Abstract
Dietary n-3 long-chain PUFAs (LC-PUFAs) are associated with improvement in the parameters of the metabolic syndrome (MetS). Glucokinase regulatory protein (GCKR) is a key protein regulating intracellular glucose disposal. Our aim was to investigate: i) the relationship between the GCKR rs1260326 (Pro446Leu) polymorphism and parameters of the MetS; and ii) a potential influence of n-3 and n-6 LC-PUFA levels on this relationship in the HELENA study (1,155 European adolescents). Linear regression analyses were performed to study the association between rs1260326 and the outcomes of interest. Interactions between rs1260326 and LC-PUFA levels on outcomes were explored. The T allele of rs1260326 was associated with higher serum TG concentrations compared with the C allele. In contrast to n-6 LC-PUFA levels, a significant interaction (P = 0.01) between rs1260326 and total n-3 LC-PUFA levels on serum TG concentrations was observed. After stratification on the n-3 LC-PUFA median values, the association between rs1260326 and TG concentration was significant only in the group with high n-3 LC-PUFA levels. In conclusion, this is the first evidence that n-3 LC-PUFAs may modulate the impact of the GCKR rs1260326 polymorphism on TG concentrations in adolescents. Several molecular mechanisms, in link with glucose uptake, could explain these findings.
Keywords: polyunsaturated fatty acid, glucokinase regulatory protein, lipid metabolism
Diet is one of the main factors involved in the modulation of serum TG concentration (1, 2). Interventions to increase the intake of n-3 long-chain PUFAs (LC-PUFAs) have been reported to improve several parameters of the metabolic syndrome (MetS), such as a decrease in the serum concentration of TG (2, 3) and insulin resistance [as measured by the homeostatic model assessment of insulin resistance (HOMA-IR)] (4) and an increase in the serum HDL-cholesterol concentration (3).
The Δ5-desaturase, encoded by the FA desaturase 1 (FADS1) gene, is required for the synthesis of LC-PUFAs in mammals (5). It has been shown that the minor allele of the FADS1 rs174546 SNP is associated with lower concentrations of HDL- and LDL-cholesterol and with higher TG concentrations in men and women of European ancestry (6).
In the liver and pancreatic islet cells, glucokinase regulatory protein (GCKR) is a key protein that binds to glucokinase (GCK) (7) and regulates intracellular glucose disposal. GCKR binds to GCK through hydrophobic interactions and inhibits GCK activity by blocking a small domain of the GCK. This inhibitory binding depends on the relative disposal of allosteric effectors: fructose-1-phosphate (weakens binding) and fructose-6-phosphate (tightens binding). Moreover, GCKR preserves the availability of GCK when glucose concentration is low. Indeed, during the fasting state, GCKR protects GCK from ubiquitin-dependent proteasomal degradation (8, 9) by maintaining GCK inside the nucleus (10), but also by readdressing GCK from cytosol to the nucleus (11) so that more GCKs are available when the rising intracytoplasmic glucose concentration (during the fed state) affords GCKR-GCK disconnection.
The minor allele of the GCKR rs1260326 (Pro446Leu) SNP has been shown to be associated with higher fasting serum TG concentration and lower glucose concentration in various populations (12–15). Teslovich et al. (6) confirmed, in a meta-analysis of 46 genome-wide association studies (GWASs) composed of more than 65,000 subjects of European ancestry (with and without coronary artery disease), a significant association between the minor allele of rs1260326 and higher circulating TG levels. In addition, in vitro experiments have shown that the Pro446Leu SNP is functional (16) and that the Leu446 allele is less sensitive to the regulation by fructose 6 phosphate esters than is the Pro446 allele (16). Consequently, GCKR is less able to maintain GCK inactive in the nucleus, resulting in an increase in GCK activity.
A recent study reported an interaction between the GCKR rs1260326 SNP and n-3 LC-PUFA concentration when considering insulin resistance parameters in subjects with MetS (17). Indeed, compared with individuals with the minor T allele, homozygous for the major C allele carriers with n-3 PUFA levels below the median showed higher plasma concentrations of fasting insulin, C-peptide, HOMA-IR, and C-reactive protein. There is no available information about an interaction between n-3 LC-PUFAs and rs1260326 in an adolescent sample. Indeed, it would be of interest to know whether the GCKR rs1260326 SNP is associated with other traits related to MetS, and whether n-3 LC-PUFA concentration could modulate this relationship.
The aims of this study were to investigate: i) the potential relationship between the GCKR rs1260326 and parameters of the MetS in European adolescents; and ii) the potential influence of n-3 and n-6 LC-PUFA concentration on this relationship.
RESEARCH DESIGN AND METHODS
Subjects
Data were derived from the Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) cross-sectional study, a multicenter study which aims to obtain reliable and comparable data on nutrition and health-related parameters in adolescents. A total of 3,528 adolescents (age range: 12–18 years) were assessed at school between 2006 and 2007 in 10 European cities from nine countries; all adolescents and schools fulfilling the general HELENA inclusion criteria (18) (Table 1). One-third of the classes were randomly selected for blood collection, producing a total of 1,155 blood samples (Table 1), including 552 boys and 603 girls (supplementary Table 1). Details of the sampling procedures and study design have been reported elsewhere (19). The ethics committee of each city involved has approved the study (20). A written informed consent was obtained from both the adolescents and their parents.
TABLE 1.
Clinical characteristics of subjects in the HELENA study
| Parameters | Whole Population | Blood Samples | P |
| N (n girls) | 3,528 (1845) | 1,155 (603) | — |
| Age (years) | 14.7 (1.2) | 14.7 (1.4) | 0.20 |
| BMI (kg/m2) | 21.3 (3.6) | 21.3 (3.8) | 0.52 |
| Waist (cm) | 71.9 (8.7) | 71.9 (8.3) | 0.61 |
| Height (cm) | 165.8 (9.1) | 165.0 (9.6) | 0.01 |
| Waist-to-height ratio | 0.43 (0.05) | 0.44 (0.05) | 0.34 |
| Fasting glucose (mmol/l) | — | 5.0 (0.4) | — |
| Fasting insulin (μIU/ml) | — | 10.26 (7.7) | — |
| HOMA-IR | — | 2.19 (1.5) | — |
| TGs (mmol/l) | — | 0.79 (0.4) | — |
| Cholesterol (mmol/l) | — | 4.16 (0.7) | — |
| HDL-cholesterol (mmol/l) | — | 1.43 (0.3) | — |
| LDL-cholesterol (mmol/l) | — | 2.45 (0.6) | — |
| Total n-3 LC-PUFA (%) | — | 3.57 (1.1) | — |
| ALA (%) | — | 0.14 (0.1) | — |
| EPA (%) | — | 0.49 (0.3) | — |
| DHA (%) | — | 2.93 (0.9) | — |
| Total n-6 LC-PUFA (%) | — | 31.71 (2.5) | — |
| LA (%) | — | 22.07 (2.6) | — |
| ARA (%) | — | 9.67 (1.6) | — |
Data are mean (SD).
Methods
The following anthropometric parameters were measured: height (Seca 225, precision 1 mm; SECA GmbH and Co., Hamburg, Germany), weight (Seca 861 electronic scale, precision 0.05 kg), and waist circumference (Seca 200 nonelastic tape, precision 0.1 cm). These measures were obtained under standardized conditions (21). BMI and waist-to-height ratio were calculated.
A detailed description of the blood analysis was reported elsewhere (22). Briefly, venous blood samples were drawn after a 10 h overnight fast and sent to a central laboratory (the Analytical Laboratory at the University of Bonn’s Institut für Ernährungs- und Lebensmittelwissenschaften). The serum concentrations of glucose, TG, HDL-cholesterol, and LDL-cholesterol were measured enzymatically using the Dimension RxL clinical chemistry system (Dade Behring, Newark, DE). Serum insulin concentration was measured using an Immunlite 200 analyzer (DPC Behring, Schwalbach, Germany). The HOMA-IR index was calculated and used as an indicator of insulin resistance (23).
After Folch extraction of serum samples, the phospholipid fraction was separated using thin-layer chromatography. The phospholipid band was scraped off, and the FAs were converted into their methyl esters by transesterification with methanol/hydrochloric acid. The phospholipid fraction FA methyl esters were analyzed using gas chromatography (Model 3900; Varian, Palo Alto, CA) on a 30 m × 0.25 mm × 0.25 μm polyethylene glycol column (Zebron ZB-WAXplus; Phenomenex, Macclesfield, UK). Peaks of interest were identified by comparison with authentic FA methyl ester standards (Sigma-Aldrich, St. Louis, MO).
LC-PUFA concentration was determined from the percentage area, which was calculated by integrating the area under the peak and dividing it by the total area for all FAs. Coefficients of variation were <4.4% for all FA analyses (calculated on 131 samples). Total n-3 and n-6 LC-PUFA concentrations were calculated respectively as the sum of the concentrations of α-linolenic acid (ALA), EPA, and DHA for n-3 LC-PUFAs, and as the sum of the concentrations of linoleic acid (LA) and arachidonic acid (ARA) for n-6 LC-PUFAs. The n-3/n-6 ratio was calculated as the ratio between the total n-3 LC-PUFA concentration (log-transformed) divided by the total n-6 LC-PUFA concentration. In the analysis of the influence of LC-PUFA concentrations on the relationship between rs1260326 and TG concentration, the LC-PUFAs were classified into two groups according to the population median concentration (equal to or greater than versus less than the median).
SNP genotyping
Blood for DNA extraction was collected into EDTA K3 tubes, stored at the central laboratory, and then sent to the Genomic Analysis Laboratory at the Institut Pasteur of Lille. DNA was extracted from white blood cells using a Puregene kit (Qiagen, Courtaboeuf, France) and stored at −20°C. The subjects were genotyped using Illumina GoldenGate technology (Illumina, San Diego, CA). The genotyping success rates of the GCKR rs1260326 and FADS1 rs174546 SNPs were 97.4 and 99.8%, respectively.
Statistical analyses
Data were analyzed using Statistical Analysis Systems software, version 9.1 (SAS Institute, Cary, NC). Associations with P ≤ 0.05 were considered significant. The results were expressed by the means and SDs for continuous variables, and by the frequencies and percentages for categorical variables. The impact of the GCKR rs1260326 and FADS1 rs174546 SNPs were analyzed using an additive model. Before analysis, the values for total n-3 LC-PUFA, ALA, EPA, DHA, TG, and HOMA-IR index were log-transformed to obtain normal distributions. Multivariable linear regression analyses were performed to analyze the associations between the GCKR SNP and outcomes of interest (anthropometric and biological variables) adjusted for sex, age, center, and BMI (for all outcomes except for anthropometric phenotypes). Additional searches for interaction between the GCKR SNP and LC-PUFAs were integrated in the previous models by including an additional SNP × LC-PUFA interaction term with dichotomized LC-PUFAs (equal to or greater than the median versus less than the median as reference level). Then, multivariable linear regressions were performed to analyze the associations between the TG concentrations and the GCKR SNP in each group of dichotomized LC-PUFA concentrations. Afterwards, these analyses were additionally adjusted for FADS1 SNP and for total n-3 and n-6 LC-PUFAs (respectively for n-6 LC-PUFA and for n-3 LC-PUFA variables). An additional analysis was performed to investigate the relationship between the TG concentrations and rs1260326 by considering rs1260326 as a categorical variable, with adjustment for sex, age, BMI, and center. The adjusted means were computed using the linear model and the linear trend between the means was tested.
RESULTS
The baseline characteristics of the genotyped participants from the HELENA study are summarized in Table 1 and supplementary Table 1. The genotype distribution of the rs1260326 SNP was 0.32, 0.47, and 0.21 for CC, CT, and TT, respectively, and the minor allele frequency was 0.45. This distribution fulfilled the Hardy-Weinberg equilibrium (χ2 = 3.08; P = 0.08).
We found no significant associations between rs1260326 and most components of the MetS (waist circumference, HOMA-IR index, and HDL-cholesterol concentration) nor with the other tested variables (BMI; waist-to-height ratio; serum concentrations of glucose, insulin, total cholesterol, and LDL-cholesterol; total n-3 and n-6 LC-PUFA concentrations; and ALA, EPA, DHA, LA, and ARA concentrations) (Table 2). In contrast, the rs1260326 T allele was associated with higher serum TG concentrations (β = 0.06 ± 0.02 mmol/l, P = 0.0012) (Table 2).
TABLE 2.
Impact of the GCKR rs1260326 polymorphism on anthropometric and biochemical parameters
| Parameters | n | CC | CT | TT | β | SD | P | SNP × Total n-3 PUFA Interaction P | SNP × Total n-6 PUFA Interaction P |
| BMI (kg/m2) | 1,115 | 21.28 | 21.23 | 21.62 | −0.07 | 0.15 | 0.65 | 0.62 | 0.26 |
| Waist circumference (cm) | 1,015 | 72.79 | 72 | 72.84 | −0.35 | 0.36 | 0.32 | 0.31 | 0.44 |
| Waist-to-height (cm/cm) | 1,015 | 0.44 | 0.44 | 0.44 | −0.001 | 0.002 | 0.67 | 0.53 | 0.33 |
| Glucose (mmol/l)a | 1,111 | 4.99 | 5.02 | 4.98 | −0.02 | 0.02 | 0.27 | 0.39 | 0.85 |
| Insulin (μIU/ml)a | 1,078 | 10.67 | 9.98 | 10.24 | −0.02 | 0.31 | 0.42 | 0.84 | 0.59 |
| HOMA-IRa | 900 | 2.25 | 2.12 | 2.27 | −0.01 | 0.06 | 0.70 | 0.53 | 0.46 |
| TGs (mmol/l)a | 1,111 | 0.77 | 0.77 | 0.85 | 0.06 | 0.02 | 0.0012 | 0.01 | 0.28 |
| Cholesterol (mmol/l)a | 1,111 | 4.18 | 4.14 | 4.17 | 0.003 | 0.91 | 0.91 | 0.22 | 0.88 |
| HDL-C (mmol/l)a | 1,111 | 1.43 | 1.43 | 1.43 | 2 × 10−5 | 0.01 | 0.99 | 0.48 | 0.85 |
| LDL-C (mmol/l)a | 1,111 | 2.47 | 2.43 | 2.45 | −0.01 | 0.03 | 0.65 | 0.42 | 0.84 |
| Total n-3/n-6 ratioa | 982 | 0.04 | 0.04 | 0.04 | −10−4 | 4 × 10−4 | 0.77 | — | — |
| Total n-3 LC-PUFA (%)a | 990 | 3.65 | 3.57 | 3.41 | −0.01 | 0.01 | 0.32 | — | 0.79 |
| ALA (%)a | 1,003 | 0.15 | 0.14 | 0.12 | −0.01 | 0.02 | 0.40 | — | 0.67 |
| EPA (%)a | 1,000 | 0.52 | 0.49 | 0.45 | 0.004 | 0.02 | 0.83 | — | 0.63 |
| DHA (%)a | 1,002 | 2.98 | 2.94 | 2.84 | −0.01 | 0.01 | 0.41 | — | 0.76 |
| Total n-6 LC-PUFA (%)a | 1,001 | 31.68 | 31.67 | 31.8 | −0.1 | 0.09 | 0.26 | 0.75 | — |
| LA (%)a | 1,009 | 22.11 | 22.07 | 22 | −0.1 | 0.11 | 0.32 | 0.89 | — |
| ARA (%)a | 1,001 | 9.62 | 9.63 | 9.8 | −0.02 | 0.07 | 0.80 | 0.59 | — |
The mean values expressed in the table are the observed means. The β coefficients represent the effect sizes of the minor T allele. SD is the standard deviation of coefficients. Analyses were performed on variables adjusted for sex, age, and center. HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol.
Further adjusted for BMI.
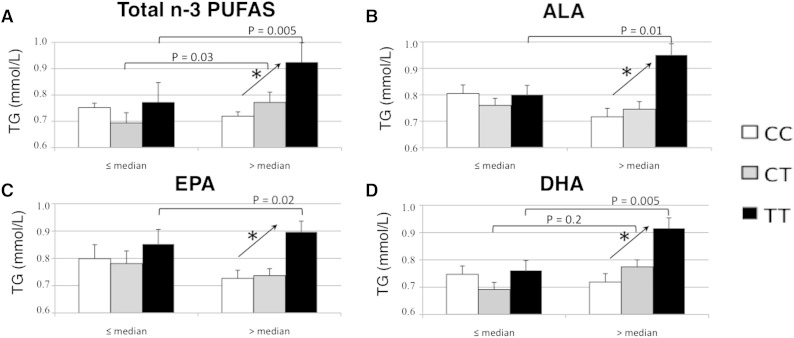
We tested for possible interactions between rs1260326 and LC-PUFA (n-3 or n-6) when considering anthropometric and biochemical parameters of the MetS. For statistical analyses, LC-PUFAs were split into two groups according to their median values (expressed as a percentages of the area of all FAs), which were 0.1% for ALA, 0.4% for EPA, 2.8% for DHA, and 3.3% for total n-3 LC-PUFAs, 22.0% for LA, 9.6% for ARA, and 31.6% for total n-6 LC-PUFAs. There was a significant interaction between n-3 LC-PUFA and rs1260326 and TG concentrations (P = 0.01) (Table 2). The association between rs1260326 and TG concentration was significant in the group with high n-3 LC-PUFA concentration (β = 0.11 ± 0.03, P = 1.6 × 10−5), but not in the group with low n-3 LC-PUFA concentration (Table 3). Adjustment for total n-6 LC-PUFA concentrations brought the same results, with significant association between rs1260326 and TG concentration in the group with high n-3 LC-PUFA concentration (P = 2 × 10−5), but not in the group with low n-3 LC-PUFA concentration. Separate n-3 LC-PUFA analysis revealed the same pattern for ALA, EPA, and DHA concentrations (Table 3, Fig. 1). Indeed, there were significant interactions between ALA or EPA or DHA and rs1260326 and TG concentrations (P = 0.04, P = 0.05, and P = 0.01, respectively) (Table 3). Compared with the C allele, the T allele of rs1260326 was associated with significantly higher TG concentrations in the subjects presenting with ALA, EPA, and DHA concentrations greater than the median value (P = 9.9 × 10−5, P = 0.0002, and P = 1.4 × 10−5, respectively) (Table 3, Fig. 1). Further adjustment for n-6 LC-PUFAs or for physical activity level did not alter these results (Table 3).
TABLE 3.
Relationship between rs1260326 and fasting TG levels by serum PUFA levels in the HELENA study
| PUFA | SNP × PUFA Interaction P | ≤Median PUFA | >Median PUFA | ||||||||||||||||
| CC | CT | TT | β | SD | P | Pa | Pb | Pc | CC | CT | TT | β | SD | P | Pa | Pb | Pc | ||
| Total n-3 PUFAs | 0.01 | 0.80 ± 0.45 | 0.72 ± 0.33 | 0.81 ± 0.41 | 0.01 | 0.03 | 0.67 | 0.67 | 0.84 | 0.59 | 0.74 ± 0.37 | 0.79 ± 0.34 | 0.93 ± 0.55 | 0.11 | 0.03 | 1.6 × 10−5 | 1.6 × 10−5 | 2 × 10−5 | 5 × 10−4 |
| n | 159 | 230 | 108 | 161 | 235 | 99 | |||||||||||||
| ALA | 0.04 | 0.80 ± 0.48 | 0.74 ± 0.35 | 0.78 ± 0.32 | 0.02 | 0.03 | 0.44 | 0.47 | 0.52 | 0.70 | 0.75 ± 0.35 | 0.77 ± 0.33 | 0.99 ± 0.63 | 0.10 | 0.03 | 9.9 ×10−5 | 1 × 10−4 | 1 × 10−4 | 1.2 × 10−3 |
| n | 142 | 236 | 125 | 183 | 234 | 85 | |||||||||||||
| EPA | 0.05 | 0.80 ± 0.43 | 0.76 ± 0.35 | 0.84 ± 0.51 | 0.02 | 0.03 | 0.39 | 0.38 | 0.52 | 0.45 | 0.75 ± 0.40 | 0.75 ± 0.33 | 0.90 ± 0.43 | 0.10 | 0.03 | 2 × 10−4 | 2 × 10−4 | 4 × 10−4 | 5 × 10−4 |
| n | 147 | 235 | 122 | 177 | 233 | 90 | |||||||||||||
| DHA | 0.01 | 0.80 ± 0.45 | 0.72 ± 0.33 | 0.81 ± 0.42 | 0.01 | 0.03 | 0.77 | 0.77 | 0.93 | 0.65 | 0.75 ± 0.37 | 0.79 ± 0.35 | 0.92 ± 0.54 | 0.11 | 0.02 | 1.4 × 10−5 | 1.5 × 10−5 | 2.8 × 10−5 | 1.7 × 10−3 |
| n | 162 | 231 | 110 | 163 | 237 | 101 | |||||||||||||
The mean values expressed in the table are the observed means. The β coefficients represent the effect sizes of the minor T allele (additive model). Tests of interaction PUFA × rs1260326 were performed using the medians of PUFAs as a cut-off. Analyses were performed on variables adjusted for age, sex, center, and BMI.
Analyses were performed on variables adjusted for age, sex, center, BMI, and FADS1 rs174546 SNP.
Analyses were performed on variables adjusted for age, sex, center, BMI, and total n-6 LC-PUFAs.
Analyses were performed on variables adjusted for age, sex, center, BMI, and physical activity level (less than or greater than or equal to 60 min per day of moderate to vigorous activity, measured by an actimeter).
Fig. 1.
TG concentrations according to rs1260326 and n-3 LC-PUFAs in the HELENA study. *P trend <0.05; rs1260326 is considered as categorical variable. Mean and P value analyses were performed on variables adjusted for sex, age, BMI, center.
Because the FADS1 rs174546 SNP is known to modulate LC-PUFA concentrations (24–26), we also adjusted all the above associations for the FADS1 rs174546 SNP, but this did not alter the results (Table 3).
In the HELENA study, fasting TG concentrations did not correlate with n-3 LC-PUFA levels, but did correlate with n-6 LC-PUFA levels, especially with LA (r2 = −0.20, P < 0.0001) (supplementary Table 2).
DISCUSSION
Among the tested parameters of the MetS, the serum concentration of TGs was the only one to be significantly associated with the GCKR rs1260326 SNP. Our study showed, for the first time, significant n-3 LC-PUFA × GCKR interactions on serum TG concentration in adolescents. This finding suggests that n-3 LC-PUFA levels modulate the association between GCKR and TG levels.
The T allele was associated with higher TG concentrations only in individuals with a high n-3 LC-PUFA concentration compared with the C allele. Further adjustment of the data obtained with n-3 LC-PUFAs for total n-6 LC-PUFAs did not alter the results, suggesting that this association was independent from n-6 LC-PUFA concentrations.
Our study is the first to investigate the influence of n-3 and n-6 LC-PUFAs on the relationship between rs1260326 and TG concentrations in an unselected population and in adolescents. Perez-Martinez et al. (17) have shown in the LIPGENE cohort (n = 379 adults with MetS) that homozygous subjects for the major allele with n-3 PUFA levels below the median had higher plasma concentrations of fasting insulin, C-peptide, HOMA-IR, and C-reactive protein, as compared with individuals with the minor allele. In contrast to our findings, Perez-Martinez et al. (17) did not report any significant interaction between rs1260326 and n-3 LC-PUFA concerning serum TG concentrations. In our study, there was no interaction between the GCKR rs1260326 polymorphism and plasma n-3 PUFA levels in the modulation of insulin resistance markers. This could be explained by the characteristics of our population, which consisted of young and healthy subjects who were therefore not insulin resistant.
Another study investigated potential interactions between rs1260326 and the major food items characterizing the Mediterranean diet in relation to TG concentration, but found no significant results (27). This study included people aged above 55 years with high cardiovascular risk, and was based on general Mediterranean diet patterns with no specific focus on n-3 LC-PUFAs.
A recent meta-analysis of 21 GWASs showed that the major allele of the GCKR rs780094 SNP was associated with an increase in fasting glucose and insulin levels, and with a higher HOMA-IR index (12). In nondiabetic Asian adults and adolescents, the minor allele of rs1260326 was associated with a lower HOMA-IR index (14).
The reason for this discrepancy with our results is not known, but it could be explained by the divergence of genetic backgrounds or lifestyles. We also cannot exclude a lack of power of our study to detect significant association between rs1260326 and HOMA-IR index.
A major finding of our study is the modulation of the association between rs1260326 and serum fasting TG concentration by n-3 LC-PUFA concentration. Our data suggest that both precursors and final metabolites of n-3 LC-PUFAs can modulate the observed association between the GCKR rs1260326 SNP and serum TG concentrations. Several molecular mechanisms could explain our findings; they are based on the increase in glucose uptake related to the GCKR SNP. An increase in intracellular glucose concentration would overflow several cellular regulatory mechanisms, including those of n-3 LC-PUFA. One possible mechanism involves an increase in the expression and activity of FAS induced by the increased glucose concentration (28). FAS activation is the first step in TG production. Hepatic GCK mRNA expression is positively associated with lipogenic gene expression [FAS, acetyl-CoA carboxylase (ACC)] and with the de novo lipogenesis index (29). A GWAS showed that the GCKR rs780093 SNP [of which the minor allele is in strong linkage disequilibrium with the minor allele of rs1260326 (30)] is associated with increased de novo lipogenesis (31). LC-PUFAs [mainly DHA (32)] may act by downregulating FAS activation both directly (33) and by inhibiting sterol regulatory element-binding protein (34, 35) and carbohydrate responsive element-binding protein. Another possible mechanism is that n-3 LC-PUFAs inhibit ACC (36), whereas the increase in glucose concentration competes to activate ACC (37). With ACC activation, malonyl-CoA production would lead to inhibition of carnitine palmitoyltransferase 1 and β-oxidation (38), and thus to an increase in TG production as the oxidative pathway is downregulated.
Our study has several strengths. First, it includes a large sample of phenotyped healthy European adolescents. Second, we have also adjusted our analysis for the FADS1 rs174546 SNP. FADS1, encoding the Δ5-desaturase, is involved in LC-PUFA synthesis, and the FADS1 rs174546 SNP, by modulating the Δ5 FA desaturase activity, is associated with serum LC-PUFA and fasting TG concentrations (6, 39). Adjustment of the GCKR data for the FADS1 SNP did not change the results, suggesting that the effects of the GCKR and FADS1 SNPs are independent from each other. Third, we used the serum LC-PUFA levels as an objective surrogate of dietary FA intake (40, 41). Indeed FA dietary intake can be misreported by questionnaires (42) and FAs have then to be converted into LC-PUFAs according to food-tables, which can translate into unreliable measures of dietary FA intake. Plasma or serum phospholipid FAs (like in the HELENA study) and cholesterol ester FAs can reflect intake of several PUFAs over the past few days or more (42–44), as well as endogenous FA turnover. Thus, circulating FAs are better markers of biological effects than the dietary FA intakes could be.
Our study also has some limitations. First, the cross-sectional design of the study only allows us to report associations, without demonstrating causality. Second, the fact that we studied a population where TG levels remained within the normal range prevents us from extending our results to subjects with hypertriglyceridemia.
In conclusion, our study provides the first evidence that n-3 LC-PUFA levels modulate the association between GCKR variants and TG levels in healthy adolescents. These elements should be considered in future studies on lipid metabolism.
Supplementary Material
Footnotes
Abbreviations:
- ACC
- acetyl-CoA carboxylase
- ALA
- α-linolenic acid
- ARA
- arachidonic acid
- FADS1
- FA desaturase 1
- GCK
- glucokinase
- GCKR
- glucokinase regulatory protein
- GWAS
- genome-wide association study
- HELENA
- Healthy Lifestyle in Europe by Nutrition in Adolescents
- HOMA-IR
- homeostatic model assessment of insulin resistance
- LA
- linoleic acid
- LC-PUFA
- long-chain PUFA
- MetS
- metabolic syndrome
The HELENA study has taken place with the Financial support of the European Community Sixth RTD Framework Programme (Contract FOOD-CT-2005-007034). The content of this article reflects only the authors’ views and the European Community is not liable for any use that may be made of the information contained therein. The authors declare no financial conflicts of interest.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Funtikova A., Baena-Diez J. M., Koebnick C., Gomez S. F., Covas M. I., Goday A., Schroder H. 2012. Validity of a short diet-quality index to predict changes in anthropometric and cardiovascular risk factors: a simulation study. Eur. J. Clin. Nutr. 66: 1369–1371. [DOI] [PubMed] [Google Scholar]
- 2.Lopez-Huertas E. 2012. The effect of EPA and DHA on metabolic syndrome patients: a systematic review of randomised controlled trials. Br. J. Nutr. 107(Suppl 2): S185–S194. [DOI] [PubMed] [Google Scholar]
- 3.Balk E. M., Lichtenstein A. H., Chung M., Kupelnick B., Chew P., Lau J. 2006. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis. 189: 19–30. [DOI] [PubMed] [Google Scholar]
- 4.Akinkuolie A. O., Ngwa J. S., Meigs J. B., Djousse L. 2011. Omega-3 polyunsaturated fatty acid and insulin sensitivity: a meta-analysis of randomized controlled trials. Clin. Nutr. 30: 702–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura M. T., Nara T. Y. 2004. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu. Rev. Nutr. 24: 345–376. [DOI] [PubMed] [Google Scholar]
- 6.Teslovich T. M., Musunuru K., Smith A. V., Edmondson A. C., Stylianou I. M., Koseki M., Pirruccello J. P., Ripatti S., Chasman D. I., Willer C. J., et al. 2010. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 466: 707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rees M. G., Wincovitch S., Schultz J., Waterstradt R., Beer N. L., Baltrusch S., Collins F. S., Gloyn A. L. 2012. Cellular characterisation of the GCKR P446L variant associated with type 2 diabetes risk. Diabetologia. 55: 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjørkhaug L., Molnes J., Søvik O., Njølstad P. R., Flatmark T. 2007. Allosteric activation of human glucokinase by free polyubiquitin chains and its ubiquitin-dependent cotranslational proteasomal degradation. J. Biol. Chem. 282: 22757–22764. [DOI] [PubMed] [Google Scholar]
- 9.Farrelly D., Brown K. S., Tieman A., Ren J., Lira S. A., Hagan D., Gregg R., Mookhtiar K. A., Hariharan N. 1999. Mice mutant for glucokinase regulatory protein exhibit decreased liver glucokinase: a sequestration mechanism in metabolic regulation. Proc. Natl. Acad. Sci. USA. 96: 14511–14516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Iglesia N., Veiga-da-Cunha M., Van Schaftingen E., Guinovart J. J., Ferrer J. C. 1999. Glucokinase regulatory protein is essential for the proper subcellular localisation of liver glucokinase. FEBS Lett. 456: 332–338. [DOI] [PubMed] [Google Scholar]
- 11.Shiota C., Coffey J., Grimsby J., Grippo J. F., Magnuson M. A. 1999. Nuclear import of hepatic glucokinase depends upon glucokinase regulatory protein, whereas export is due to a nuclear export signal sequence in glucokinase. J. Biol. Chem. 274: 37125–37130. [DOI] [PubMed] [Google Scholar]
- 12.Dupuis J., Langenberg C., Prokopenko I., Saxena R., Soranzo N., Jackson A. U., Wheeler E., Glazer N. L., Bouatia-Naji N., Gloyn A. L., et al. 2010. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat. Genet. 42: 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingelsson E., Langenberg C., Hivert M. F., Prokopenko I., Lyssenko V., Dupuis J., Magi R., Sharp S., Jackson A. U., Assimes T. L., et al. 2010. Detailed physiologic characterization reveals diverse mechanisms for novel genetic Loci regulating glucose and insulin metabolism in humans. Diabetes. 59: 1266–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Y., Wu L., Xi B., Liu X., Zhao X., Cheng H., Hou D., Wang X., Mi J. 2013. GCKR variants increase triglycerides while protecting from insulin resistance in Chinese children. PLoS One. 8: e55350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orho-Melander M., Melander O., Guiducci C., Perez-Martinez P., Corella D., Roos C., Tewhey R., Rieder M. J., Hall J., Abecasis G., et al. 2008. Common missense variant in the glucokinase regulatory protein gene is associated with increased plasma triglyceride and C-reactive protein but lower fasting glucose concentrations. Diabetes. 57: 3112–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beer N. L., Tribble N. D., McCulloch L. J., Roos C., Johnson P. R., Orho-Melander M., Gloyn A. L. 2009. The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Hum. Mol. Genet. 18: 4081–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez-Martinez P., Delgado-Lista J., Garcia-Rios A., Mc Monagle J., Gulseth H. L., Ordovas J. M., Shaw D. I., Karlstrom B., Kiec-Wilk B., Blaak E. E., et al. 2011. Glucokinase regulatory protein genetic variant interacts with omega-3 PUFA to influence insulin resistance and inflammation in metabolic syndrome. PLoS One. 6: e20555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreno L. A., De Henauw S., Gonzalez-Gross M., Kersting M., Molnar D., Gottrand F., Barrios L., Sjostrom M., Manios Y., Gilbert C. C., et al. 2008. Design and implementation of the Healthy Lifestyle in Europe by Nutrition in Adolescence Cross-Sectional Study. Int. J. Obes. (Lond). 32(Suppl 5): S4–S11. [DOI] [PubMed] [Google Scholar]
- 19.Moreno L. A., Gonzalez-Gross M., Kersting M., Molnar D., de Henauw S., Beghin L., Sjostrom M., Hagstromer M., Manios Y., Gilbert C. C., et al. 2008. Assessing, understanding and modifying nutritional status, eating habits and physical activity in European adolescents: the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) study. Public Health Nutr. 11: 288–299. [DOI] [PubMed] [Google Scholar]
- 20.Béghin L., Castera M., Manios Y., Gilbert C. C., Kersting M., De Henauw S., Kafatos A., Gottrand F., Molnar D., Sjöström M., et al. 2008. Quality assurance of ethical issues and regulatory aspects relating to good clinical practices in the HELENA cross-sectional study. Int. J. Obes. (Lond). 32(Suppl 5): S12–S18. [DOI] [PubMed] [Google Scholar]
- 21.Lohman T. G., Roche A. F., Martorell R. 1988. Anthropometric Standardization Reference Manual. Human Kinetics, Champaign, IL. [Google Scholar]
- 22.González-Gross M., Breidenassel C., Gómez-Martínez S., Ferrari M., Béghin L., Spinneker A., Díaz L. E., Maiani G., Demailly A., Al-Tahan J., et al. 2008. Sampling and processing of fresh blood samples within a European multicenter nutritional study: evaluation of biomarker stability during transport and storage. Int. J. Obes. (Lond). 32(Suppl 5): S66–S75. [DOI] [PubMed] [Google Scholar]
- 23.Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., Turner R. C. 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 24.Kettunen J., Tukiainen T., Sarin A. P., Ortega-Alonso A., Tikkanen E., Lyytikainen L. P., Kangas A. J., Soininen P., Wurtz P., Silander K., et al. 2012. Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat. Genet. 44: 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bokor S., Dumont J., Spinneker A., Gonzalez-Gross M., Nova E., Widhalm K., Moschonis G., Stehle P., Amouyel P., De Henauw S., et al. 2010. Single nucleotide polymorphisms in the FADS gene cluster are associated with delta-5 and delta-6 desaturase activities estimated by serum fatty acid ratios. J. Lipid Res. 51: 2325–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dumont J., Huybrechts I., Spinneker A., Gottrand F., Grammatikaki E., Bevilacqua N., Vyncke K., Widhalm K., Kafatos A., Molnar D., et al. ; HELENA Study Group. 2011. FADS1 genetic variability interacts with dietary alpha-linolenic acid intake to affect serum non-HDL-cholesterol concentrations in European adolescents. J. Nutr. 141: 1247–1253. [DOI] [PubMed] [Google Scholar]
- 27.Sotos-Prieto M., Guillen M., Vicente Sorli J., Portoles O., Guillem-Saiz P., Ignacio Gonzalez J., Qi L., Corella D. 2013. Relevant associations of the glucokinase regulatory protein/glucokinase gene variation with TAG concentrations in a high-cardiovascular risk population: modulation by the Mediterranean diet. Br. J. Nutr. 109: 193–201. [DOI] [PubMed] [Google Scholar]
- 28.Foufelle F., Girard J., Ferre P. 1996. Regulation of lipogenic enzyme expression by glucose in liver and adipose tissue: a review of the potential cellular and molecular mechanisms. Adv. Enzyme Regul. 36: 199–226. [DOI] [PubMed] [Google Scholar]
- 29.Peter A., Stefan N., Cegan A., Walenta M., Wagner S., Konigsrainer A., Konigsrainer I., Machicao F., Schick F., Haring H. U., et al. 2011. Hepatic glucokinase expression is associated with lipogenesis and fatty liver in humans. J. Clin. Endocrinol. Metab. 96: E1126–E1130. [DOI] [PubMed] [Google Scholar]
- 30.Lin Y. C., Chang P. F., Chang M. H., Ni Y. H. 2014. Genetic variants in GCKR and PNPLA3 confer susceptibility to nonalcoholic fatty liver disease in obese individuals. Am. J. Clin. Nutr. 99: 869–874. [DOI] [PubMed] [Google Scholar]
- 31.Wu J. H., Lemaitre R. N., Manichaikul A., Guan W., Tanaka T., Foy M., Kabagambe E. K., Djousse L., Siscovick D., Fretts A. M., et al. 2013. Genome-wide association study identifies novel loci associated with concentrations of four plasma phospholipid fatty acids in the de novo lipogenesis pathway: results from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium. Circ Cardiovasc Genet. 6: 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jump D. B., Botolin D., Wang Y., Xu J., Demeure O., Christian B. 2008. Docosahexaenoic acid (DHA) and hepatic gene transcription. Chem. Phys. Lipids. 153: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teran-Garcia M., Rufo C., Nakamura M. T., Osborne T. F., Clarke S. D. 2002. NF-Y involvement in the polyunsaturated fat inhibition of fatty acid synthase gene transcription. Biochem. Biophys. Res. Commun. 290: 1295–1299. [DOI] [PubMed] [Google Scholar]
- 34.Nakakuki M., Kawano H., Notsu T., Imada K., Mizuguchi K., Shimano H. 2014. A novel processing system of sterol regulatory element-binding protein-1c regulated by polyunsaturated fatty acid. J. Biochem. 155: 301–313. [DOI] [PubMed] [Google Scholar]
- 35.Howell G., 3rd, Deng X., Yellaturu C., Park E. A., Wilcox H. G., Raghow R., Elam M. B. 2009. N-3 polyunsaturated fatty acids suppress insulin-induced SREBP-1c transcription via reduced trans-activating capacity of LXRalpha. Biochim. Biophys. Acta. 1791: 1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tai C. C., Chen C. Y., Lee H. S., Wang Y. C., Li T. K., Mersamm H. J., Ding S. T., Wang P. H. 2009. Docosahexaenoic acid enhances hepatic serum amyloid A expression via protein kinase A-dependent mechanism. J. Biol. Chem. 284: 32239–32247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y., Yin L., Hillgartner F. B. 2003. SREBP-1 integrates the actions of thyroid hormone, insulin, cAMP, and medium-chain fatty acids on ACCalpha transcription in hepatocytes. J. Lipid Res. 44: 356–368. [DOI] [PubMed] [Google Scholar]
- 38.Schreurs M., Kuipers F., van der Leij F. R. 2010. Regulatory enzymes of mitochondrial beta-oxidation as targets for treatment of the metabolic syndrome. Obes. Rev. 11: 380–388. [DOI] [PubMed] [Google Scholar]
- 39.Cormier H., Rudkowska I., Paradis A. M., Thifault E., Garneau V., Lemieux S., Couture P., Vohl M. C. 2012. Association between polymorphisms in the fatty acid desaturase gene cluster and the plasma triacylglycerol response to an n-3 PUFA supplementation. Nutrients. 4: 1026–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dennis L. K., Snetselaar L. G., Smith B. J., Stewart R. E., Robbins M. E. 2004. Problems with the assessment of dietary fat in prostate cancer studies. Am. J. Epidemiol. 160: 436–444. [DOI] [PubMed] [Google Scholar]
- 41.Cantwell M. M. 2000. Assessment of individual fatty acid intake. Proc. Nutr. Soc. 59: 187–191. [DOI] [PubMed] [Google Scholar]
- 42.Arab L. 2003. Biomarkers of fat and fatty acid intake. J. Nutr. 133(Suppl 3): 925S–932S. [DOI] [PubMed] [Google Scholar]
- 43.Ma J., Folsom A. R., Shahar E., Eckfeldt J. H. 1995. Plasma fatty acid composition as an indicator of habitual dietary fat intake in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Am. J. Clin. Nutr. 62: 564–571. [DOI] [PubMed] [Google Scholar]
- 44.Hodge A. M., Simpson J. A., Gibson R. A., Sinclair A. J., Makrides M., O’Dea K., English D. R., Giles G. G. 2007. Plasma phospholipid fatty acid composition as a biomarker of habitual dietary fat intake in an ethnically diverse cohort. Nutr. Metab. Cardiovasc. Dis. 17: 415–426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.