses in DPBS, cultures were incubated 10 min with 10 μg/ml streptavidin Alexa Fluor 488 diluted in DPBS. Cultures were then rinsed three times with DPBS and mounted in Vectashield hard-set mounting medium with DAPI nuclear stain in preparation for digital imaging using an Olympus IX81 fluorescence microscope. Because macrophages were not permeabilized, MAb 58B1 staining represents cell surface or extracellular staining. No staining was observed when the control MAb was substituted for the anti-cholesterol microdomain MAb.
Microscopic analysis
Cells were identified using phase-contrast microscopy, or by locating DAPI-stained nuclei. The pattern and intensity of MAb 58B1 staining were then analyzed for cultures from each experimental parameter, and these data were compared with one another. We considered MAb 58B1 labeling cellular if it was located within cell membrane boundaries, as identified on the corresponding phase-contrast view. Labeling was considered extracellular if it was located outside the cell membrane boundaries seen on phase-contrast view. Different planes of focus were visualized before acquiring images to confirm that only a monolayer of cells was present, thereby ensuring that labeling seen outside cell membrane boundaries did not represent cellular labeling from cells lying in a different plane of focus. As we reported before (15), MAb 58B1 labeling of mouse macrophage cultures showed extracellular rather than plasma membrane staining. The immunostained cells shown in the figures are representative of a minimum of five microscopic fields viewed in one culture well.
Quantification and statistical analysis of MAb 58B1 immunofluorescence
For each condition shown in the figures, including additional control images where macrophages were incubated without AcLDL, we quantified MAb 58B1 immunofluorescence in three separate digital images using Image J software (version 1.37) developed by the National Institutes of Health. Control image fluorescence values were subtracted from non-control image fluorescence values. Statistical analysis of the obtained fluorescence data was carried out with SigmaPlot for Windows (version 11.0). One-way ANOVA using the Holm-Sidak method was employed for comparisons of three groups (Figs. 1–3), and the unpaired t-test was used for comparison of two groups (Figs. 4–6). P ≤ 0.05 was considered significant.
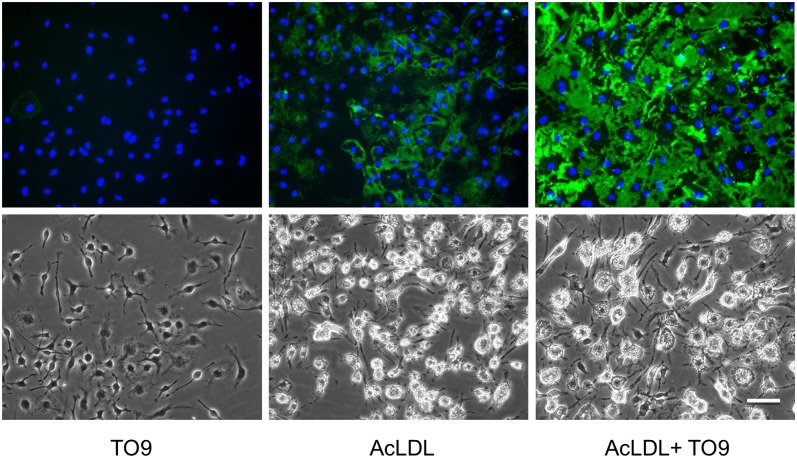
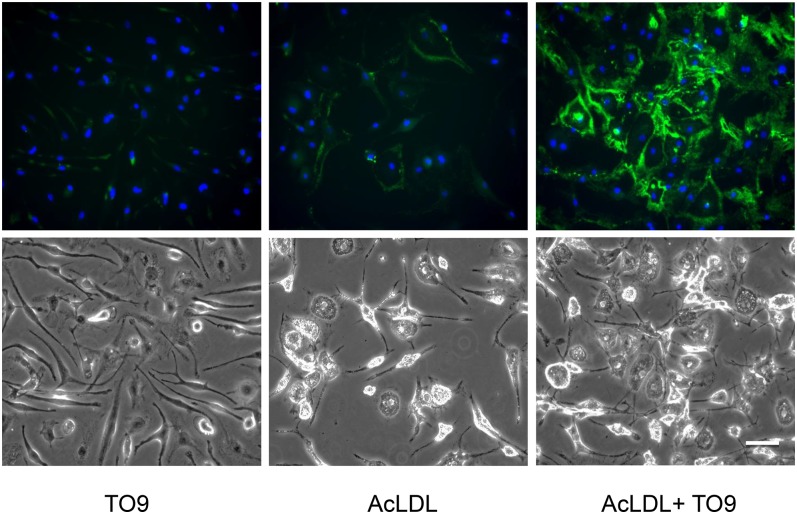
Fig. 1.
Cholesterol-enriched wild-type macrophages deposit extracellular cholesterol without TO9, but deposition is increased with TO9. Wild-type mouse bone marrow-derived macrophages were incubated for 4 days with either TO9 (5 μM), AcLDL (50 μg/ml), or AcLDL + TO9 before cultures were immunostained with anti-cholesterol microdomain MAb 58B1 (green fluorescence) and DAPI nuclear stain (blue fluorescence). Upper and lower rows are, respectively, the fluorescence and phase photomicrographs. Scale bar = 50 μm and applies to all.
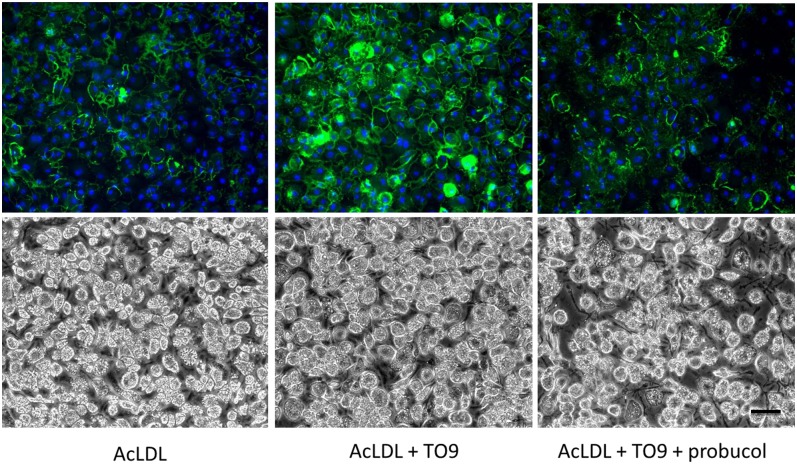
Fig. 3.
Inhibiting ABCA1 with probucol in cholesterol-enriched wild-type macrophages blocks TO9-stimulated cholesterol deposition. Wild-type mouse bone marrow-derived macrophages were incubated for 4 days with either AcLDL (50 μg/ml), AcLDL + TO9 (5 μM), or AcLDL + TO9 + probucol (10 μM) before cultures were immunostained with anti-cholesterol microdomain MAb 58B1 (green fluorescence) and DAPI nuclear stain (blue fluorescence). Upper and lower rows are, respectively, the fluorescence and phase photomicrographs. Scale bar = 50 μm and applies to all.
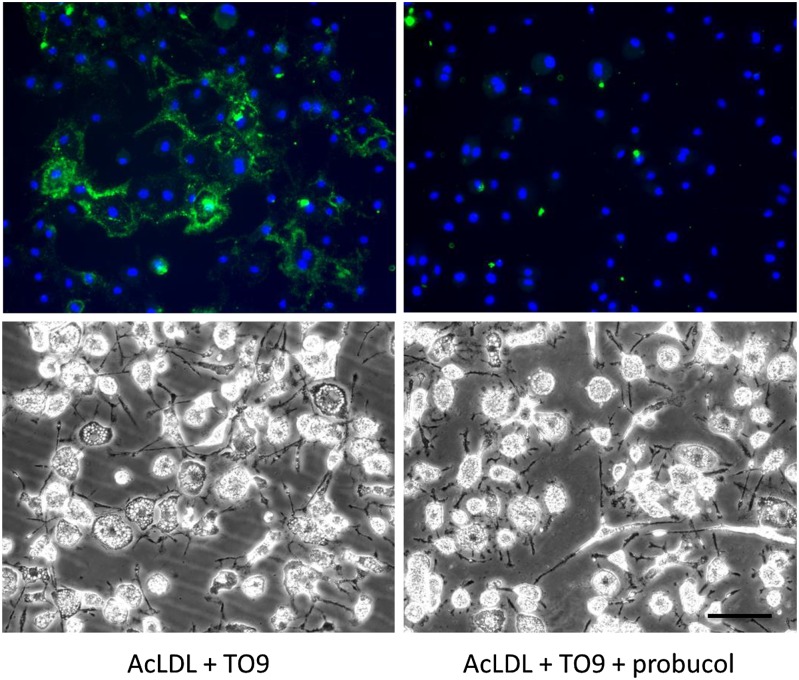
Fig. 4.
Inhibiting ABCA1 with probucol in cholesterol-enriched ABCG1−/− macrophages blocks TO9-stimulated cholesterol deposition. ABCG1−/− mouse bone marrow-derived macrophages were incubated for 4 days with either AcLDL (50 μg/ml) + TO9 (5 μM) or AcLDL + TO9 + probucol (10 μM) before cultures were immunostained with anti-cholesterol microdomain MAb 58B1 (green fluorescence) and DAPI nuclear stain (blue fluorescence). Upper and lower rows are, respectively, the fluorescence and phase photomicrographs. Scale bar = 50 μm and applies to all.
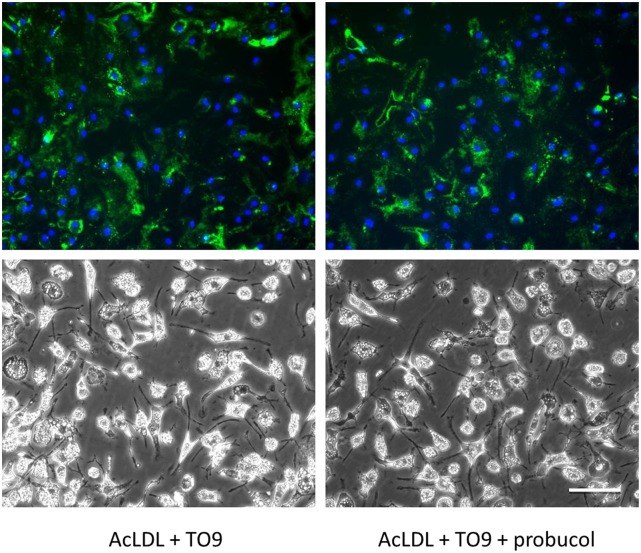
Fig. 6.
Probucol does not block cholesterol deposition in TO9-treated cholesterol-enriched ABCA1−/− macrophages. ABCA1−/− mouse bone marrow-derived macrophages were incubated for 4 days with AcLDL (50 μg/ml) + TO9 (5 μM) without or with probucol (10 μM) before cultures were immunostained with anti-cholesterol microdomain MAb 58B1 (green fluorescence) and DAPI nuclear stain (blue fluorescence). Upper and lower rows are, respectively, the fluorescence and phase photomicrographs. Scale bar = 50 μm and applies to all.
RESULTS
In an earlier study, we observed that cholesterol-enriched ABCG1−/− mouse bone marrow-derived macrophages, in contrast to cholesterol-enriched wild-type mouse bone marrow-derived macrophages, excreted very little cholesterol into the extracellular matrix (15). This suggested that ABCG1 mediated the extracellular cholesterol deposition process in the mouse. However, probucol inhibited cholesterol-enriched human monocyte-derived macrophage deposition of extracellular cholesterol (15). Because probucol inhibits ABCA1 (27, 28), this suggested the possibility that besides ABCG1, ABCA1 may also contribute to macrophage deposition of extracellular cholesterol. Although cholesterol-enriched wild-type mouse bone marrow-derived macrophages showed extracellular cholesterol deposition (AcLDL treatment in Fig. 1), the liver X receptor agonist, TO9, increased this extracellular cholesterol deposition 3.5-fold, as estimated from quantification of the MAb 58B1 immunofluorescence (AcLDL + TO9 treatment in Fig. 1).
Cholesterol-enriched ABCG1−/− mouse macrophages (AcLDL treatment in Fig. 2) showed very little extracellular cholesterol deposition, as we reported previously (15). However, when stimulated with TO9 (AcLDL + TO9 treatment in Fig. 2), these macrophages then deposited cholesterol into the extracellular matrix (7.6-fold more MAb 58B1 immunofluorescence compared with macrophages incubated with AcLDL without TO9). Given that TO9 is known to upregulate ABCA1 expression (29), that probucol inhibits human macrophage extracellular cholesterol deposition (15), and that TO9 stimulates extracellular cholesterol deposition by ABCG1−/− mouse macrophages (Fig. 2), we considered the possibility that with TO9 treatment, ABCA1, in addition to ABCG1, would contribute to macrophage extracellular cholesterol deposition.
Fig. 2.
In contrast to cholesterol-enriched wild-type macrophages, cholesterol-enriched ABCG1−/− macrophages deposit little cholesterol unless treated with TO9. ABCG1−/− mouse bone marrow-derived macrophages were incubated for 4 days with either TO9 (5 μM), AcLDL (50 μg/ml), or AcLDL + TO9 before cultures were immunostained with anti-cholesterol microdomain MAb 58B1 (green fluorescence) and DAPI nuclear stain (blue fluorescence). Upper and lower rows are, respectively, the fluorescence and phase photomicrographs. Scale bar = 50 μm and applies to all.
If ABCA1 contributes to extracellular cholesterol deposition by TO9-treated cholesterol-enriched mouse macrophages, then probucol should inhibit the component of cholesterol deposition stimulated by TO9, as probucol inhibits ABCA1 but does not inhibit ABCG1 (27, 28, 30). We tested this by incubating cholesterol-enriched wild-type mouse macrophages with TO9 in the presence and absence of probucol. We observed that TO9 increased MAb 58B1 immunofluorescence 2.5-fold compared with macrophages incubated with AcLDL alone (Fig. 3). However, when probucol was added to AcLDL + TO9, there was no significant difference in MAb 58B1 immunofluorescence compared with macrophages treated with AcLDL alone. Thus, probucol blocked the increment of macrophage extracellular cholesterol deposition that was stimulated by TO9, consistent with ABCA1 mediating a portion of this macrophage cholesterol deposition.
We further tested the function of ABCA1 in macrophage cholesterol deposition by incubating TO9-treated cholesterol-enriched ABCG1−/− macrophages with probucol. Given that ABCG1 would not be contributing to cholesterol deposition in these macrophages, we expected that probucol should completely block macrophage cholesterol deposition through its inhibition of ABCA1. That is what we observed, in that probucol blocked the TO9-stimulated cholesterol deposition that occurred with cholesterol-enriched ABCG1−/− macrophages (Fig. 4). Quantified MAb 58B1 immunofluorescence levels for macrophages incubated with AcLDL + TO9 + probucol were similar to macrophages incubated with AcLDL (not shown).
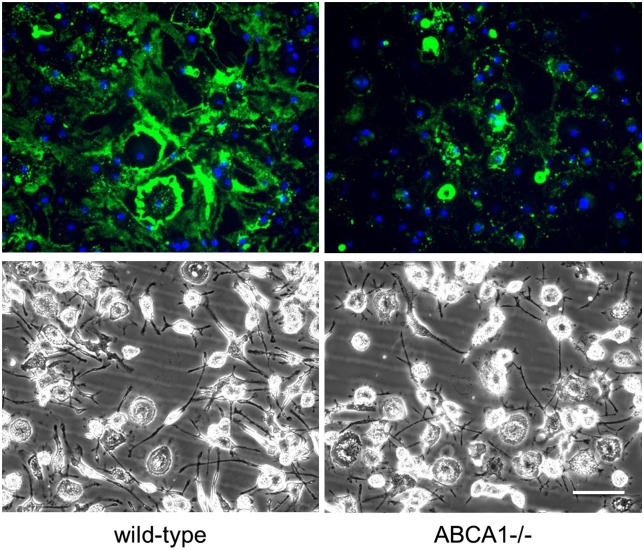
Next, we directly confirmed that ABCA1 functioned in macrophage cholesterol deposition. Cholesterol deposition was partially reduced in TO9-treated cholesterol-enriched ABCA1−/− macrophages compared with TO9-treated cholesterol-enriched wild-type macrophages (Fig. 5). MAb 58B1 immunofluorescence levels of ABCA1−/− macrophages were 34 ± 2% of wild-type macrophages. This partial reduction would be expected in TO9-treated cholesterol-enriched ABCA1−/− macrophages if both ABCA1 and ABCG1 were contributing to macrophage cholesterol deposition by TO9-treated cholesterol-enriched wild-type macrophages. This is because TO9 stimulation of ABCA1 could not occur with the ABCA1−/− macrophages.
Fig. 5.
Cholesterol-enriched ABCA1−/− mouse macrophages show less extracellular cholesterol deposition than cholesterol-enriched wild-type macrophages. Wild-type and ABCA1−/− mouse bone marrow-derived macrophages were incubated for 4 days with AcLDL (50 μg/ml) + TO9 (5 μM) before cultures were immunostained with anti-cholesterol microdomain MAb 58B1 (green fluorescence) and DAPI nuclear stain (blue fluorescence). Upper and lower rows are, respectively, the fluorescence and phase photomicrographs. Scale bar = 50 μm and applies to all. This experiment was repeated two additional times with similar results.
Lastly, we tested the effect of probucol on cholesterol deposition by TO9-treated cholesterol-enriched ABCA1−/− macrophages. If probucol’s effect of inhibiting macrophage cholesterol deposition was mediated by ABCA1, then we would expect no effect of probucol on macrophage cholesterol deposition by these macrophages. Indeed, that is what we observed (Fig. 6). There was no quantitative difference between MAb 58B1 immunofluorescence of macrophages incubated with AcLDL + TO9 and macrophages incubated with AcLDL + TO9 + probucol.
DISCUSSION
While in some studies ABCA1 mediates cholesterol efflux to mature HDL as well as nascent HDL (11, 31, and references contained therein), this cholesterol efflux has been attributed to ApoA-I that dissociates from the mature HDL and then interacts with ABCA1 generating nascent HDL. In this scenario, nascent HDL functions as the true cholesterol acceptor (32). Recently, this point of view has been challenged based on no evidence for dissociation of ApoA-I from HDL3b particles, a very efficient acceptor of cholesterol effluxed by ABCA1 (31). Previously we reported that ABCG1 mediates macrophage deposition of cholesterol into the extracellular matrix (15). Our new finding that ABCA1 as well as ABCG1 mediate macrophage deposition of cholesterol into the extracellular matrix can explain how ABCA1 and ABCG1 both mediate cholesterol efflux to mature HDL: by mature HDL mobilizing extracellular cholesterol, which can occur even in the absence of macrophages (17).
Although both ABCA1 and ABCG1 mediated deposition of cholesterol into the extracellular matrix, they functioned independently. Absence of one or the other did not eliminate cholesterol deposition by TO9-treated cholesterol-enriched macrophages. A block in cholesterol deposition would be expected if the two proteins were functioning in a sequential fashion. Rather, elimination of either protein partially decreased the extent of cholesterol deposition compared with that occurring with TO9-treated cholesterol-enriched wild-type macrophages. Thus, there was an additive effect of ABCA1 and ABCG1 in mediating macrophage cholesterol deposition. Similarly, ABCA1 and ABCG1 produce an additive effect in their mediation of reverse cholesterol transport in vivo (3).
ABCA1 and ABCG1 induction of cholesterol microdomains that label with MAb 58B1 in the plasma membrane of human macrophages and the extracellular matrix surrounding human and mouse macrophages may occur through enrichment of the plasma membrane with cholesterol (8, 33). Cholesterol microdomains form in both model and cell membranes when these membranes are enriched with cholesterol (23, 32–35). This is due to lateral phase separation of cholesterol within the membrane as certain critical membrane cholesterol concentrations are reached. We previously showed that SU6656, a Src kinase inhibitor, causes human macrophage cholesterol microdomains to accumulate in association with the plasma membrane rather than deposit into the extracellular matrix (17). Thus, there could be a two-step process in which ABCA1 and ABCG1 mediate transport of cholesterol to the plasma membrane, and then some other process mediates shedding of these microdomains into the extracellular matrix. In support of an independently regulated two-step process, we have observed that cholesterol-enrichment of fibroblasts induces plasma membrane-associated cholesterol microdomains that do not shed (16). Furthermore, cholesterol enrichment of human macrophages grown on certain substrates also blocks the shedding process of plasma membrane cholesterol microdomains detected with MAb 58B1 (unpublished observation).
The cholesterol microdomains we detect here in the extracellular matrix could be related to previously observed plasma membrane-associated structures that form with cholesterol enrichment of cells or increased expression of cellular ABCA1. Upregulation of ABCA1 in fibroblasts and macrophages induces the formation of ApoA-I binding to plasma membrane-associated (≤200 nm diameter generally spherical) structures (34, 35). Possibly also related to the extracellular lipid particles that we have observed are the lipid-containing binding sites for ApoA-I that underlie cultured J774 mouse and THP-1 human macrophages (36).
Without liver X receptor stimulation of cholesterol efflux ABC transporters, ABCA1 mediates extracellular cholesterol deposition by human macrophages as deposition is eliminated by probucol, an ABCA1 inhibitor (17), while ABCG1 mediates extracellular cholesterol deposition by mouse macrophages (15). A similar difference in mouse and human macrophage efflux to HDL has been reported (11). ABCG1 predominantly mediates mouse macrophage cholesterol efflux to HDL, while ABCA1 predominantly mediates human macrophage cholesterol efflux to HDL. The low levels of ABCG1 compared with ABCA1 in cholesterol-enriched human monocyte-derived macrophages may contribute to this difference [see Supplemental Fig. IIIA in (11)].
In conclusion, we have shown that in addition to ABCG1, ABCA1 independently mediates deposition of cholesterol into the extracellular matrix by cholesterol-enriched macrophages. Our findings show a novel function for both ABCA1 and ABCG1 that results in excretion of cholesterol from the cell that is not mediated by formation of classical HDL cholesterol acceptor lipoproteins. While macrophage export of excess cholesterol into the extracellular matrix may be a protective cellular mechanism, if not mobilized through reverse cholesterol transport, buildup of this extracellular cholesterol possibly promotes atherosclerosis.
Acknowledgments
The authors thank the Department of Transfusion Medicine, Clinical Center, National Institutes of Health, for providing elutriated monocytes and Dr. Michael Fessler for providing the ABCG1−/− mice.
Footnotes
Abbreviations:
- AcLDL
- acetylated LDL
- DAPI
- 4′,6 diamidino-2-phenylindole
- DPBS
- Dulbecco’s phosphate-buffered saline
- MAb
- monoclonal antibody
- TO9
- TO901317
This work was supported by the Intramural Research Program, National Heart, Lung, and Blood Institute, National Institutes of Health, and by the Binational Science Foundation (Grant 2013045).
REFERENCES
- 1.Rosenson R. S., Brewer H. B., Jr, Davidson W. S., Fayad Z. A., Fuster V., Goldstein J., Hellerstein M., Jiang X. C., Phillips M. C., Rader D. J., et al. 2012. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 125: 1905–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher E. A., Feig J. E., Hewing B., Hazen S. L., Smith J. D. 2012. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 32: 2813–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X., Collins H. L., Ranalletta M., Fuki I. V., Billheimer J. T., Rothblat G. H., Tall A. R., Rader D. J. 2007. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 117: 2216–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips M. C. 2014. Molecular mechanisms of cellular cholesterol efflux. J. Biol. Chem. 289: 24020–24029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcel Y. L., Ouimet M., Wang M. D. 2008. Regulation of cholesterol efflux from macrophages. Curr. Opin. Lipidol. 19: 455–461. [DOI] [PubMed] [Google Scholar]
- 6.Wang N., Lan D., Chen W., Matsuura F., Tall A. R. 2004. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. USA. 101: 9774–9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura K., Kennedy M. A., Baldan A., Bojanic D. D., Lyons K., Edwards P. A. 2004. Expression and regulation of multiple murine ATP-binding cassette transporter G1 mRNAs/isoforms that stimulate cellular cholesterol efflux to high density lipoprotein. J. Biol. Chem. 279: 45980–45989. [DOI] [PubMed] [Google Scholar]
- 8.Vaughan A. M., Oram J. F. 2005. ABCG1 redistributes cell cholesterol to domains removable by high density lipoprotein but not by lipid-depleted apolipoproteins. J. Biol. Chem. 280: 30150–30157. [DOI] [PubMed] [Google Scholar]
- 9.Mulya A., Lee J. Y., Gebre A. K., Thomas M. J., Colvin P. L., Parks J. S. 2007. Minimal lipidation of pre-beta HDL by ABCA1 results in reduced ability to interact with ABCA1. Arterioscler. Thromb. Vasc. Biol. 27: 1828–1836. [DOI] [PubMed] [Google Scholar]
- 10.Sankaranarayanan S., Oram J. F., Asztalos B. F., Vaughan A. M., Lund-Katz S., Adorni M. P., Phillips M. C., Rothblat G. H. 2009. Effects of acceptor composition and mechanism of ABCG1-mediated cellular free cholesterol efflux. J. Lipid Res. 50: 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larrede S., Quinn C. M., Jessup W., Frisdal E., Olivier M., Hsieh V., Kim M. J., Van Eck M., Couvert P., Carrie A., et al. 2009. Stimulation of cholesterol efflux by LXR agonists in cholesterol-loaded human macrophages is ABCA1-dependent but ABCG1-independent. Arterioscler. Thromb. Vasc. Biol. 29: 1930–1936. [DOI] [PubMed] [Google Scholar]
- 12.Nandi S., Ma L., Denis M., Karwatsky J., Li Z., Jiang X. C., Zha X. 2009. ABCA1-mediated cholesterol efflux generates microparticles in addition to HDL through processes governed by membrane rigidity. J. Lipid Res. 50: 456–466. [DOI] [PubMed] [Google Scholar]
- 13.Duong P. T., Collins H. L., Nickel M., Lund-Katz S., Rothblat G. H., Phillips M. C. 2006. Characterization of nascent HDL particles and microparticles formed by ABCA1-mediated efflux of cellular lipids to apoA-I. J. Lipid Res. 47: 832–843. [DOI] [PubMed] [Google Scholar]
- 14.Kruth H. S., Skarlatos S. I., Gaynor P. M., Gamble W. 1994. Production of cholesterol-enriched nascent high density lipoproteins by human monocyte-derived macrophages is a mechanism that contributes to macrophage cholesterol efflux. J. Biol. Chem. 269: 24511–24518. [PubMed] [Google Scholar]
- 15.Freeman S. R., Jin X., Anzinger J. J., Xu Q., Purushothaman S., Fessler M. B., Addadi L., Kruth H. S. 2014. ABCG1-mediated generation of extracellular cholesterol microdomains. J. Lipid Res. 55: 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruth H. S., Ifrim I., Chang J., Addadi L., Perl-Treves D., Zhang W. Y. 2001. Monoclonal antibody detection of plasma membrane cholesterol microdomains responsive to cholesterol trafficking. J. Lipid Res. 42: 1492–1500. [PubMed] [Google Scholar]
- 17.Ong D. S., Anzinger J. J., Leyva F. J., Rubin N., Addadi L., Kruth H. S. 2010. Extracellular cholesterol-rich microdomains generated by human macrophages and their potential function in reverse cholesterol transport. J. Lipid Res. 51: 2303–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perl-Treves D., Kessler N., Izhaky D., Addadi L. 1996. Monoclonal antibody recognition of cholesterol monohydrate crystal faces. Chem. Biol. 3: 567–577. [DOI] [PubMed] [Google Scholar]
- 19.Kessler N., Perl-Treves D., Addadi L. 1996. Monoclonal antibodies that specifically recognize crystals of dinitrobenzene. FASEB J. 10: 1435–1442. [DOI] [PubMed] [Google Scholar]
- 20.Scheffer L., Futerman A. H., Addadi L. 2007. Antibody labeling of cholesterol/ceramide ordered domains in cell membranes. ChemBioChem. 8: 2286–2294. [DOI] [PubMed] [Google Scholar]
- 21.Ziblat R., Fargion I., Leiserowitz L., Addadi L. 2012. Spontaneous formation of two-dimensional and three-dimensional cholesterol crystals in single hydrated lipid bilayers. Biophys. J. 103: 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Addadi L., Geva M., Kruth H. S. 2003. Structural information about organized cholesterol domains from specific antibody recognition. Biochim. Biophys. Acta. 1610: 208–216. [DOI] [PubMed] [Google Scholar]
- 23.Preston Mason R., Tulenko T. N., Jacob R. F. 2003. Direct evidence for cholesterol crystalline domains in biological membranes: role in human pathobiology. Biochim. Biophys. Acta. 1610: 198–207. [DOI] [PubMed] [Google Scholar]
- 24.Tulenko T. N., Chen M., Mason P. E., Mason R. P. 1998. Physical effects of cholesterol on arterial smooth muscle membranes: evidence of immiscible cholesterol domains and alterations in bilayer width during atherogenesis. J. Lipid Res. 39: 947–956. [PubMed] [Google Scholar]
- 25.Kennedy M. A., Barrera G. C., Nakamura K., Baldan A., Tarr P., Fishbein M. C., Frank J., Francone O. L., Edwards P. A. 2005. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 1: 121–131. [DOI] [PubMed] [Google Scholar]
- 26.Marim F. M., Silveira T. N., Lima D. S., Jr, Zamboni D. S. 2010. A method for generation of bone marrow-derived macrophages from cryopreserved mouse bone marrow cells. PLoS One. 5: e15263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu C. A., Tsujita M., Hayashi M., Yokoyama S. 2004. Probucol inactivates ABCA1 in the plasma membrane with respect to its mediation of apolipoprotein binding and high density lipoprotein assembly and to its proteolytic degradation. J. Biol. Chem. 279: 30168–30174. [DOI] [PubMed] [Google Scholar]
- 28.Favari E., Zanotti I., Zimetti F., Ronda N., Bernini F., Rothblat G. H. 2004. Probucol inhibits ABCA1-mediated cellular lipid efflux. Arterioscler. Thromb. Vasc. Biol. 24: 2345–2350. [DOI] [PubMed] [Google Scholar]
- 29.Repa J. J., Turley S. D., Lobaccaro J. A., Medina J., Li L., Lustig K., Shan B., Heyman R. A., Dietschy J. M., Mangelsdorf D. J. 2000. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 289: 1524–1529. [DOI] [PubMed] [Google Scholar]
- 30.Stefulj J., Panzenboeck U., Becker T., Hirschmugl B., Schweinzer C., Lang I., Marsche G., Sadjak A., Lang U., Desoye G., et al. 2009. Human endothelial cells of the placental barrier efficiently deliver cholesterol to the fetal circulation via ABCA1 and ABCG1. Circ. Res. 104: 600–608. [DOI] [PubMed] [Google Scholar]
- 31.Du X. M., Kim M. J., Hou L., Le Goff W., Chapman M. J., Van Eck M., Curtiss L. K., Burnett J. R., Cartland S. P., Quinn C. M., et al. 2015. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ. Res. 116: 1133–1142. [DOI] [PubMed] [Google Scholar]
- 32.Okuhira K., Tsujita M., Yamauchi Y., Abe-Dohmae S., Kato K., Handa T., Yokoyama S. 2004. Potential involvement of dissociated apoA-I in the ABCA1-dependent cellular lipid release by HDL. J. Lipid Res. 45: 645–652. [DOI] [PubMed] [Google Scholar]
- 33.Vaughan A. M., Oram J. F. 2003. ABCA1 redistributes membrane cholesterol independent of apolipoprotein interactions. J. Lipid Res. 44: 1373–1380. [DOI] [PubMed] [Google Scholar]
- 34.Lin G., Oram J. F. 2000. Apolipoprotein binding to protruding membrane domains during removal of excess cellular cholesterol. Atherosclerosis. 149: 359–370. [DOI] [PubMed] [Google Scholar]
- 35.Vedhachalam C., Duong P. T., Nickel M., Nguyen D., Dhanasekaran P., Saito H., Rothblat G. H., Lund-Katz S., Phillips M. C. 2007. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J. Biol. Chem. 282: 25123–25130. [DOI] [PubMed] [Google Scholar]
- 36.Burgess J. W., Kiss R. S., Zheng H., Zachariah S., Marcel Y. L. 2002. Trypsin-sensitive and lipid-containing sites of the macrophage extracellular matrix bind apolipoprotein A-I and participate in ABCA1-dependent cholesterol efflux. J. Biol. Chem. 277: 31318–31326. [DOI] [PubMed] [Google Scholar]