Abstract
Palmitic acid (PA) is associated with higher blood concentrations of medium-chain acylcarnitines (MCACs), and we hypothesized that PA may inhibit progression of FA β-oxidation. Using a cross-over design, 17 adults were fed high PA (HPA) and low PA/high oleic acid (HOA) diets, each for 3 weeks. The [1-13C]PA and [13-13C]PA tracers were administered with food in random order with each diet, and we assessed PA oxidation (PA OX) and serum AC concentration to determine whether a higher PA intake promoted incomplete PA OX. Dietary PA was completely oxidized during the HOA diet, but only about 40% was oxidized during the HPA diet. The [13-13C]PA/[1-13C]PA ratio of PA OX had an approximate value of 1.0 for either diet, but the ratio of the serum concentrations of MCACs to long-chain ACs (LCACs) was significantly higher during the HPA diet. Thus, direct measurement of PA OX did not confirm that the HPA diet caused incomplete PA OX, despite the modest, but statistically significant, increase in the ratio of MCACs to LCACs in blood.
Keywords: dehydrogenases, diet and dietary lipids, fatty acid, fatty acid/oxidation, inflammation, lipids/oxidation, macrophages/monocytes, mass spectrometry, nutrition, palmitic acid, oleic acid, incomplete β-oxidation of fatty acids, stable isotopes, innate immunity, cytokines
There is an increased risk of type 2 diabetes in obese people who consume a diet rich in both the saturated FA, palmitic acid (PA; C16:0), and the monounsaturated FA, oleic acid (OA; 18:1 cis 9) (1, 2). Diets high in PA may create particular health risks in people who are excessively accreting body fat (obesity) via an imbalance of fat intake versus fat oxidation (1, 2).
Relatively impaired oxidation of FAs, including PA, may lead to the accumulation of lipids (diacylglycerol, ceramide), which activate serine kinases inhibiting activity of insulin-receptor substrate 1 (2–4). In addition, PA may be pro-inflammatory (5, 6). However, the literature also supports the hypothesis that excessive entry of FA into the mitochondria of skeletal muscle causes oxidative stress via an imbalance of NADH production relative to the level of ATP demand, leading to the accumulation of reactive oxygen species and activation of c-Jun N-terminal kinase, accompanied also by serine phosphorylation of insulin-receptor substrate 1 (2, 7, 8). In rodents fed a high saturated fat diet, insulin resistance was linked to “incomplete oxidation of FA,” with relative accumulation of chain-shortened acyl-CoAs esterified to carnitine. Relative to a high OA and low PA (HOA) diet, during a high PA (HPA) diet, insulin sensitivity in humans was inversely correlated with the serum concentration of medium-chain acylcarnitines (MCACs) measured in the fed state (2), but the HPA diet did not affect muscle expression in the fed state of medium-chain acyl-CoA dehydrogenase, which catalyzes the β-oxidation of acyl-CoAs with carbon length of C12 down to C4 (9). Thus, it is not known whether accumulation of MCACs on a HPA diet is related to impaired oxidation of FA or to other unknown mechanisms. It is also not clear whether MCACs per se impair insulin signaling or whether MCACs are biomarkers for excessive production of reduced nucleotides (10). Thus, the primary purpose of the present study was to investigate, in humans, the hypothesis that a HPA diet, relative to a HOA diet, would impair complete β-oxidation as manifested by a higher oxidation rate of PA labeled on the first carbon compared with oxidation of PA labeled on the thirteenth carbon (representing the first carbon of the butyryl-CoA moiety corresponding to PA).
In our previous study (2), we used principal components analysis to show that insulin sensitivity was inversely correlated with the HPA diet-induced increased PA/OA ratio in serum phospholipids and ACs, as well as skeletal muscle lipids. However, studies of the liver in pigs (11), rats (12), and mice (13, 14) suggest that the PA and OA composition of neutral or “total” lipids, but not phospholipids, are directly affected by PA and OA intake (11). Moreover, in mice, it appears that a high OA intake cannot mitigate the OA-lowering effects of inhibition of stearoyl-CoA desaturase on the OA content of LDL. Therefore, in order to gain insight into how a HPA diet leads to PA enrichment of body lipids, we also felt it was important to quantify the effects of a high or low PA intake on PA oxidation (PA OX), as well as the “retention” of PA in the fed state (intake minus oxidation).
The stimulation of cell surface pattern recognition receptors, such as toll-like receptor-4 (TLR4), by molecules, including lipopolysaccharide (LPS) and PA, results in the activation of intracellular signaling cascades, including nuclear factor-κB (NF-κB), leading to the transcription and translation of pro-interleukin (IL)-1β and pro-IL-18, as well as other inflammatory mediators (including TNFα) (5). We (2) recently showed that a HPA diet in humans is associated with increased blood and muscle concentration of ceramides. Therefore, it is of interest that activation of intracellular nucleotide oligomerization domain-like receptors [e.g., nucleotide oligomerization domain-like receptor protein 3 (NLRP3)] by a variety of stimuli, such as PA and ceramides, leads to the formation of a molecular platform called the inflammasome (6), which in the case of NLRP3 mediates activation of caspase-1 and the cleavage of pro-IL-1β and pro-IL-18 into the mature IL-1β and IL-18 that can be secreted (6). Both C12:0 and C14:0 ACs have been shown to be pro-inflammatory in cell culture models (15, 16). Thus, we also sought to correlate serum ACs with the LPS-stimulated production of cytokines by peripheral blood mononuclear cells (PBMCs) isolated from the same subjects (15).
MATERIALS AND METHODS
Subjects, screening, and overall design
The research protocol was approved by institutional committees associated with the University of Vermont Clinical Research Center, where the clinical study was carried out. This trial was registered at clinicaltrials.gov as University of Vermont Protocol Record R01DK082803.
We employed a double-masked cross-over study evaluating two diets, one based on the typical FA composition of the diet consumed by our subjects prior to our study, which was high in PA (HPA), and one reflecting more the Mediterranean diet FA composition, low in PA and high in OA (HOA) (2).
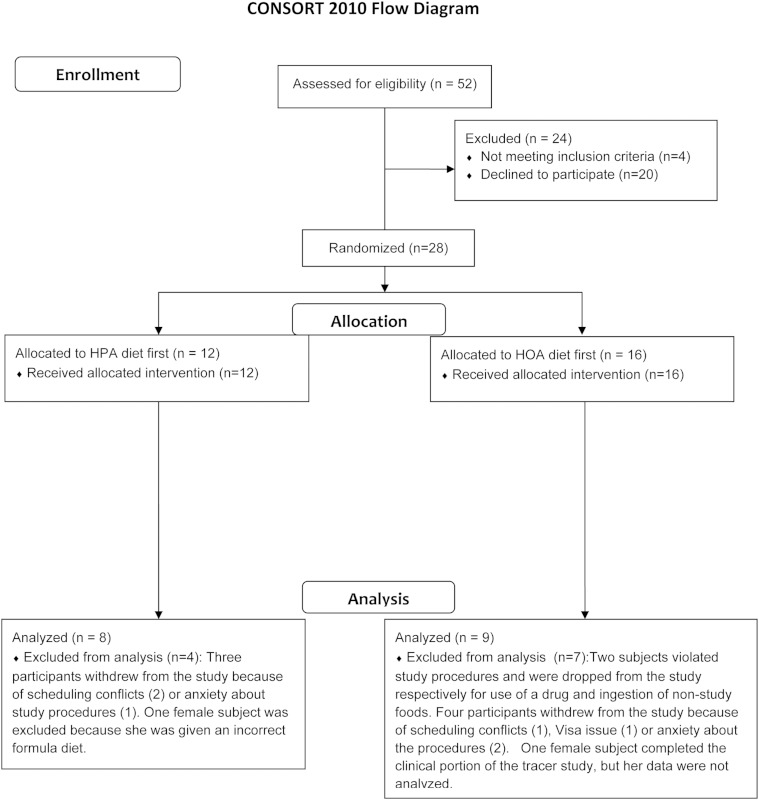
Figure 1 depicts the consort diagram for this trial. There were no changes to the design after the study was initiated. We studied healthy males (n = 8) and females (n = 9), aged 18–40 years, with a body mass index greater than 18 and less than 25 (n = 11) or greater than 30 (n = 6). In this cohort, on the basis of race, there was 1 South Asian (male), 3 blacks (2 males, one of whom was a native African), 1 mixed-race subject (white and Native American), and 12 whites. There were 3 Hispanics (one black and two white) and 14 non-Hispanics. Exclusion criteria included use of prescription medication, except for contraception; regular use of nonprescription medication; regular aerobic exercise training; dyslipidemia (17); and evidence of type 2 diabetes or insulin resistance (18). Screening for study eligibility included: history and physical; laboratory tests in the fasted state for evidence of diabetes (glucose, HbA1C), miscellaneous metabolic dysfunction, dyslipidemia, anemia, and hepatitis and HIV infection. Females were enrolled, whether they received hormonal contraception or not, but studies on the experimental diet were conducted 4 weeks apart. Seventeen subjects completed the whole body tracer studies, including all analyses, but because of catheter problems, a few subjects did not have muscle oxidation studies (sample sizes are given in the figure legends).
Fig. 1.
Consort diagram for the trial.
After screening, all subjects ingested a baseline/control diet for 7 days. The baseline/control diet was low in fat (protein, 19.7% kcal; carbohydrate, 51.6% kcal; fat, 28.4% kcal; PA, 5.3% kcal; OA, 15.9% kcal) (17). Oil mixtures appropriate to this diet, as well as the experimental HPA and HOA diets, were mixed with each meal according to suggestions provided by our dietary staff. For the baseline/control diet, two daily menus were used. On day 8, while in the fasted state, blood was drawn for immunology studies, and then a biopsy of the vastus lateralis was carried out for purposes not related to this report. The subjects then ingested a solid food breakfast consistent with the baseline/control diet (1/3 of daily kilocalories). In view of a previous report noting that nonfat components of a meal may affect the inflammatory response (19), we note that both breakfasts during the control diet contained one possible anti-inflammatory factor, orange juice. Three hours after this meal was provided, blood was also obtained for immunology studies. Beginning with the noon meal that day, the subjects ingested the low-glycemic load “experimental” HPA or HOA diet in random order for 3 weeks; the first 3 week diet period was followed again by the control diet for 1 week (2, 9). Studies of inflammation were also conducted at the end of each of the HPA and HOA diets, as noted in more detail below. The HPA diet resembled the habitual diet of the subjects (20) (fat, 40.4% kcal; PA, 16.0% kcal; OA, 16.2% kcal). The HOA diet was low in PA and high in OA (fat, 40.1% kcal; PA, 2.4% kcal; OA, 28.8% kcal). For the HPA and HOA diets, we used a 3 day rotated menu consisting of three different breakfasts, lunches, and dinners. These menus were identical for each subject during both the HPA and HOA diets, except for the FA composition and amount of oil, which was added to each meal for the baseline/control, HPA, and HOA diets to achieve the desired FA composition.
During the baseline/control diet phases, as well as during experimental diet phases (HPA or HOA), of the study, all solid food and liquids, except water, were given to the subject to ingest, and they were requested to entirely ingest each meal, which consisted of one-third of daily energy needs and which contained approximately the same proportion of macronutrients, including FAs, as a proportion of energy as the entire daily diet. Total daily energy intake was varied, if necessary, to maintain constant body weight. Except for beverages (e.g., orange juice), the meals consisted of lean turkey and chicken, fat-free dairy products, and fruits and vegetables. We enhanced and monitored compliance with the diets in three ways: written attestation, daily verbal guidance, and diet history; monitoring any food returns (usually absent or minimal) (2, 9, 20).
In summary, the overall dietary design for the 8 week study was as follows: 1) control diet for 7 days; 2) either the HPA or HOA diet for 21 days (random order); 3) repeat of the control diet for 7 days; and 4) either the HPA or HOA diet for 21 days. Eight subjects received the HPA diet first and nine received the HOA diet first.
On day 19 of each experimental diet, at 10:00 AM, each subject underwent a study of PA OX (21). Details pertaining to the stable isotope models are described in a separate section below. Briefly, each subject received a eucaloric oral formula, administered over a 9 h period as a series of oral meals every 20 min. During the last 7 h of the formula feeding, the subject received an equal dose of PA tracer with each feeding, either [1-13C]PA or [13-13C]PA in random order on two consecutive days. On day 20, at approximately 10:00 AM, the subject underwent an identical tracer study with the other PA isotope. On both days, we inserted a retrograde catheter in the hand vein (heated before blood withdrawal) and a retrograde catheter in the deep brachial vein of the opposite arm. Blood samples from both catheters and breath samples were used to estimate whole body and forearm muscle oxidation of PA (22). Blood and breath samples were obtained just prior to formula administration and again just prior to isotope administration for determining the isotopic enrichment of plasma PA and breath and blood CO2. During the PA tracer study, blood samples for determining PA and CO2 concentration and enrichment and breath samples for assessment of CO2 enrichment were obtained at the following times after beginning isotope administration: 180, 240, 300, 360, 380, 400, and 420 min. During each PA tracer study, blood flow was measured by venous occlusion plethysmography (345, 365, 385, and 405 min) (23), and AC profiling was carried out on blood samples collected at the end of each tracer study (in the fed state). Measurements (15 min) of oxygen consumption and CO2 production (Vmax SPECTRA 29; Sensor Medics Corp., Yorba Linda, CA) were performed at 245 min and 305 min, after the beginning of PA tracer administration.
On day 21, the subjects underwent an identical feeding protocol, as on days 19 and 20, except [1-13C]acetate was administered with each 20 min formula feeding (20 μmol/kg/20 min), and only breath samples were obtained (preformula, pretracer, and 60, 120, 180, 240, 300, 360, 380, 400, and 420 min after beginning tracer administration). Measurements (15 min) of oxygen consumption and CO2 production were performed at 180, 240, 300, and 360 min after beginning the acetic acid tracer administration and once again the next morning in the fasted state (50 min measurement at 5:00 AM).
Then, on day 22, while still in the fasted state, we again collected blood for immunology studies, and then a biopsy of the vastus lateralis muscle was obtained for unrelated studies of muscle expression of redox-sensitive genes, not further discussed here. The subject then ate a solid food breakfast consistent with the diet that the subject had been ingesting for the previous 3 weeks (HPA or HOA) (1/3 of daily energy intake). The breakfast meals were identical for each subject on each diet, except for the composition of the oil added to the meal. Three hours after the subject began to ingest the breakfast meal, we again collected blood for immunology studies.
During both the PA and acetic acid tracer studies, the subjects were allowed to use the bathroom with assistance and to sit in a chair, but in part because of the need to maintain two venous catheters during most of the day for the PA tracer studies, subjects remained at rest without exercise. Because their formula energy intake was based on their usual intake prior to these studies, it is likely that the subjects received a surfeit energy intake during these tracer studies.
On the first day of the baseline diet and on day 16 of the HPA and HOA diets, body composition was assessed by dual-energy X-ray absorptiometry, including upper body (android) and lower body (gynoid) regions (GE Lunar Prodigy densitometer, version 5.6) (24). In extremely obese individuals who were too wide to fit adequately on the scanning table, we used the technique of Tataranni and Ravussin (25) to perform a half-body scan to estimate body fat and fat-free mass.
Detailed tracer study procedures and calculations
At time zero on days 19 and 20 of each experimental diet (HPA, HOA), the subjects received an oral formula of the same composition as the experimental diet which they were currently ingesting; this liquid diet was administered over a 9 h period as a series of oral meals every 20 min. During the entire 9 h protocol, the energy intake was matched to the eucaloric energy intake the subject received on the day before the first tracer study. During the last 7 h of formula feeding, the subject received a PA tracer with every 20 min feeding, either [1-13C]PA or [13-13C]PA in random order (2.33 μmol/kg body weight/20 min, equal to 0.1165 μmol/kg body weight/min) (both purchased from Isotec Inc., Sigma-Aldrich, Miamisburg, OH). During the HPA diet, 7 subjects received the [1-13C]PA first and 10 subjects received the [13 -13C]PA first, and during the HOA diet, 10 subjects received the [1-13C]PA first and 7 subjects received the [13-13C]PA first. We based our tracer dosages on body weight in an effort to attain similar plateau isotopic enrichments of plasma PA and breath and blood CO2 in individuals with varying body size. However, when comparing diets, we are comparing the results with each tracer within each individual during each of the two diets; therefore, we did not use a body weight correction to express PA OX in order to limit extraneous experimental error. The enrichments and position of the label for the two tracers was confirmed by a combination of GC/MS and nuclear magnetic resonance.
For the studies of PA OX across the forearm muscle bed, we followed the procedures of Rasmussen et al. (22) closely, except for two differences: 1) For each diet period, we carried-out a separate acetic acid tracer recovery study (26–28); and 2) Instead of utilizing the femoral artery for blood sampling, we obtained arterialized-venous blood using the heated hand vein technique (29, 30), and instead of femoral vein blood sampling, we used a second contralateral deep forearm catheter (31–33). Both catheters were placed in the retrograde position, i.e., tip toward the fingers. Generally, assurance of the intended position of the deep brachial catheter was obtained using both ultrasound and a blood sample from the catheter showing an O2 saturation ≤70% (34). We did complete 1 of the 34 studies in a volunteer in whom the O2 saturation was 76.7%. The “forearm” uptake and oxidation of PA included a contribution from the hand and fingers of that limb, and despite our efforts using the deep brachial catheter to exclude subcutaneous adipose tissue from the estimates, there were some adipocytes between muscle fibers, which metabolized the PA tracer.
During each PA tracer study, blood flow was measured by venous occlusion plethysmography (EC6 strain gauge and photo plethysmograph; D. E. Hokanson, Inc., Bellevue, WA) (23). This procedure included using “blood pressure cuffs” during the measurement of forearm volume. Specifically, we prevented arterial and venous blood flow to and from the hand distal to the strain gauge, using a wrist cuff inflated at 200 mm Hg (above arterial blood pressure). We excluded venous return proximal to the strain gauge by inflating an upper arm cuff to a pressure of 50 mm Hg.
We used standard equations for estimating the rates of both whole body PA OX (21) and skeletal muscle PA OX and skeletal muscle PA uptake (22). However, several caveats need to be mentioned before providing detailed equations. First of all, we elected to study PA OX in the fed state because our previous study suggested that MCAC accumulation in the fed state correlated with insulin sensitivity (2). Second, we corrected the rate of breath CO2 excretion of the PA-derived label for isotope exchange of both acetyl-CoA and CO2 per se using a [1-13C]acetate breath test (as both PA tracers label carbon one of acetyl-CoA after β-oxidation) (26, 27, 35). However, while we acknowledge that the use of [1-13C]4-methyl-2-oxovaleric (2-ketoisocaproic acid), from which acetyl-CoA is generated within the mitochondria, may be ideal for assessing isotope exchange in the TCA cycle, we were uncertain of the absorption of this compound. Third, skeletal muscle oxidation of PA was based on blood sampling (22) and not intramyocellular palmitoyl-CoA enrichment. Because PA derived from intramyocellular triacylglycerol probably only accounts for about 10% of skeletal muscle FA oxidation (FA OX) in the resting nonexercising state (36), the likely underestimation of the isotopic enrichment of the “true” precursor pool of PA for oxidation was probably not great. Finally, we were interested in estimating retention of PA in the whole body, as well as in muscle, because, presumably, excess palmitoyl-CoA would be more likely to activate NLRP3 or lead to enhanced ceramide synthesis (2). Whole body “retention” of PA (body PA retention) was equated to PA intake minus PA OX, measured, for this calculation, only with the [1-13C]PA tracer. However, on either diet, we overestimated retention to the degree that food-derived PA was not completely absorbed. Similarly, skeletal muscle PA retention was equated to net uptake of PA across the forearm muscle minus skeletal muscle PA OX, with both skeletal muscle PA uptake and skeletal muscle PA retention calculated from data using the [1-13C]PA tracer. Thus, we constructed a model of PA balance that is analogous to the term “leucine balance” (37). An important caveat of this approach to estimating PA retention on either the whole body or skeletal muscle level is to realize that the ultimate fate of the labeled carbon per se beyond incorporation into CO2, has no bearing on our model or the meaning of “PA retention.” Because we correct for the oxidative fate of acetyl-CoA using the acetate tracer (and incorporation of acetyl-CoA into other products such as acetylcarnitine, as well as isotope exchange of the one carbon of acetyl-CoA within the tricarboxylic acid cycle), our estimates of both the whole body and skeletal muscle rates of PA oxidation are, in reality, estimates of the molar rate of removal of acetyl-CoA (carbons 1 and 2 using the [1-13C]PA tracer and carbons 13 and 14 using the [13-13C]PA tracer). Thus, once the first two carbons are removed via β-oxidation, the resultant chain-shortened acyl-CoA cannot, to our knowledge, be resynthesized into PA. Assuming, then, that PA oxidation can be accurately assessed using our tracer model, we can estimate retention of PA (as palmitoyl-CoA) in tissues during the course of the tracer study.
Typically, in substrate oxidation studies, one calculates an isotope recovery factor, c, equal to the fractional recovery of labeled carbon from [1-13C]acetic acid or H13CO3– (22, 35). When the [1-13C]PA and the [13-13C]PA tracers are oxidized via β-oxidation, the 13C of both tracers will become [1-13C]acetyl-CoA. We also used this construct in our interpretation of the muscle oxidation studies described below. Then, the fraction of acetic acid tracer-derived 1-13C that was recovered in exhaled CO2 (c) was calculated as follows:
| (Eq. 1) |
where dACET is the rate of administration of the [1-13C]acetic acid tracer (μmol/min) and IE CO2 ACET is the plateau isotopic enrichment of CO2 (atoms fraction excess) at the end of this study (atoms fraction excess equals atoms percent excess/100).
When c is substituted into the denominator of the conventional equation for FA OX (22), VCO2 cancels in both the numerator and denominator (35). So, in estimating whole body PA OX using either tracer, we defined a new parameter, RaCO2 ACET, which equals the rate appearance of CO2 (35) corrected for isotope exchange of acetyl-CoA in the TCA cycle (26, 27).
| (Eq. 2) |
Whole body [or “plasma” (22)] oxidation of PA (PAox) then was calculated using equation 3:
| (Eq. 3) |
where IE CO2 PA is the plateau isotopic enrichment of CO2 (atoms fraction excess) at the end of the respective PA tracer study and IE PA is the plateau isotopic enrichment of PA (moles fraction excess) at the end of the study in the nonesterified plasma FA pool (arterialized hand vein).
In Fig. 2, we also expressed the whole body rate of oxidation of PA during both diets as a ratio (Total Serum FA OX) between PAox (μmol/min) and the fraction of PA in total serum FAs (PA Fract Serum FA):
| (Eq. 4) |
where PA Fract Serum FA is defined as follows:
| (Eq. 5) |
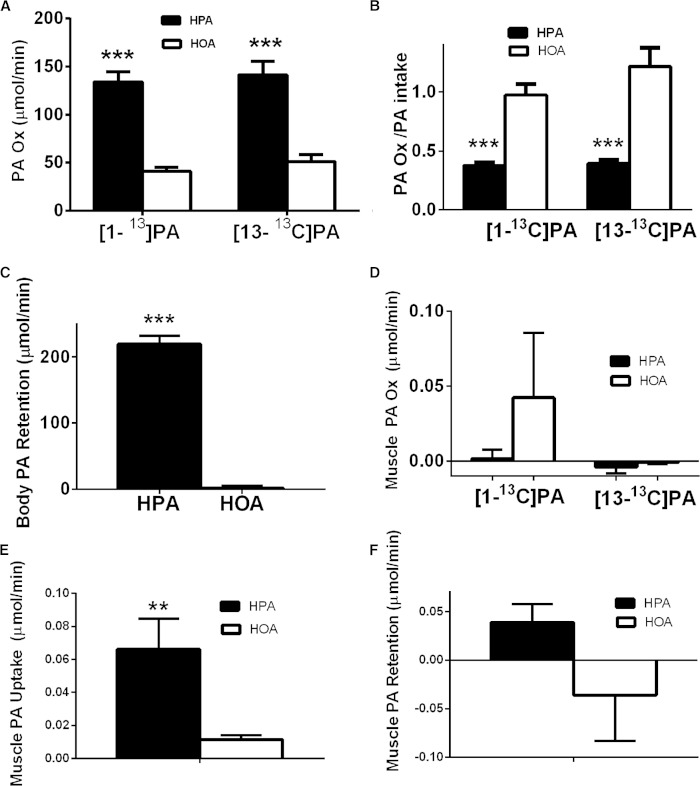
Fig. 2.
PA oxidation and retention is increased on the HPA diet. As described in detail in the Materials and Methods, studies of the oxidation of [1-13C]PA and [13-13C]PA tracers were carried out in random order, in the fed state, using an oral tracer administration protocol for the HPA, low PA, and HOA diets. Both whole body and muscle parameters are calculated based on data obtained from blood samples (see the Materials and Methods, as well as detailed equations in the online data supplement). Black bars indicate the HPA diet and white bars indicate the HOA diet. **P ≤ 0.01 or ***P ≤ 0.0001 for diet differences. A: The whole body rate of oxidation of PA (PA OX) based either on the [1-13C]PA tracer or the [13-13C]PA tracer (micromoles per minute) (n = 17). B: The whole body rate of oxidation of PA, using either tracer, expressed as a fraction of PA intake (n = 17). C: Whole body PA retention (PA intake − PA oxidation) (micromoles per minute). Data are presented for the [1-13C]PA tracer only as described in the text (n = 17). D: Skeletal muscle oxidation of PA (micromoles per minute, across the forearm muscle) based either on the [1-13C]PA tracer (n = 15) or the [13-13C]PA tracer (n = 12). E: Skeletal muscle PA uptake (micromoles per minute, across the forearm muscle). Average uptake on both tracer days for each diet is presented because position of label has no effect (n = 16 for the [1-13C]PA tracer and n = 13 for the [13-13C]PA tracer). The values for both tracer days were averaged when both values were available. F: Skeletal muscle PA retention based on the [1-13C]PA tracer (micromoles per minute, across the forearm muscle) (n = 15).
Total Serum FA OX provides an index of whether the rate of PA oxidation was lower or higher during each diet in proportion to the relative circulating PA concentration, but is also an estimate of total FA OX as measured by the tracer technique (38).
For the estimate of muscle PA oxidation, the major comparison was the isotopic enrichment of CO2 derived from deep venous and arterialized venous blood. Although the other measurements implied in the technique (22) basically cancel out in the comparison between PA tracers (except for daily variation), we measured plasma PA concentration, isotopic enrichment of PA, concentration of CO2, and blood flow. The equations we used for estimating the fraction of PA extracted by the forearm muscle, forearm muscle PA oxidation, forearm muscle uptake of PA, and forearm muscle retention of PA were adapted from Rasmussen et al. (22) and are provided in the online supplementary data file.
Concentration of PA and other FAs and isotopic enrichment of PA
PA enrichment and concentration in plasma samples was determined by methane chemical ionization GC/MS (Agilent 5973; Agilent Technologies, Palo Alto, CA). Please see the online supplementary data file for more details. The concentrations of nonesterified serum FAs were analyzed by GC using recently described methods (39).
Isotopic enrichment of CO2 and the concentration of CO2 in blood
Determination of 13C/12C ratios in carbon dioxide in expired breath samples and in blood was performed using a gas isotope ratio mass spectrometer (PDZ Europa, Cheshire, UK; model 20/20 with an automated breath carbon analyzer module) (40). More details are found in the online supplementary data file.
The blood concentration of CO2 was assessed by isotope dilution using 13C aqueous sodium carbonate solution (99% 13C) (MSD-3105; MSD Isotope, Montreal, PQ, Canada) and gas isotope ratio MS, as noted above for assessment of blood CO2 enrichment. See the online data supplement for more details.
Serum concentrations of AC
Serum AC concentrations were measured by direct-injection electrospray tandem MS (8) at the Duke Molecular Physiology Institute Metabolomics Laboratory; we averaged the values for the two tracer studies on each diet before comparing the diets. Despite our ability to detect statistically significant effects of the diets (presented below), a caveat related to these comparisons is that the concentrations of C6, C8, C10, C12:0, C12:1, C14:1, and C16:1 ACs are at the lower limit of detectability (0.01–0.02 μM). Also, in trying to interpret the relative effects of the HPA and HOA diets on saturated and monounsaturated long-chain AC (LCAC) species, respectively, our analytical method presently cannot distinguish between cis species of monounsaturated ACs (derived from OA primarily in this study) and trans species of monounsaturated ACs, derived from the acyl-CoA dehydrogenase step of β-oxidation (41, 42). For example, C12:1 AC may be derived from OA or PA.
Measurement of cytokines secreted by PBMCs isolated in the fasted and fed states
In 16 subjects (n = 8 males and n = 8 females), we collected blood in the fasted state, as well as in the fed state, 3 h after a breakfast meal (1/3 of daily kilocalories). Then, we enriched the PBMC population (consisting of lymphocytes and monocytes) from blood using LSM lymphocyte separation medium (MP Biomedicals, Solon, OH) according to manufacturer’s directions, from which the mononuclear cell layer was collected, washed, and counted. Freshly-collected PBMCs were plated at 106 cells/ml and treated in duplicate under control conditions or were stimulated by the addition of 1 ng/ml ultra-pure LPS (from Escherichia coli 0111:B4; InvivoGen, San Diego, CA). After 24 h, the supernatants were separated and stored at −80°C until analysis. The following cytokines were measured in these supernatants using a Luminex-based assay: TNFα, IL-1β, and IL-18 (Bio-Rad, Hercules, CA) (2). These particular cytokines were selected for correlation analysis with AC concentrations because pilot studies showed that the secretion of these cytokines was enhanced by LPS and because the synthesis of these cytokines (TNFα, pro-IL-1β, and pro-IL-18) were stimulated by activation of TLR4 and/or cleaved to the active form by the NLRP3 inflammasome (IL-1β and IL-18) (5, 6). AC and cytokine concentrations, depicted as “HPA − HOA” [the value (AC or cytokine concentration) during the HPA diet minus the respective value during the HOA diet] differences in the Results, represent values obtained during the HOA diet subtracted from the values obtained from subjects during the HPA diet. A positive correlation between the HPA − HOA difference in the concentration of a particular AC and the respective difference in the secretion of a cytokine by PBMCs implies that a larger relative concentration of the AC (higher value during HPA and/or lower value during HOA) was associated with a higher respective relative secretion of the particular cytokine.
Data analysis and statistics
Analyses were performed with SAS, version 9.2. All data are expressed as mean ± SEM, and P < 0.05 was selected as statistically significant. We defined MCACs as consisting of the following species: C6:0, C8:0, C10:0, C12:0, and C12:1; and the LCAC as consisting of the following species representing dietary and chain-shortened species derived from linoleic acid (18:2), OA (18:1), stearic acid (18:0), PA (16:0), palmitoleic acid (16:1), and myristic acid (14:0): 18:2, 18:1, 18:0, 16:0, 16:1, 16:2, 14:0, and 14:1.
This study employed a two-treatment two-period two-sequence cross-over design. Generally, diet effects were analyzed using a repeated measures ANOVA, including period, sequence, and treatment effects, with the baseline value as a covariate, when available. However, diet effects on body weight, fat oxidation, and AC profiles, without baseline data, were analyzed using the paired t-test. Because gender-specific immune responses to the diets were anticipated (2), males and females were analyzed both as a group and separately. For some analyses, we employed the same model using ranks. All correlations reported are Spearman rank correlations.
Subjects were randomly assigned to a diet order and tracer order using the random allotment procedures in SAS by the Clinical Research Center informatics staff, with block size 4 stratified within gender and obesity group. Tracer order was randomized within gender, obesity status, and diet. The diet order randomization was viewable by the Clinical Research Center bionutritionist, who assigned the diet order, but the diet order spreadsheet, as well as the tracer order spreadsheet, was also viewable by the staff preparing the liquid diets and adding tracer to the liquid diets. However, during both the laboratory analyses and the process of determining that the resultant data were valid, the investigators were masked to both the order of the diets and the order of the tracer administration. The sample size for the trial was estimated prior to beginning the study using methods detailed in the online data supplement.
RESULTS
Body composition and indirect calorimetry
There were no differences between the HPA and HOA diets for body mass index, body weight, body fat, or percent body fat. Fat-free mass (in kilograms) was 1% higher during the HOA diet compared with HPA diet (54.1 ± 3.1 kg versus 53.5 ± 3.1 kg) (P = 0.024). Expressed as a percent of resting energy expenditure, fat oxidation rate in the fed state was 147% higher during the HPA diet (11.6 ± 3.0%) compared with the HOA diet (4.7 ± 2.4%) (P = 0.044). In the fed state, a higher mean rate of FA OX during the HPA diet (in grams per minute) was not statistically significant (P = 0.058), nor was a lower mean respiratory exchange ratio (P = 0.06). In the fasted state, there were no diet differences in fat oxidation.
Whole body oxidation and retention of PA
Figure 2A shows that the rate of whole body PA oxidation, based either on the [1-13C]PA or the [13-13C]PA tracer, was increased 226% and 176%, respectively, during the HPA diet. Essentially all of the dietary PA was oxidized during the HOA diet, but only about 38–39% was oxidized during the HPA diet, based on estimates from either tracer (Fig. 2B). When expressed as a fraction of the serum long-chain FA, PA oxidation (total serum FA acid oxidation) ([1-13C]PA tracer) was greater during the HPA diet (134.1 ± 10.6 μmol/min) compared with the HOA diet (41.1 ± 4.0 μmol/min) (P < 0.05). Whole body PA retention, based on the [1-13C]PA tracer oxidation data, was higher during the HPA diet (Fig. 2C). Expressed as a fraction of intake, whole body PA retention was 62.1 ± 2.4% for the HPA diet and only 2.7% ± 9.3% for the HOA diet (P < 0.001).
Because the two PA tracer studies were randomized within each diet, effects of “tracer spillover” on day 2 from the tracer administered on day 1, was unlikely to have had a systematic effect on our conclusions. Still, there was evidence that the ratio of M + 1/M + 0 for the enrichment of plasma PA, just prior to the beginning of administering the PA tracer (110 min of formula feeding), was respectively 1.6–3% higher on day 2 compared with day 1 for the HPA and HOA diets (P < 0.001). In view of these data on spillover and the results presented below indicating that the rate of oxidation of PA was unaffected by the tracer employed, we examined whole body PA oxidation rate expressed either as micromoles per minute or as a fraction of PA intake, using only data from the day 1 study (independent of the tracer used); on this basis, whole body PA oxidation rate during the HOA diet was respectively 198% increased (in micromoles per minute) or 35% increased (fraction of intake), values which are very similar to those presented above where both tracers and both tracer days were considered. Thus, spillover of labeled PA from day 1 to day 2 did not affect our conclusions.
Skeletal muscle oxidation, uptake, and retention of PA
There was no difference between diets in forearm blood flow on either tracer day. As noted in the Materials and Methods, skeletal muscle PA oxidation and uptake are based on assessment of blood flow and measurements of PA and CO2 concentration and enrichment across the forearm muscle bed (based on blood obtained from the heated dorsal hand vein and deep brachial vein). Forearm skeletal muscle oxidation of PA was very low on both diets, independent of the tracer, and was not different from zero for either tracer or diet condition (Fig. 2D). Fractional extraction of either the [1-13C]PA or [13-13C]PA tracer was different from zero (P values) but not affected by diet: 1) [1-13C]PA: HPA, 0.18 ± 0.07 (P = 0.027); HOA, 0.20 ± 0.05 (P = 0.001); and 2) [13-13C]PA: HPA, 0.31 ± 0.03 (P < 0.0001); HOA, 0.28 ± 0.03 (P < 0.0001). Forearm skeletal muscle uptake of PA was higher during the HPA diet (Fig. 2E) and was different from zero on either diet, using either tracer (data not shown). Thus, mean forearm skeletal muscle PA retention was higher during the HPA diet, but not to a statistically significant state (P = 0.06) (Fig. 2 F). Therefore, with the higher PA intake, there appeared to be a relative expansion, at least temporarily, in the intramyocellular pool of palmitoyl-CoA or its metabolites, which could have functional consequences.
Comparison of oxidation of the PA carbon on the first and thirteenth position
Just prior to the beginning of tracer administration, the isotopic abundance of 13CO2/(13CO2 + 12CO2) in breath was 0.2% higher (P < 0.001) on day 2 compared with day 1 during both the HOA (1.092 ± 0.00034 versus 1.091 ± 0.00034 atoms %) and the HPA diets (1.093 ± 0.00028 versus 1.0904 ± 0.00028 atoms %). Thus, although detectable statistically in breath CO2, the tracer spillover was very low in comparison to plasma PA (which was still low, as shown above). So, we computed the ratio of the plateau rate of breath excretion of 13CO2 to the rate of administration of tracer for each of the two tracer studies on each formula (both expressed in molar units). This estimate of relative oxidation of the two carbon positions of PA is independent of plasma PA enrichment. As with the more informed model for assessing the rate of oxidation of PA using either tracer, this comparison of the relative oxidation of the thirteenth carbon compared with the first carbon of PA assumes equal acetate kinetics on both days; however, in addition, we assume here with this simpler approach that the absorption of tracer and its dilution by dietary PA is equal on both days of tracer administration. Because the subjects were studied on each tracer day for each diet under identical conditions, including formula intake, this is a reasonable approximation of the relative rate of oxidation of these two PA carbons. For the HOA diet, the respective average gross recovery of label in expired CO2 was 17.7 ± 0.9% for the [13-13C]PA tracer and 17.2 ± 1.1% for the [1-13C]PA tracer (not significantly different); and for the HPA diet, the respective values were 21.3 ± 1.2% and 19.7 ± 1.1% (also not significantly different). Therefore, recovery of label from the first carbon of PA did not exceed that from the thirteenth carbon.
We then proceeded to estimate the rate of oxidation of the two labeled PA tracers using the more informed stable isotope model described in the Materials and Methods. Figure 3A shows that there was no diet difference in the ratio of the rate of oxidation derived from the [13-13C]PA tracer to that using the [1-13C]PA tracer with an approximate value of 1.0 for either diet. There was no diet by sex interaction for this parameter.
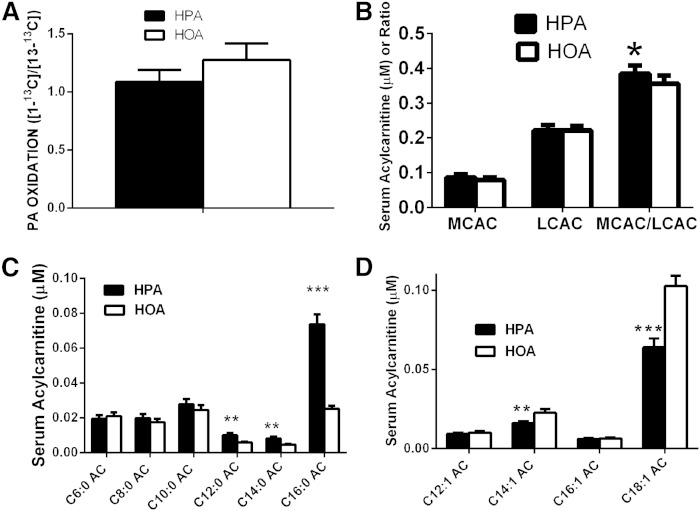
Fig. 3.
Discordant interpretation of completeness of FA OX based on tracer data and AC profiling. The oxidation of PA was assessed during a 7 h protocol in which tracers for PA and diet formula were administered by mouth every 20 min. The [1-13C]PA and [13-13C]PA tracers were administered in random order for each of the experimental diets (HPA, HOA). As described in Materials and Methods, oxidation of each tracer was based on plateau samples obtained during the last hour of tracer administration. AC profiling was conducted on blood obtained at the end of the tracer administration protocol (n = 17 for all diet comparisons). Black bars indicate the HPA diet and white bars indicate HOA diet. *P ≤ 0.05, **P < 0.01, and ***P < 0.001, for differences between tracers (repeated measures ANOVA, except for AC statistics, which were based on a paired t-test). A: Ratio of the whole body rates of PA oxidation measured with tracers for PA labeled on positions C1 and C13 (C13/C1 data presented). B: Serum AC profile averaged for the two tracer days on each diet (see text for calculation). C: Effects of diets on the serum concentrations of specific saturated ACs. D: Effects of diets on the serum concentrations of specific monounsaturated ACs.
ACs
As noted in the Materials and Methods, AC profiling was carried out on blood samples collected at the end of each tracer study (in the fed state). There were no differences between diet groups in the serum AC concentration or in the sum of serum concentrations of either MCAC or LCAC, but the MCAC/LCAC ratio was 8% higher during the HPA diet (Fig. 3B). We next examined whether the diets affected various species of MCAC; there were no differences in C6:0, C8:0, and C10:0 (Fig. 3C). However, during the HPA diet, the serum concentration of the C12:0 AC was 70% higher, the C14:0 AC was 72% higher, and the C16:0 AC was 193% higher (Fig. 3C). The serum concentrations of the C12:1 and C16:1 ACs were only 8% and 7% higher during the HOA diet and not statistically different from the HPA diet, but the C14:1 and C18:1 ACs were respectively 40 and 61% higher during the HOA diet (Fig. 3D).
Both C12:0 and C14:0 AC have been shown to be pro-inflammatory in cell culture models (15, 16); therefore, we examined how the AC profile, obtained only in the fed state at the end of each tracer study, correlated with secretion of cytokines by PBMCs activated by LPS. As noted in the Materials and Methods, ACs were measured at the conclusion of a feeding regimen consisting of repeated (every 20 min) feedings of formula diets, appropriate to the particular diet, HPA or HOA. On the other hand, the secretion of cytokines by PBMCs was measured using blood collected about 11 h later in the fasted state, as well as 3 h after a breakfast meal that was preceded by a muscle biopsy. With these caveats in mind, in males and females considered together, there were no correlations between diet differences in C6:0, C8:0, and C10:0 ACs and IL-1β, IL-18, or TNFα secretion by PBMCs. Table 1 shows that there were only modest scattered correlations between diet differences (HPA − HOA) in longer-chain ACs and secretion of IL-1β, IL-18, and TNFα in males and females considered together. However, in view of our previous observation that the HPA diet seemed to cause enhanced inflammatory and redox stress in females but not males (2), we also examined these correlations separately in males and females. Table 1 indicates that in females there were correlations between diet differences (HPA − HOA) in LPS-induced secretion of IL-1β and TNFα from PBMCs during both the fasted state and the fed state (post-breakfast) versus the respective differences in C12:0, C14:0, and C16:0 ACs. Because these particular saturated ACs were all higher in the HPA diet versus the HOA diet, these correlations could be simple manifestations of an effect of PA per se on secretion of IL-1β, IL-18, and TNFα (5, 6), but neither the diet difference in whole body rate of oxidation of PA nor the diet difference in whole body PA retention correlated with diet differences in the secretion of these three cytokines by PBMCs. Table 1 also shows that, in males, diet differences in unsaturated ACs (C12:1, C14:1, C16:1, and C18:1) correlated with respective differences in PBMC secretion of IL-1β and IL-18 in the fasted state, despite the fact that there were no diet effects on the serum concentration of either the C12:1 or C16:1 ACs.
TABLE 1.
Correlation matrix for diet differences (HPA − HOA) in AC species and respective diet differences in cytokines secreted by peripheral blood PBMCs
| IL-1β Fasted | IL-1β Fed | IL-18 Fasted | IL-18 Fed | TNF α Fasted | TNF α Fed | |
| Males and Females | ||||||
| Saturated ACs | ||||||
| C12:0 | NS | NS | NS | NS | NS | NS |
| C14:0 | NS | NS | NS | NS | NS | NS |
| C16:0 | NS | 0.58a | NS | NS | 0.50a | 0.50a |
| C18:0 | NS | NS | NS | NS | NS | NS |
| Unsaturated ACs | ||||||
| C12:1 | 0.51a | NS | 0.58a | NS | 0.53a | NS |
| C14:1 | NS | NS | 0.59a | NS | 0.49a | NS |
| C16:1 | 0.50a | NS | 0.62b | NS | NS | NS |
| C18:1 | NS | NS | 0.53a | NS | NS | NS |
| Females | ||||||
| Saturated ACs | ||||||
| C12:0 | 0.81a | NS | NS | NS | NS | NS |
| C14:0 | 0.79a | 0.76a | NS | NS | 0.74a | NS |
| C16:0 | NS | 0.93b | NS | 0.79a | 0.90b | 0.79a |
| C18:0 | NS | NS | NS | NS | NS | NS |
| Unsaturated ACs | ||||||
| C12:1 | NS | 0.71a | NS | NS | NS | NS |
| C14:1 | NS | NS | NS | NS | NS | NS |
| C16:1 | NS | NS | NS | NS | NS | NS |
| C18:1 | NS | NS | NS | NS | NS | NS |
| C18:2 | NS | NS | NS | NS | NS | NS |
| Males | ||||||
| Saturated ACs | ||||||
| C12:0 | NS | NS | NS | NS | NS | NS |
| C14:0 | NS | NS | NS | NS | NS | NS |
| C16:0 | NS | NS | NS | NS | NS | NS |
| C18:0 | NS | NS | NS | NS | NS | NS |
| Unsaturated ACs | ||||||
| C12:1 | 0.78a | NS | 0.81a | NS | NS | NS |
| C14:1 | 0.76a | NS | 0.88b | NS | NS | NS |
| C16:1 | 0.74a | NS | 0.79a | NS | NS | NS |
| C18:1 | NS | NS | 0.72a | NS | NS | NS |
| C18:2 | NS | NS | NS | NS | NS | NS |
AC species were measured in the fed state at the end of each tracer study and cytokines secreted by PBMCs were collected in the fasted and fed states. When statistically significant, the Spearman rank r value is shown for each relevant comparison. AC profiling was conducted at the end of 9 h of repeated (every 20 min) feedings of a formula diet (HPA or HOA) on days 20 and 21 (fed state) and then averaged for each species. Immunology studies were conducted on day 22 of each experimental diet in both the fasted state and 3 h after a breakfast meal consisting of one-third of the total energy content of the daily diet (fed state). Each breakfast was identical during each of the HPA and HOA diets, except for the FA composition, which reflected that of the particular diet.
P < 0.05.
P ≤ 0.01.
DISCUSSION
Here we show that although whole body PA oxidation is increased on the HPA diet, the increase is not commensurate with the proportion of PA in the diet. While the ratio of PA intake (as percent energy) in the HPA and HOA diets was 6.76, the relative ratio in PA retention was 168. Because OA appears to be preferentially oxidized compared with PA, when both FAs are approximately equal in the diet (as is the case with the HPA diet) (21), it is not surprising that the rate of PA oxidation might be diminished in proportion to its contribution to the diet and, thus, much lower during meal-feeding with the HOA diet pattern. That is, the HOA diet contained very little PA; therefore, the lower rate of PA oxidation during this diet (in the fed state) could be related to the effects of the liquid meals per se, and not necessarily mainly or only due to effects of the chronic (3 week) HOA diet.
From a perusal of the rodent literature, it may not be self-evident that changing the PA/OA ratio of the diet would affect the content of tissue lipids (2, 9) or PA retention. The PA and OA contents of aortic triglycerides were not affected by HPA or HOA diets in mice (13). In contrast, our data suggest that, in humans, accumulation of palmitoyl-CoA in cellular pools is likely during a typical HPA diet, at least temporarily in the fed state. Thus, a high PA intake could be detrimental to optimal cellular function and might affect macrophage content of PA and OA, as well as TLR4-responsive inflammatory gene expression (13). It is important to note that there were, however, no diet effects on body fat as measured by dual-energy X-ray absorptiometry.
Estimates of total FA OX measured with either indirect calorimetry or the [1-13C]PA tracer both suggested that total oxidation of FAs was increased during the HPA diet. These findings reflect a similar observation from another cross-over study conducted on a completely different cohort (9). The sterol regulatory element-binding proteins (SREBPs), SREBP-1a and SREBP-1c, enhance the transcription of a number of enzymes involved in de novo FA synthesis, including acetyl-CoA carboxylase, which produces malonyl-CoA, an important inhibitor of carnitine palmitoyl transferase 1 and, thus, FA OX (43–46). SREBPs synthesized in the endoplasmic reticulum (ER) must be proteolytically processed in the Golgi apparatus to be active; and for that to occur, SREBP cleavage-activating protein (Scap) must escort SREBP to the Golgi, a process that is inhibited if the insulin induced gene-1 protein (Insig-1) binds to Scap. High cholesterol concentration in the ER membrane enhances the binding of Insig-1 with Scap (43). Low ER membrane free cholesterol not only lowers this interaction of Insig-1 with Scap, but also enhances degradation of the Insig-1 protein (43). Thus, raising the OA content of the diet and consequently increasing the esterification of cholesterol with OA, a preferential substrate for acyl-CoA:cholesterol acyltransferase, is apt to lower total FA OX and enhance de novo FA synthesis by enhancing cellular activity of SREBPs (9, 43). The transcription of insig-1 is enhanced by increased activity of SREBPs (43). Thus, it is relevant that the HOA diet, compared with the HPA diet, appeared to increase muscle mRNA expression of INSIG-1 (9), again providing evidence of increased SREBP activity during this diet. This line of evidence, while consistent with the lower rate of FA OX during the HOA diet, contrasts with the persistent oxidation of PA on the low PA HOA diet, such that there was no retention of PA during the HOA diet. Thus, there is no apparent mechanism for conserving palmitoyl-CoA in cells under conditions of limited intake. Because PA is an integral part of lipid rafts and palmitoylation of signaling proteins and transmembrane receptors is critical to cell function, it is interesting to speculate whether upregulated PA (and probably OA) synthesis, under conditions of increased activation of SREBP-1a and SREBP-1c, is sufficient to provide the necessary cell membrane content of PA. Potential limited PA intake over a time period of a few weeks would likely be buffered by PA present in adipose tissue triacylglycerols, which did not change over a period of 3 weeks (2).
The fractional extraction of PA by forearm skeletal muscle for the [1-13C]PA tracer on either diet (means, 18 and 20%) was similar to what has been reported in the single comparable study of forearm muscle oxidation of PA in the literature (47). Skeletal muscle oxidation of PA was negligible during both diets. However, energy intake was estimated from each subject’s food intake just prior to the tracer studies. Undoubtedly this intake was above that required for energy balance during the tracer studies because although the subjects were not at complete bedrest, they were allowed only limited physical movement during the protocol, particularly with respect to the forearms, because of the intravenous catheters placed in the contralateral hand vein and deep brachial vein. Thus, muscle contraction in the arms was limited and fuel utilization likely also was low. Although muscle uptake was higher during the HPA diet, the P value for skeletal muscle retention of PA did not quite reach statistical significance during the HPA diet. This persistent uptake of PA without augmented oxidation during the HPA diet could explain a previous finding that during the HPA diet, there was a higher muscle concentration of ceramide, which is synthesized from PA (2). However, as noted in the Materials and Methods, lack of equilibration of blood entering and exiting the skeletal muscle with unlabeled palmitoyl-CoA derived from intramyocellular triacylglycerol probably resulted in a minor underestimation of muscle PA oxidation rate, even with these studies of nonexercising subjects.
A specific and unique goal of the present study, though, was to determine whether a higher PA intake inhibited the progressive oxidation of the carbon chain derived from PA, and we employed a dynamic isotopic assessment of the rate of PA oxidation, rather than relying only on static measurements of AC concentrations. Clearly, in the present study, the rate of whole body oxidation of the thirteenth carbon of PA was not less than that of the first carbon during either diet. A recent study in myotubes showed that OA (but not PA) specifically stimulated the deacetylation and, thus, activation of PGC-1α and expression of MCAD, as well as other targets of PPARα or -δ moreover, OA increased complete oxidation of either PA or OA (48). In our previous study of humans fed the HPA and HOA diets, we found, however, no evidence that the diets altered the mRNA expression of PGC-1α or known targets of PPARα or -δ (9). However, our present findings related to the MCAC/LCAC ratio, as well as our previous results (2), are partially consistent with these in vitro effects, to the extent that accumulation of MCACs may reflect incomplete FA OX (48). Although the MCAC/LCAC ratio was increased on the HPA diet, the overall pattern of ACs, especially the relatively high C10:0 (during both diets) (Fig. 3), was not specifically characteristic of MCAD deficiency (41), nor is our previous observation that muscle mRNA expression of MCAD was not differentially affected by these diets (9). The high concentration of C14:1 AC during both diets (Fig. 3), compared with either C16:1 AC or C12:1 AC, is consistent with relatively impaired activity of LC acyl-CoA dehydrogenase (41). However, these ACs are present at very low concentrations and at the limit of detectability. So, caution in interpreting the AC profiling is needed.
It is also important to consider whether initial peroxisomal β-oxidation of PA and OA could have contributed to the observed discordance between “completeness” of FA OX assessed with our tracers and that assessed using AC profiling. In this study, we measured AC concentrations only in blood, not in muscle. In the heart, and presumably also in the liver, kidney, and other tissues, OA tends to undergo up to three cycles of β-oxidation in peroxisomes (generating C12:0 acyl-CoA) compared with one to two cycles for PA (generating 14:0 and 12:0 acyl-CoAs) (49). The first step of peroxisomal β-oxidation of FAs (dehydrogenation) is distinct from that occurring in mitochondria in that FAD is not the hydrogen acceptor, meaning that oxidation is effectively uncoupled from ATP production at this step (50). This observation could have thermogenic implications, particularly because we have observed relatively higher resting energy expenditure in subjects during the HOA diet (20). However, relatively more peroxisomal β-oxidation of OA would not explain why the MCAC/LCAC ratio was higher during the HPA diet, because this ratio is mainly dependent on the accumulation of ACs with chain lengths of less than 12 carbons.
In vitro, both C12:0 AC and C14:0 AC activated NF-κB in a murine monocytic cell line (15, 16), but in a recent study, this group did not detect evidence of increased cytokine release from human myotubes treated with C14:0 or C16:0 ACs (10). In this study, we examined whether diet differences in serum concentrations of MCLCs or LCACs correlated with diet differences in the secretion of NF-κB-regulated pro-inflammatory cytokines by PBMCs. Because we (2) previously reported strong sex differences in the differential effects of HPA and HOA diets on inflammatory and oxidant stress, as well as on serum AC profiles, we especially examined correlations between dietary differences in the concentrations of AC measured in the blood after about 9 h of repeated feedings of small meals (every 20 min) versus respective differences in cytokine secretion by PBMCs, collected in both the fasted state, about 11 h later, and then again 3 h after a breakfast meal (and a skeletal muscle biopsy conducted just prior to this meal). Our results must be considered preliminary because the sample sizes for women and men considered separately are small, although the Spearman rank correlation is not affected by outliers. In men, relatively higher concentrations of unsaturated ACs during the HPA diet were associated with enhanced NLRP3 inflammasome activity, assessed in the fasted state. However, in women, the relative accumulation of saturated ACs during the same diet seemed to be associated with enhanced activation of both the NLRP3 inflammasome and TLR4, because we observed correlations between diet differences in ACs and PBMC secretion of both IL-1β and TNFα in the fasted and fed (post-breakfast) states (5, 15). Interestingly, these correlations between diet differences in ACs and cytokine secretion by PBMCs could indeed relate to specific effects of ACs on inflammation (15) and not merely due to effects of higher PA retention on the HPA diet because: 1) there were no correlations between diet changes in whole body PA retention and respective changes in cytokine secretion by PBMCs; and 2) in men, cytokine secretion seemed to be affected by two ACs, which were not even different between diets (C12:1, C16:1). However, despite these potentially interesting correlations, we wish to reemphasize, as noted just above, that the AC studies and the PBMC studies were performed at different time points and under different conditions.
There are several caveats concerning our assessment of PA metabolism by the whole body as well as the “forearm muscle”. First, as explained in both the Materials and Methods and Results, the two PA tracers were administered on consecutive days, albeit in random order; spillover of enriched PA, presumably from VLDL, induced a slightly higher pretracer M + 1/M + 0 ratio for PA and CO2 on day 2 during both diets. As shown in the Results, this effect did not alter our conclusions relative to either the effect of the diets on the whole body rate of PA oxidation or the comparability of the rates of oxidation using the two tracers. Importantly, using only day 1 tracer data, we found very similar relative effects of the HPA diet on the whole body rate of PA oxidation compared with using both tracer days (and both tracers), and we found no difference between the oxidation of the two tracers using a simpler tracer model of the fraction of the PA tracer dose that was oxidized during each diet. Second, we did not attempt to exclude venous return from the hand during our sampling of deep brachial venous blood; therefore, forearm muscle metabolism of PA will include a presumably small fractional contribution from the hand and finger muscles. Third, adipocytes reside between muscle fibers; therefore, some of the uptake and oxidation of PA by the forearm will include an unknown contribution by these cells. It is not known whether these adipocytes will function like those in the subcutaneous adipose tissue, but we also acknowledge that we did not specifically assess PA metabolism by subcutaneous adipose tissue, although our assumption is that PA metabolism by this tissue was not included in our measurements because of the use of the deep brachial venous catheter. Fourth, in order to achieve “steady-state” kinetics in our PA oxidation studies, we administered the isotope and feedings every 20 min (21). This mode of feeding is apt to disrupt the normal rise and then fall in insulin and intestinal peptide secretion theoretically achieved with normal meal feeding, although individual differences in gastric emptying and intestinal motility will blur this distinction somewhat. However, our observation of a similar relative increase in the MCAC/LCAC ratio during the HPA diet (8%), as reported previously with meal feeding (10%) (2), suggests that this aspect of fat metabolism is not particularly different from that seen with normal feeding. Finally, we acknowledge that, in our protocol, the “effects” of the HOA diet on PA oxidation included those induced by 2–3 weeks of diet (e.g., changes in liver and muscle FA composition) and by the low PA intake per se during the tracer studies. There were practical and logistical issues related to other goals of our project, which in our view, necessitated that the subjects remain on the same diet for the entire 3 weeks. Moreover, a plausible effect of a low PA or HPA diet includes the acute effects of the FAs during each period of food ingestion. So, our project was designed to “capture” these various effects. Also, our previous study did not reveal effects of these diets on the expression of genes in skeletal muscle affecting FA OX (9); thus, we suggest that the “acute” effects of each meal ingested, differing in the FA composition, may play an important role on the biological and health effects of diets of varying FA composition.
In conclusion, our data show that a HPA diet leads to disproportionate retention of PA in cellular pools, but associated with both a higher total FA and PA oxidation rate. Also, for the first time, we have compared tracer data relating to completeness of FA OX with AC profiling in a relevant human population (two prevalent dietary patterns). The diets did not differentially affect the ratio of oxidation measured using the [13-13C]PA and [1-13C]PA tracers, suggesting that completeness of PA oxidation per se was not affected by the diets.
Supplementary Material
Acknowledgments
The authors are extremely grateful to the many research volunteers for their patience and hard work in enduring the rigorous protocol. They thank the staff of the University of Vermont College of Medicine Clinical Research Center for dietary, nursing, body composition and exercise services, administration, and informatics support. The authors are especially grateful to Betsy Cutler, RN, at the University of Vermont College of Medicine Clinical Research Center, for inserting the deep brachial catheters and to both Gene Barrett, MD and Linda Jahn, RN at the University of Virginia for helpful advice regarding this technique. Finally, the authors thank Michael Toth, PhD for his helpful advice in planning this project.
Footnotes
Abbreviations:
- AC
- acylcarnitine
- ER
- endoplasmic reticulum
- FA OX
- FA oxidation
- HOA
- low palmitic/high oleic acid
- HPA
- high palmitic acid
- IL
- interleukin
- Insig-1
- insulin induced gene-1 protein
- LCAC
- long-chain acylcarnitine
- LPS
- lipopolysaccharide
- MCAC
- medium-chain acylcarnitine
- NF-κB
- nuclear factor-κB
- NLRP3
- nucleotide oligomerization domain-like receptor protein 3
- OA
- oleic acid
- PA
- palmitic acid
- PA OX
- palmitic acid oxidation
- PBMC
- peripheral blood mononuclear cell
- Scap
- sterol regulatory element-binding protein cleavage-activating protein
- SREBP
- sterol regulatory element-binding protein
- TLR4
- toll-like receptor-4
This study was supported by National Institutes of Health Grant R01 DK082803, and these studies were conducted at the University of Vermont General Clinical Research Center, funded by grant RR00109 from the National Center for Research Resources, National Institutes of Health, US Public Health Service.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Kien C. L. 2009. Dietary interventions for metabolic syndrome: role of modifying dietary fats. Curr. Diab. Rep. 9: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kien C. L., Bunn J. Y., Poynter M. E., Stevens R., Bain J., Ikayeva O., Fukagawa N. K., Champagne C. M., Crain K. I., Koves T. R., et al. 2013. A lipidomics analysis of the relationship between dietary fatty acid composition and insulin sensitivity in young adults. Diabetes. 62: 1054–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shulman G. I. 2000. Cellular mechanisms of insulin resistance. J. Clin. Invest. 106: 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samuel V. T., Petersen K. F., Shulman G. I. 2010. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 375: 2267–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stienstra R., Tack C. J., Kanneganti T. D., Joosten L. A., Netea M. G. 2012. The inflammasome puts obesity in the danger zone. Cell Metab. 15: 10–18. [DOI] [PubMed] [Google Scholar]
- 6.Wen H., Gris D., Lei Y., Jha S., Zhang L., Huang M. T., Brickey W. J., Ting J. P. 2011. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 12: 408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muoio D. M., Newgard C. B. 2006. Obesity-related derangements in metabolic regulation. Annu. Rev. Biochem. 75: 367–401. [DOI] [PubMed] [Google Scholar]
- 8.Koves T. R., Li P., An J., Akimoto T., Slentz D., Ilkayeva O., Dohm G. L., Yan Z., Newgard C. B., Muoio D. M. 2005. PPARgamma coactivator-1alpha -mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J. Biol. Chem. 280: 33588–33598. [DOI] [PubMed] [Google Scholar]
- 9.Kien C. L., Bunn J. Y., Stevens R., Bain J., Ikayeva O., Crain K., Koves T. R., Muoio D. M. 2014. Dietary intake of palmitate and oleate has broad impact on systemic and tissue lipid profiles in humans. Am. J. Clin. Nutr. 99: 436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguer C., McCoin C. S., Knotts T. A., Thrush A. B., Ono-Moore K., McPherson R., Dent R., Hwang D. H., Adams S. H., Harper M. E. 2015. Acylcarnitines: potential implications for skeletal muscle insulin resistance. FASEB J. 29: 336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olivares A., Daza A., Rey A. I., Lopez-Bote C. J. 2010. Effect of diet saturation on growth performance, carcass characteristics and fat quality of heavy pigs. Food Sci. Technol. Int. 16: 321–327. [DOI] [PubMed] [Google Scholar]
- 12.Aoun M., Feillet-Coudray C., Fouret G., Chabi B., Crouzier D., Ferreri C., Chatgilialoglu C., Wrutniak-Cabello C., Cristol J. P., Carbonneau M. A., et al. 2012. Rat liver mitochondrial membrane characteristics and mitochondrial functions are more profoundly altered by dietary lipid quantity than by dietary lipid quality: effect of different nutritional lipid patterns. Br. J. Nutr. 107: 647–659. [DOI] [PubMed] [Google Scholar]
- 13.Brown J. M., Chung S., Sawyer J. K., Degirolamo C., Alger H. M., Nguyen T., Zhu X., Duong M. N., Wibley A. L., Shah R., et al. 2008. Inhibition of stearoyl-coenzyme A desaturase 1 dissociates insulin resistance and obesity from atherosclerosis. Circulation. 118: 1467–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X., Cao Y., Fu Y., Guo G., Zhang X. 2011. Liver fatty acid composition in mice with or without nonalcoholic fatty liver disease. Lipids Health Dis. 10: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams S. H., Hoppel C. L., Lok K. H., Zhao L., Wong S. W., Minkler P. E., Hwang D. H., Newman J. W., Garvey W. T. 2009. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J. Nutr. 139: 1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutkowsky J. M., Knotts T. A., Ono-Moore K. D., McCoin C. S., Huang S., Schneider D., Singh S., Adams S. H., Hwang D. H. 2014. Acylcarnitines activate proinflammatory signaling pathways. Am. J. Physiol. Endocrinol. Metab. 306: E1378–E1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. 2001. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- 18.Stern S. E., Williams K., Ferrannini E., Defronzo R. A., Bogardus C., Stern M. P. 2005. Identification of individuals with insulin resistance using routine clinical measurements. Diabetes. 54: 333–339. [DOI] [PubMed] [Google Scholar]
- 19.Ghanim H., Sia C. L., Upadhyay M., Korzeniewski K., Viswanathan P., Abuaysheh S., Mohanty P., Dandona P. 2010. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am. J. Clin. Nutr. 91: 940–949. [Erratum. 2011. Am. J. Clin. Nutr. 93: 674.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kien C. L., Bunn J. Y., Tompkins C. L., Dumas J. A., Crain K. I., Ebenstein D. B., Koves T. R., Muoio D. M. 2013. Substituting dietary monounsaturated fat for saturated fat is associated with increased daily physical activity and resting energy expenditure and with changes in mood. Am. J. Clin. Nutr. 97: 689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt D. E., Allred J. B., Kien C. L. 1999. Fractional oxidation of chylomicron-derived oleate is greater than that of palmitate in healthy adults fed frequent small meals. J. Lipid Res. 40: 2322–2332. [PubMed] [Google Scholar]
- 22.Rasmussen B. B., Holmback U. C., Volpi E., Morio-Liondore B., Paddon-Jones D., Wolfe R. R. 2002. Malonyl coenzyme A and the regulation of functional carnitine palmitoyltransferase-1 activity and fat oxidation in human skeletal muscle. J. Clin. Invest. 110: 1687–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper B. C., Sites C. K., Fairhurst P. A., Toth M. J. 2006. Evidence against a role for ovarian hormones in the regulation of blood flow. Fertil. Steril. 86: 440–447. [DOI] [PubMed] [Google Scholar]
- 24.Heymsfield S. B., Smith R., Aulet M., Bensen B., Lichtman S., Wang J., Pierson R. N., Jr 1990. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am. J. Clin. Nutr. 52: 214–218. [DOI] [PubMed] [Google Scholar]
- 25.Tataranni P. A., Ravussin E. 1995. Use of dual-energy X-ray absorptiometry in obese individuals. Am. J. Clin. Nutr. 62: 730–734. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe R. R., Jahoor F. 1990. Recovery of labeled CO2 during the infusion of C-1- vs C-2-labeled acetate: Implications for tracer studies of substrate oxidation. Am. J. Clin. Nutr. 51: 248–252. [DOI] [PubMed] [Google Scholar]
- 27.Mittendorfer B., Sidossis L. S., Walser E., Chinkes D. L., Wolfe R. R. 1998. Regional acetate kinetics and oxidation in human volunteers. Am. J. Physiol. 274: E978–E983. [DOI] [PubMed] [Google Scholar]
- 28.Sidossis L. S., Coggan A. R., Gastaldelli A., Wolfe R. R. 1995. A new correction factor for use in tracer estimations of plasma fatty acid oxidation. Am. J. Physiol. 269: E649–E656. [DOI] [PubMed] [Google Scholar]
- 29.Copeland K. C., Kenney F. A., Nair K. S. 1992. Heated dorsal hand vein sampling for metabolic studies: a reappraisal. Am. J. Physiol. 263: E1010–E1014. [DOI] [PubMed] [Google Scholar]
- 30.Toth M. J., Sites C. K., Cefalu W. T., Matthews D. E., Poehlman E. T. 2001. Determinants of insulin-stimulated glucose disposal in middle-aged, premenopausal women. Am. J. Physiol. Endocrinol. Metab. 281: E113–E121. [DOI] [PubMed] [Google Scholar]
- 31.Abumrad N. N., Rabin D., Diamond M. P., Lacy W. W. 1981. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism. 30: 936–940. [DOI] [PubMed] [Google Scholar]
- 32.Jensen M. D., Heiling V. J. 1991. Heated hand vein blood is satisfactory for measurements during free fatty acid kinetic studies. Metabolism. 40: 406–409. [DOI] [PubMed] [Google Scholar]
- 33.Baltzan M. A., Andres R., Cader G., Zierler K. L. 1962. Heterogeneity of forearm metabolism with special reference to free fatty acids. J. Clin. Invest. 41: 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Astrup A., Simonsen L., Bulow J., Christensen N. J. 1988. Measurement of forearm oxygen consumption: role of heating the contralateral hand. Am. J. Physiol. 255: E572–E578. [DOI] [PubMed] [Google Scholar]
- 35.Kien C. L. 1989. Isotopic dilution of CO2 as an estimate of CO2 production during substrate oxidation studies. Am. J. Physiol. 257: E296–E298. [DOI] [PubMed] [Google Scholar]
- 36.Bonen A., Luiken J. J., Liu S., Dyck D. J., Kiens B., Kristiansen S., Turcotte L. P., van der Vusse G. J., Glatz J. F. 1998. Palmitate transport and fatty acid transporters in red and white muscles. Am. J. Physiol. 275: E471–E478. [DOI] [PubMed] [Google Scholar]
- 37.Cortiella J., Matthews D. E., Hoerr R. A., Bier D. M., Young V. R. 1988. Leucine kinetics at graded intakes in young men: quantitative fate of dietary leucine. Am. J. Clin. Nutr. 48: 998–1009. [DOI] [PubMed] [Google Scholar]
- 38.Sidossis L. S., Wolfe R. R. 1996. Glucose and insulin-induced inhibition of fatty acid oxidation: the glucose-fatty acid cycle reversed. Am. J. Physiol. 270: E733–E738. [DOI] [PubMed] [Google Scholar]
- 39.Kien C. L., Everingham K. I., Stevens R. D., Fukagawa N. K., Muoio D. M. 2011. Short-term effects of dietary fatty acids on muscle lipid composition and serum acylcarnitine profile in human subjects. Obesity (Silver Spring). 19: 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Downey R. S., Mellone A., Matthews D. E. 1986. Effect of tracer infusion site on measurement of bicarbonate-carbon dioxide metabolism in dogs. J. Appl. Physiol. 60: 1248–1253. [DOI] [PubMed] [Google Scholar]
- 41.Roe C. R., Coates P. M. 1995. Mitochondrial fatty acid oxidation disorders. In The Metabolic and Molecular Basis of Inherited Disease. 7th edition. C. R. Scriver, A. L. Beaudet, W. S. Sly, et al., editors. McGraw-Hill, Inc., New York. 1501–1533. [Google Scholar]
- 42.Devlin T. M. 2002. Textbook of Biochemistry with Clinical Correlations, 5th edition. Wiley-Liss, New York. [Google Scholar]
- 43.Goldstein J. L., DeBose-Boyd R. A., Brown M. S. 2006. Protein sensors for membrane sterols. Cell. 124: 35–46. [DOI] [PubMed] [Google Scholar]
- 44.Horton J. D., Shah N. A., Warrington J. A., Anderson N. N., Park S. W., Brown M. S., Goldstein J. L. 2003. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. USA. 100: 12027–12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foufelle F., Ferre P. 2002. New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose: a role for the transcription factor sterol regulatory element binding protein-1c. Biochem. J. 366: 377–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Unger R. H. 2003. Lipid overload and overflow: metabolic trauma and the metabolic syndrome. Trends Endocrinol. Metab. 14: 398–403. [DOI] [PubMed] [Google Scholar]
- 47.Hagenfeldt L., Wahren J. 1968. Human forearm muscle metabolism during exercise. II. Uptake, release and oxidation of individual FFA and glycerol. Scand. J. Clin. Lab. Invest. 21: 263–276. [DOI] [PubMed] [Google Scholar]
- 48.Lim J. H., Gerhart-Hines Z., Dominy J. E., Lee Y., Kim S., Tabata M., Xiang Y. K., Puigserver P. 2013. Oleic acid stimulates complete oxidation of fatty acids through protein kinase A-dependent activation of SIRT1–PGC1alpha complex. J. Biol. Chem. 288: 7117–7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reszko A. E., Kasumov T., David F., Jobbins K. A., Thomas K. R., Hoppel C. L., Brunengraber H., Des R. C. 2004. Peroxisomal fatty acid oxidation is a substantial source of the acetyl moiety of malonyl-CoA in rat heart. J. Biol. Chem. 279: 19574–19579. [DOI] [PubMed] [Google Scholar]
- 50.Baillie R. A., Takada R., Nakamura M., Clarke S. D. 1999. Coordinate induction of peroxisomal acyl-CoA oxidase and UCP-3 by dietary fish oil: a mechanism for decreased body fat deposition. Prostaglandins Leukot. Essent. Fatty Acids. 60: 351–356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.