Abstract
Resolvins, maresins, and protectins can be formed from fish oils. These specialized pro-resolving mediators (SPMs) have been implicated in the resolution of inflammation. Synthetic versions of such SPMs exert anti-inflammatory effects in vitro and when administered to animal models. However, their importance as endogenous products formed in sufficient amounts to exert anti-inflammatory actions in vivo remains speculative. We biased our ability to detect SPMs formed in healthy volunteers by supplementing fish oil in doses shown previously to influence blood pressure and platelet aggregation under placebo-controlled conditions. Additionally, we sought to determine the relative formation of SPMs during an acute inflammatory response and its resolution, evoked in healthy volunteers by bacterial lipopolysaccharide (LPS). Bioactive lipids, enzymatic epoxyeicosatrienoic acids (EETs), and free radical-catalyzed prostanoids [isoprostanes (iPs)] formed from arachidonic acid and the fish oils, served as comparators. Despite the clear shift from ω-6 to ω-3 EETs and iPs, we failed to detect a consistent signal, in most cases, of SPM formation in urine or plasma in response to fish oil, and in all cases in response to LPS on a background of fish oil. Our results question the relevance of these SPMs to the putative anti-inflammatory effects of fish oils in humans.
Keywords: inflammation, resolution, lipids
The clinical cardiovascular utility of a diet rich in fish oils, particularly EPA and DHA, has been debated over the past 50 years (1–3). Large clinical outcome trials, such as the open-labeled GISSI-Prevenzione (4) or JELIS (5) studies, supported the notion that fish oil supplements confer therapeutic benefit on patients with cardiovascular disease. However, an overview analysis of results of more than 50 randomized controlled trials and cohort studies addressing this question yielded equivocal results (6, 7). Consequently, the adoption of the dietary interventions with fish oil into clinical guidelines has been limited (8). Fish oil supplementation does influence a series of cardiovascular biomarkers: it decreases blood levels of triglycerides in patients with hypertriglyceridemia, an effect primarily driven by lowering the production of triglycerides from nonesterified fatty acids (9); high doses reduce blood pressure in patients with essential hypertension (10, 11) and modestly inhibit indices of platelet activation (12). The mechanisms involved are unclear, but may involve a shift in formation of enzymatic and free radical-catalyzed prostanoids, reflecting utilization of EPA and DHA rather than arachidonic acid (AA) as a substrate. It has been speculated also that cardiovascular benefit might derive, in part, from anti-inflammatory actions of fish oils: families of bioactive lipids which favor resolution of inflammation have been suggested to be of particular importance (13). Synthetic versions of such specialized pro-resolving mediators (SPMs), products of transcellular metabolism of fish oils, exert anti-inflammatory effects in vitro and when administered in vivo in several animal models (14–17). Quantities of exogenous SPMs in these models, however, are substantial; 0.6 μg resolvin (Rv)D1 per mouse, for example, was used to attenuate the lipopolysaccharide (LPS)-induced inflammatory response in lung (18). These quantities are in marked contrast to the low picograms per milliliter concentrations reported in humans (19). Thus, the importance of SPMs as endogenous products formed in sufficient amounts to exert an anti-inflammatory action in vivo remains speculative. A particular limitation has been the use of assays in biological systems related to capacity, such as serum, rather than actual biosynthesis in a field where a marked discrepancy between the two approaches has long been recognized (20). Here, the fact that detection of SPMs is confounded by cells activated ex vivo in the test tube often remains unrecognized. An additional limitation has been of a technical nature. There are few data attesting to their formation in humans based on rigorous mass spectrometric methodology. Most clinical investigations reporting the formation of SPMs rely on immunoassays (21) or MS without synthetic internal standards structurally similar to the SPMs under investigation (22–27, 29–32), often in ex vivo stimulated systems (17, 33–36).
Here we biased our ability to detect SPMs formed in healthy volunteers by administering to them high doses of fish oils, such as have been shown to influence blood pressure and platelet aggregation under placebo-controlled conditions (11, 12). As a comparator, we also analyzed enzymatic [epoxyeicosatrienoic acids (EETs)] and free radical [isoprostanes (iPs)] catalyzed prostanoids formed from AA and the fish oils. Additionally, we sought to determine the relative formation of SPMs and these bioactive lipids during an acute inflammatory response and its resolution, evoked in healthy volunteers by administration of LPS. Despite a clear shift from ω-6 to ω-3 EETs and iPs, we failed to detect a consistent signal in most cases of SPM formation in urine or plasma in response to fish oil supplementation and in all cases in response to LPS on a background of fish oil supplementation.
METHODS
Human studies
Samples were available from a human study (clinicaltrials.gov registration number: NCT00682318) assessing the interaction of high doses of marine lipids delivered as Lovaza fish oil with ethanol on lipid peroxidation. Healthy volunteers (n = 12) received, in an open single-arm design, seven capsules LovazaTM three times a day (tid) for 24.2 ± 2.3 days (Fig. 1A). This dose delivered a total of 17.6 g/day ω-3 PUFA, consisting of 55.1% EPA (9.7 g/day) and 44.9% DHA (7.9 g/day). Prior to study enrollment, informed consent, approval by the Institutional Review Board, and authorization by the Food and Drug Administration (IND#79,750) had been obtained. Subjects were nonsmokers, not pregnant, and abstained from the use of high-dose vitamins, NSAIDs, and illicit drugs as assessed by cotinine (Craig Medical, Vista, CA) and pregnancy tests, history, platelet aggregometry (37), and a urine drug screen (RDI, Poteau, OK), respectively, for at least 2 weeks before enrollment and throughout the study. Health status and safety was assessed by routine medical history, physical exam, and laboratory work (hematology, biochemistry, and urinalysis) at the time of screening and on completion of the study. Subjects were counseled not to make major changes in their diet, and to refrain from consuming any additional fish foods to minimize exposure to contaminant heavy metals, including mercury, apparent in fish products. Compliance with this regimen was assessed daily by real-time text messaging or emailing the intake of LovazaTM capsules, weekly by capsule count in the Clinical and Translational Research Center, by the change of lipid ratios in red blood cell (RBC) membranes (Fig. 1C), and by diversion from AA-derived iPs and epoxides toward EPA/DHA-derived species (Fig. 1D–H).
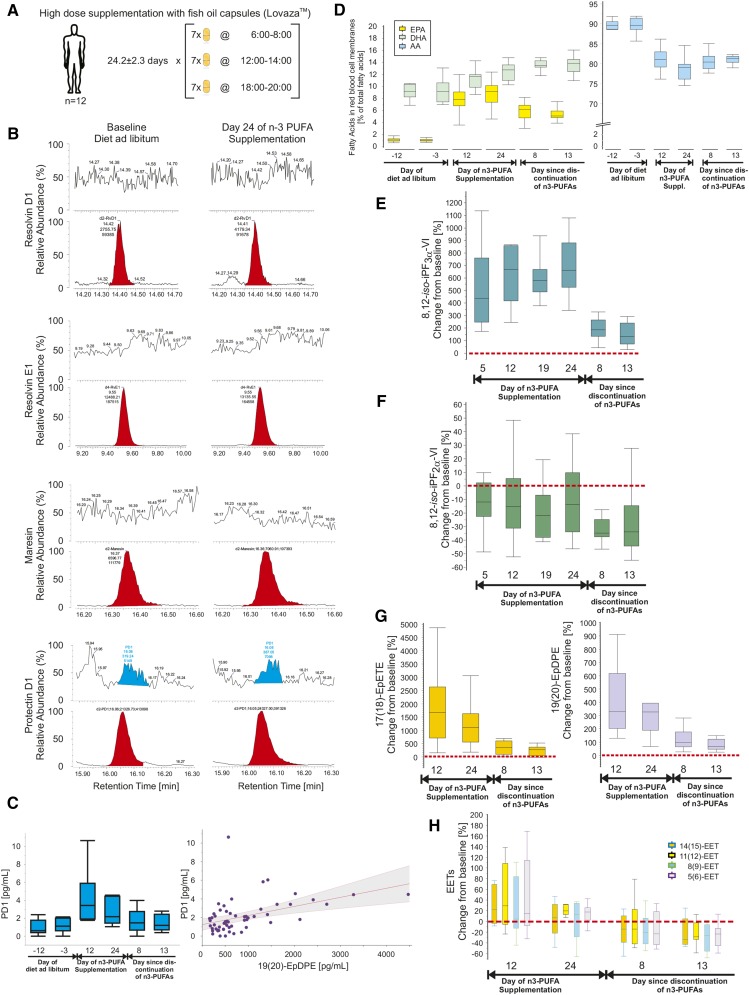
Fig. 1.
A: Study design of High Doses of Lovaza Fish Oils. Healthy volunteers, n = 12 (7 females, 58%; 30.8 ± 11.6 years of age) were supplemented with 17.6 g/day n-3 PUFAs (seven capsules tid of LovazaTM) for 24.2 ± 2.3 days. Timed blood and urine sampling were collected from 2 weeks prior until 2 weeks after the intervention. B: Representative chromatograms from plasma samples of a single study participant before and after supplementation with fish oil. The first panel shows signals for d2-RvD1 in red and no identifiable authentic RvD1; transitions monitored m/z 375→141 (RvD1), m/z 377→141 (d2-RvD1), CE 14 eV. The second panel shows signals for d4-RvE1 in red and no identifiable authentic RvE1; transitions monitored m/z 349→161 (RvE1), m/z 353→162 (d4-RvE1), CE 18 eV. The third panel shows signals for d2-maresin in red and no identifiable authentic maresin; transitions monitored m/z 359→177 (maresin), m/z 361→179 (d2-maresin), CE 14 eV. The fourth panel shows signals for d2-PD1 in red and authentic PD1 in blue; transitions monitored m/z 359→153 (PD1), m/z 361→153 (d2-PD1), CE 15 eV. Authentic PD1 appears in plasma in concentrations close to the level of detection; limitations are, however, that wide peaks compromise clear identification and that concentrations are not dose dependent. C: Mean ± SD plasma concentrations of plasma PD1 before, during, and after supplementation of healthy volunteers (n = 12) with high doses of fish oil; the red dotted line represents the approximated limit of detection for PD1 (left). The relationship of PD1 (ordinate) and plasma 19(20)-EpDPE (abscissa) shows a weak association between the two species derived from DHA; the area shaded in gray depicts the confidence curve fit (right). D: Ratios of EPA/AA and DHA/AA in RBC membranes before, during, and after supplementation with high doses of fish oil. E: Urinary 8,12-iso-iPF3α-VI, derived from EPA and DHA, as percent change from baseline (red dotted line). F: Urinary 8,12-iso-iPF2α-VI, derived from AA, as percent change from baseline (red dotted line). Baseline was averaged from n = 3 measurements during the 2 weeks prior to the start of supplementation with fish oil. G: Plasma 17(18)-EpETE (left), derived from EPA, and plasma 19(20)-EpDPE (right), derived from DHA, as percent change from baseline (red dotted line). H: Several EETs in plasma, derived from AA, as percent change from baseline (red dotted line). Boxplots indicate median, 25% and 75% quartiles and whiskers drawn to the furthest point within 1.5× interquartile range from the box. Note that the unit range on the ordinates varies considerably.
Plasma samples were also available from a subset of healthy volunteers (n = 6), studied to evaluate the systemic inflammatory response to experimental endotoxemia in subjects treated with lower doses of marine lipids delivered as Lovaza fish oil; please refer to (38) for further details (clinicaltrials.gov registration number: NCT01048502). In brief, this group of subjects was randomized to receive two capsules LovazaTM twice a day (bid) for 8 weeks in a double-blinded fashion, followed by an intravenous bolus of US standard reference endotoxin (LPS; lot number CCRE-LOT-1 +2; Clinical Center, Pharmacy Department at the National Institutes of Health, Bethesda, MD) dosed at 0.6 ng/kg body weight (Fig. 2A).
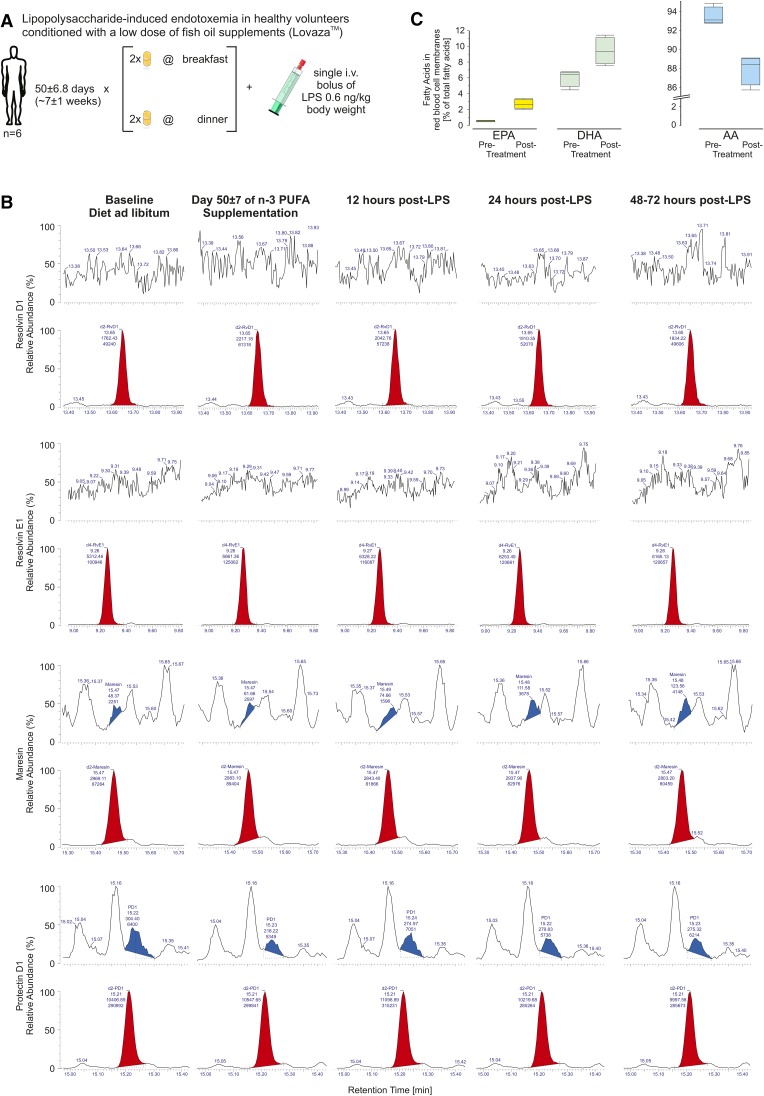
Fig. 2.
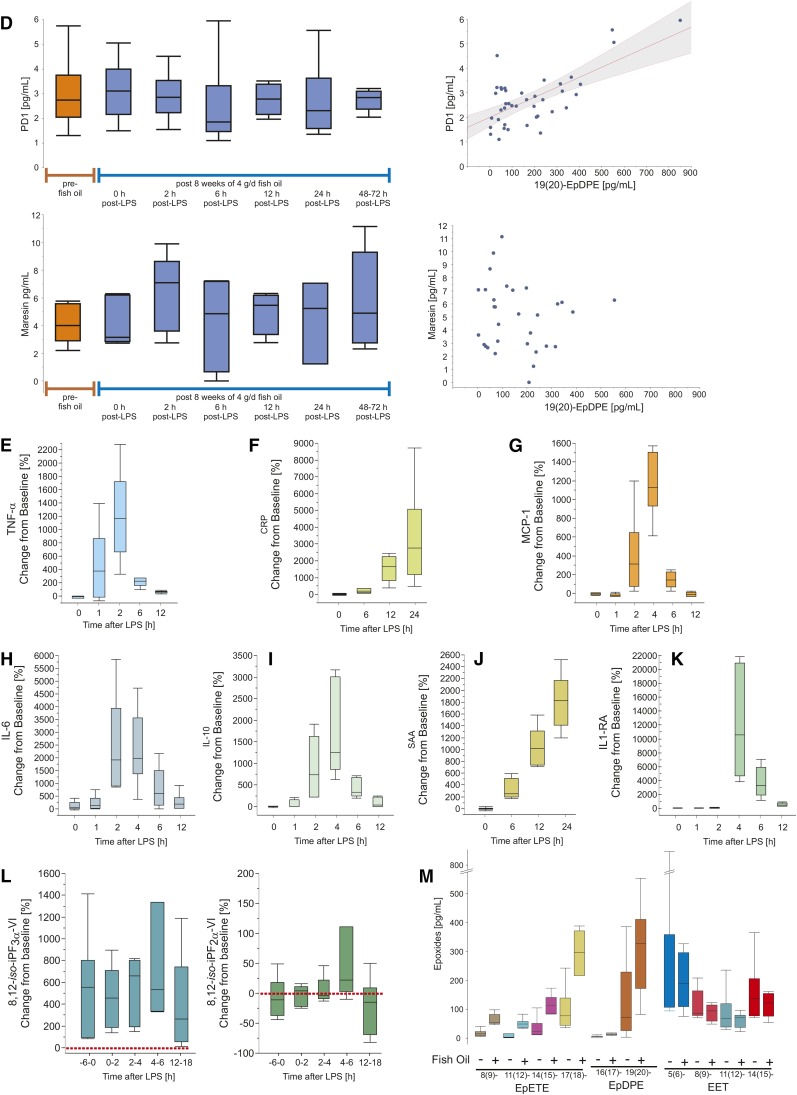
A: Study design of Evoked Endotoxemia. Healthy volunteers, n = 6 (3 females, 50%; 24.3 ± 4.3 years of age) were supplemented with 3.4 g/day n-3 PUFAs (two capsules bid of LovazaTM) for 50 ± 6.8 days followed by an intravenous bolus of 0.6 ng/kg body weight bacterial LPS. Timed blood and urine sampling were collected at enrollment, at the end of supplementation with n-3 PUFAs (corresponding to pre-LPS), and in short intervals during the intervention with LPS. B: Representative chromatograms from plasma samples of a single study participant before and at the end of the supplementation period with fish oil, as well as 12, 24, and 48–72 h post-intervention with LPS. The first panel shows signals for d2-RvD1 in red and no identifiable authentic RvD1; transitions monitored m/z 375→141 (RvD1), m/z 377→141 (d2-RvD1), CE 14 eV. The second panel shows signals for d4-RvE1 in red and no identifiable authentic RvE1; transitions monitored m/z 349→161 (RvE1), m/z 353→162 (d4-RvE1), CE 18 eV. The third panel shows signals for d2-maresin in red and authentic maresin in blue; transitions monitored m/z 359→177 (maresin), m/z 361→179 (d2-maresin), CE 14 eV. The fourth panel shows signals for d2-PD1 in red and authentic PD1 in white and with earlier retention time than its isomer PDX marked in blue; transitions monitored m/z 359→153 (PD1), m/z 361→153 (d2-PD1), CE 15 eV. Authentic maresin and PD1 appear in plasma in concentrations close to the level of detection; limitations are, however, that wide peaks compromise clear identification and that concentrations are not dose dependent. Chromatograms not shown for time points 2 and 6 h post-LPS. C: EPA and DHA percentage (left) and AA percentage (right) of total fatty acids in RBC membranes before and after supplementation with fish oil. D: Plasma concentrations of PD1 (top left) and maresin (bottom left) prior to and after conditioning with a clinical dose of fish oil (4 g/day), and subsequent acute challenge with LPS. The relationship of PD1 (ordinate) and 19(20)-EpDPE (abscissa) shows a weak association between the two species derived from DHA; area shaded in gray depicts the confidence curve fit (top right). Formation of maresin, also derived from DHA, is not associated with 19(20)-EpDPE (bottom right). E–K: The time course of TNF-α (E), CRP (F), MCP-1 (G), IL-6 (H), IL-10 (I), SAA (J), and IL1-RA (K) in plasma is shown as percent change from baseline, i.e., pre-fish oil measurements. Note that time point zero (0) denotes the condition pre-LPS which coincides with day 50 ± 6.8 of supplementing the healthy volunteers with two capsules LovazaTM bid. L: Urinary 8,12-iso-iPF3α-VI, derived from EPA and DHA, as percent change from baseline (pre-fish oil measurements, red dotted line) (left); urinary 8,12-iso-iPF2α-VI, derived from AA, as percent change from baseline (pre-fish oil measurements, red dotted line) (right). Note that time point zero (0) denotes the condition pre-LPS which coincides with day 50 ± 6.8 of supplementing the healthy volunteers with two capsules LovazaTM bid. M: Plasma concentrations in picograms per milliliter of EETs formed from AA (left), EpETEs derived from EPA (center), and EpDPEs formed from DHA (right) before and after 8 weeks of supplementing with two capsules LovazaTM bid. Boxplots indicate median, 25% and 75% quantiles and whiskers drawn to the furthest point within 1.5× interquartile range from the box. Note that the unit range on the ordinates varies considerably.
A pilot study in 21 healthy participants established baseline values of urinary 5-epi-8,12-iso-iPF3α-VI, 8,12-iso-iPF2α-VI, and iPF2α-III. All studies were approved by the Institutional Review Board and informed consent was obtained from the participants prior to study initiation.
Biospecimens
Blood (plasma).
For the study High Doses of Lovaza Fish Oils, venous blood was drawn into Na-heparin vacutainer tubes after an overnight fast on six occasions: two times as baseline (12.2 ± 6.0 days and 3.0 ± 0.0 days before the start of fish oil supplementation), two times during fish oil supplementation (12.0 ± 0.0 days and 24.2 ± 2.3 days after the start of fish oil supplementation), and two times after discontinuation of fish oil supplementation (7.9 ± 0.3 days and 13.3 ± 0.8 days after the stop of taking fish oil). Plasma was separated by immediate centrifugation (3,000 rpm, 15 min, 4°C) and stored at −80°C until extraction.
For the study Evoked Endotoxemia, venous blood was drawn into citrate vacutainer tubes after an overnight fast on seven occasions: one time as baseline (before the start of fish oil supplementation), one time after 50.0 ± 6.8 days of fish oil supplementation (which coincided as baseline prior to the injection of LPS), and five times after the intravenous injection of LPS (time course of 2, 6, 12, and 24 h post-LPS administration, as well as a follow-up any time between 48 and 72 h post-LPS). Plasma was separated by immediate centrifugation (3,000 rpm, 15 min, 4°C) and stored at −80°C until extraction.
Blood (RBC membranes).
For the study High Doses of Lovaza Fish Oils, venous blood was drawn into EDTA vacutainer tubes after an overnight fast on six occasions: two times as baseline (12.2 ± 6.0 days and 3.0 ± 0.0 days before the start of fish oil supplementation), two times during fish oil supplementation (12.0 ± 0.0 days and 24.2 ± 2.3 days after the start of fish oil supplementation), and two times after discontinuation of fish oil supplementation (7.9 ± 0.3 days and 13.3 ± 0.8 days after the stop of taking fish oil). The tubes were immediately centrifuged at 3,000 rpm for 15 min (4°C) to separate the RBCs from plasma and the buffy coat. RBCs were transferred to a clean tube and washed twice with ice-cold isotonic saline, followed each time by centrifugation for 10 min (3,000 rpm, 4°C). Washed RBCs were stored in saline (33:67 v/v) at −80°C until extraction. For the study Evoked Endotoxemia, RBCs were obtained from venous blood as described in (38).
Urine.
For the study High Doses of Lovaza Fish Oils, 12 h overnight urine samples were collected on eight occasions: two times as baseline (12.2 ± 6.0 days and 3.0 ± 0.0 days before the start of fish oil supplementation), four times during fish oil supplementation (5.0 ± 0.0 days, 12.0 ± 0.0 days, 19.1 ± 0.3 days, and 24.2 ± 2.3 days after the start of fish oil supplementation), and two times after discontinuation of fish oil supplementation (7.9 ± 0.3 days and 13.3 ± 0.8 days after the stop of taking fish oil supplementation). Urine aliquots with quality control samples were stored until extraction at −80°C.
MS
Materials.
The d4-RvE1, d2-RvD1, d2-PD1, and d2-maresin were synthesized and kindly furnished by Dr. Bernd Spur (39, 40). Maresin, RvD1, RvD2, PD1, 5(6)-EET, d11-5(6)-EET, 8(9)-EET, d11-8(9)-EET, 11(12)-EET, d11-11(12)-EET, 14(15)-EET, d11-14(15)-EET, 8(9)-EpETE, 11(12)-EpETE, 14(15)-EpETE, 17(18)-EpETE, 16(17)-EpDPE, 19(20)-EpDPE, AA, d8-AA, EPA, d5-EPA, DHA, and d5-DHA were purchased from Cayman Chemical Co. (Ann Arbor, MI). We verified that the deuterated internal standards for EETs, DHA, and EPA were not contaminated by authentic unlabeled lipids (supplementary Fig. 6a). Please note that protectin D1 (PD1) and neuroprotectin D1 are structurally equivalent, while protectin DX (PDX) is an isomer (41). The d3-creatinine was purchased from CDN Isotopes (Quebec, Canada). Synthetic d4-8,12-iso-iPF2α-VI and d4-5-epi-8,12-iso-iPF3α-VI were synthesized by Joshua Rokach, PhD, Florida Institute of Technology, Melbourne, FL, as previously described, and used as internal standards (42–46). Burdick and Jackson solvents were purchased from Honeywell. All water was freshly generated Millipore, 18.2 MΩ.
Sample preparation.
Plasma.
To 1 ml of plasma, a mix of deuterium-labeled internal standards in 50 μl acetonitrile (AcN) was added. Included in the internal standard was 1 ng each of d4-RvE1, d11-5(6)-EET, d11-8(9)-EET, d11-11(12)-EET, d11-14(15)-EET, and 0.2 ng each of d2-RvD1, d2-PD1, d2-maresin. The sample was gently mixed and allowed to equilibrate for 15 min. Formic acid (50 μl) was added immediately before applying the sample to a solid phase extraction (SPE) cartridge (StrataX, 30 mg; Phenomenex, Torrance, CA) that had been conditioned with 1 ml AcN followed by 0.25 ml water. The cartridge was washed with 1 ml water, dried by application of vacuum, and eluted with 1 ml 50% AcN/ethyl acetate. The eluate was dried under a stream of N2 and dissolved in 15 μl AcN. For analysis, 135 μl water was added and 75 μl was injected into the tandem mass spectrometer.
Urine.
Deuterium-labeled internal standards dissolved in 50 μl AcN were added to 2 ml of urine, gently mixed, and allowed to equilibrate for 15 min. The internal standard consisted of 5 ng each of d4-RvE1, d2-RvD1, d2-PD1, and d2-maresin. The sample was then acidified with 20 μl formic acid and applied to a StrataX SPE cartridge which was washed and eluted as described above. iPs were quantitated as described previously (47). In brief, 5 ng of each internal standard was added to 1 ml of urine. The SPE cartridge was conditioned with 1 ml of AcN and equilibrated with 0.25 ml of water. The sample was applied to the cartridge, which was then washed with 1 ml of 5% AcN in water and dried with vacuum for 15 min. The analyte and internal standards were eluted from the cartridge using 1 ml of 5% AcN in ethyl acetate. The eluate was collected and dried under a gentle stream of nitrogen. The resulting residue was reconstituted in 200 μl of 20% AcN in water and filtered by centrifugation using 0.2 μm Nylon Microspin filters (Spin-X HPLC Filter; Fisher Scientific). One hundred microliters were injected. Metabolite levels were corrected for urinary creatinine.
Urinary creatinine.
To 20 μl urine, 1 ml AcN containing 10 μg d3-creatinine was added. Ten microliters of this solution was injected into the tandem mass spectrometer. Mobile phase A consisted of AcN and mobile phase B consisted of 5 mM ammonium formate at pH 4.0. A Waters XBridge BEH HILIC (2.1 mm × 50 mm × 2.5 um) was used as the LC column. The LC was programmed at 3.5% mobile phase B at 350 μl/min flowrate.
Lipids from RBC membranes.
Lipids were extracted using a modified Folch method (48). RBCs (50 μl), mixed in 1:3 ratio with saline, were used per sample, and further diluted with an additional 950 μl of saline after thawing from the freezer. The RBCs were added to an internal standard (dried using nitrogen gas) containing labeled fatty acids (d8-AA, d5-EPA, and d5-DHA). The RBCs were homogenized in chloroform and methanol (2:1 v/v) containing 0.01% butylated hydroxytoluene as an antioxidant. The samples were then base hydrolyzed (KOH 7.5% in water) for 30 min. The pH was adjusted with formic acid to ∼2.5. The samples were purified by SPE.
LC/MS/MS analysis.
All lipid analyses were performed on a Xevo TQ-S tandem mass spectrometer interfaced with an Acquity ultra-performance liquid chromatograph (Waters, Milford, MA), except for the analysis of urinary iPs, which was run on a TSQ Quantum Ultra instrument (Thermo; please see instrument parameters below). The mass spectrometer was operated in the negative ion ESI multiple reaction monitoring (MRM) mode using argon as the collision gas. The ESI probe capillary potential was 2.8 kV. The source was maintained at 150°C; the desolvation temperature was 350°C. All samples were chromatographed on a BEH C18 UPLC column (150 mm × 2.1 mm × 1.7 μ particle size; Waters) with a flow rate of 350 μl/min. Mobile phase A consisted of Millipore water containing 0.05% acetic acid, adjusted to pH 5.7 with ammonium hydroxide. Mobile phase B was AcN/methanol (95:5). Solvent AcN Optima® (Fisher Scientific) and methanol (Burdick and Jackson) were HPLC grade and met ACS specifications.
LC/MS/MS analysis of SPMs in urine.
The UPLC gradient was held at 10% B for 1 min, followed by a linear gradient to 22% at 10 min, 38% at 13 min, and 48% at 20 min. The column was then washed for 2 min with 100% B before returning to initial conditions, which were held for a 5 min equilibration period. Transitions monitored were m/z 349→161 (RvE1), m/z 353→162 (d4-RvE1) collision energy (CE) 18 eV; m/z 375→141 (RvD1/RvD2), m/z 377→141 (d2-RvD1) CE 14 eV; m/z 359→153 (PD1), m/z 361→153 (d2-PD1) CE 15 eV; m/z 359→177 (maresin), m/z 361→179 (d2-maresin) CE 14 eV.
LC/MS/MS analysis of iPs in urine.
The HPLC included an Accela solvent delivery system (Thermo) and a Hypersil GOLD C18 (2), 200 mm × 2.1 mm, 1.9 μm particle size column (Thermo). The mobile phase consisted of water (solvent A) and acetonitrile:methanol (95:5, solvent B), both with 0.005% acetic acid adjusted to pH 5.7 with ammonium hydroxide. The flow rate was 350 μl/min. The mobile phase gradient began at 5% B, increased linearly to 18.5% B at 45 min, then to 38% B at 65 min. A TSQ Quantum Ultra instrument (Thermo) equipped with a heated electrospray source and a triple quadrupole analyzer were used in these studies. The ESI source used nitrogen for both sheath and auxiliary gas, set to 70 and 0 arbitrary units, respectively. The mass spectrometer was operated in the negative ion mode with a capillary temperature of 350°C and a spray voltage of 0 kV. The source offset was 6 V. The analyzer was operated in the MRM mode for the analysis of urinary iPs. The transitions monitored were: m/z 353 > 115 for the endogenous 8,12-iso-iPF2α-VI and m/z 357 > 115 for the corresponding tetradeuterated internal standard. The CE was 24 eV. For the endogenous 5-epi-8,12-iso-iPF3α-VI, the transitions monitored were m/z 351 > 115 and m/z 355 > 115 for the corresponding tetradeuterated internal standard. The CE was 23 eV.
LC/MS/MS analysis of SPMs/epoxides in plasma.
The UPLC was held at 10% B for 1 min, and then programmed linearly to 22% at 10 min, 40% at 14.5 min, and 53% at 27.5 min. The column was then washed with 100% B for 2 min and allowed to equilibrate at initial conditions for 5 min prior to the next injection. Transitions monitored were those of urine (above) and m/z 319→191 [5(6)-EET], m/z 330→202 [d11-5(6)-EET] CE 11 eV; m/z 319→155 [8(9)-EET], m/z 330→155 [d11-8(9)-EET] CE 14 eV; m/z 319→167 [11(12)-EET], m/z 330→167 [d11-11(12)-EET] CE 14 eV; m/z 319→257 [14(15)-EET], m/z 330→268 [d11-14(15)-EET] CE 14 eV; m/z 343→233 [16(17)-EpDPE] CE 12 eV; and m/z 343→299 [19(20)-EpDPE] CE 12 eV.
LC/MS/MS analysis of creatinine in urine.
A Thermo Ultima LC/MS/MS interfaced to an Accela UPLC (Thermo) was used in the positive ion chemical ionization MRM mode using argon as the collision gas. The MS interface capillary was held at 350°C. The chemical ionization probe was set at 350°C. The discharge current was 4.0 μA. The column was an XBridge BEH HILIC, 50 mm × 2.1 mm × 2.5 μ particle size (Waters). Mobile phase A was AcN; mobile phase B was 100 mM ammonium formate, adjusted to pH 4.0 with acetic acid. The UPLC program was isocratic at 7.5% B. Transitions monitored were m/z 114→86 (creatinine) and m/z 117→89 (d3-creatinine) at CE 12 eV.
LC/MS/MS analysis of lipids from RBC membranes.
The analyzer was operated in the MRM mode for the analysis of the fatty acids EPA, DHA, and AA. The transitions monitored were m/z 301.2 > 257.2 for the endogenous EPA and m/z 306.2 > 262.2 for d5-EPA; m/z 327.2 > 283.2 for the endogenous DHA and m/z 332.2 > 288.2 for d5-DHA; m/z 303.2 > 259.2 for the endogenous AA and m/z 311.2 > 267.2 for d8-AA. CE was 12 eV for all compounds.
Limit of detection calculations
RvD1, RvE1, and maresin were not observed by UPLC/MS/MS in the study involving administration of high doses of Lovaza fish oils. To determine the actual limit of detection of the samples in question, we identified and integrated one or more peaks, with a signal-to-noise ratio of three or greater, that eluted near the expected retention time for the compound in question. When the areas of these peaks are divided by the areas of the deuterated standards, we obtain a value in picograms (supplementary Fig. 3a). Because no peak was observed at the correct retention time, the amount is therefore less than that of the selected peaks. For the RvD1 samples shown in Fig. 1B, the integrated peaks ranged from 3.4 to 4.3 pg. When the volume of the sample is taken into account, this calculates to a range of 3.9 to 4.2 pg/ml of plasma. For the RvE1 samples in Fig. 1B, the limit of detection ranges from 7.8 to 10.4 pg/ml of plasma. For maresin, the limit of detection ranges from 3.4 to 3.6 pg/ml of plasma. A limit of detection of 0.2–0.3 pg/ml of plasma was approximated for PD1 using the signals depicted in Fig. 1. Noise was calculated as root mean square from selected time windows in the chromatograms. The limit of detection was then extrapolated to discriminate a signal from noise at a ratio of 3:1.
In the study of lower dose Lovaza fish oils in which volunteers were administered LPS, RvD1 and RvD2 were not observed. Calculations analogous to those above show the limit of detection for the RvD1 samples shown in Fig. 2B to range from 3.4 to 6.1 pg/ml of plasma. For the RvE1, the range is from 6.8 to 10.0 pg/ml of plasma. The limit of detection for PD1, based on the signals of Fig. 2 and determined as described above, ranged from 0.1 to 0.3 pg/ml. Notably for one sample, the limit of detection was one order of magnitude lower at 0.02 pg/ml.
Recovery
The analytes with homologous internal standards were RvE1, PD1, maresin, 5(6)-EET, 8(9)-EET, 11(12)-EET, and 14(15)-EET. The remaining nonpolar compounds were quantitated against d11-14(15)-EET, that is 8(9)-EpETE, 11(12)-EpETE, 14(15)-EpETE, 17(18)-EpETE, 16(17)-EpDPE, and 19(20)-EpDPE.
The recovery of each compound for which a homologous internal standard was available was calculated from the average area of the UPLC/MS/MS internal standard peak from each sample divided by the average area of two internal standards that were not subjected to SPE. In detail, the recoveries amounted to 76% for RvE1, 77% for PD1, 71% for maresin, 69% for 5(6)-EET, 81% for 8(9)-EET, 84% for 11(12)-EET, and 83% for 14(15)-EET. The recovery was not calculable for the compounds for which a homologous internal standard was unavailable.
Standard curve
The relative UPLC/MS/MS response of compounds, for which d11-14(15)-EET was used as a heterologous internal standard, was obtained by generating a six-point standard curve, 0, 1, 5, 10, 50, and 100 ng d11-14(15)-EET in triplicate, in which the amount of d11-14(15)-EET was constant and the amount of the target analyte varied (supplementary Fig. 5). The equations derived from the linear regression from these standard curves were used to adjust the response of the analytes in the actual samples.
Markers of inflammatory response
Details on the analysis of the panel of inflammatory responses to the administration of LPS, consisting of TNF-α, interleukin (IL)-6, IL-10, IL-1-receptor antagonist (RA), monocyte chemoattractant protein-1 (MCP-1), C-reactive protein (CRP), and serum amyloid A (SAA), have been published in (38).
Statistical analyses
Descriptive statistics were executed in JMP® Pro Version 10. Means ± SDs are depicted unless otherwise stated. Averaged baseline measurements served as the reference point to calculate percent changes from baseline. Tests of significance were not conducted due to the exploratory nature of this study.
RESULTS
Human subjects and safety
Twelve subjects concluded the study High Doses of Lovaza Fish Oils (supplementary Table 1); one subject discontinued due to personal reasons (CONSORT diagram in supplementary Fig. 1a). Three subjects deviated from the supplementation regimen of seven capsules LovazaTM tid; that is, subject identification number (ID#) 31 took six capsules instead of seven on four occasions; morning dose on days 5 and 10 of supplementation, evening dose on day 8, and midday dose on day 12 during the 24 day dosing period due to difficulties in swallowing the capsules. Subject ID# 34 missed the morning dose on day 14. Due to oily diarrhea, the period of fish oil supplementation was shortened by 11 days and reduced to four capsules LovazaTM tid (5,580 mg/day EPA and 4,500 mg/day DHA) for the last 5 days by subject ID# 34. Similar adverse events in subject ID# 37.1 accounted for the reduction to four capsules of LovazaTM tid after 5 days of protocol-conform fish oil supplementation. The safety laboratory assessments at the screening and exit visits were both within normal range for the complete blood count, blood chemistry, lipid, and coagulation panels (supplementary Table 2). Spontaneous blood pressure was, on average (across all clinical visits), 120 ± 13 mmHg over 71 ± 10 mmHg (supplementary Fig. 1b); spontaneous heart rate was 71 ± 12 beats/min (supplementary Fig. 1c); oral temperature was 97.6 ± 0.9°F. Serious adverse events did not occur. The adverse event profiles are listed in supplementary Table 6 for the High Doses of Lovaza Fish Oils study and in supplementary Table 7 for the Evoked Endotoxemia study.
Lipidomics
SPMs.
RvD1 and RvE1 were not detected in plasma samples from both clinical studies, as evident in the chromatograms of Figs. 1B, 2B. For PD1, peaks in plasma were seen which met the LC and MS/MS criteria for authentic material (chromatograms of Figs. 1B, 2B). Baseline PD1 concentrations in plasma amounted to 1.0 ± 0.8 pg/ml and 1.2 ± 0.8 pg/ml, which rose to 4.1 ± 2.8 pg/ml and 2.7 ± 1.4 pg/ml after 12 and 24 days, respectively, of supplementation with high doses of fish oil; PD1 dropped to 1.7 ± 1.3 pg/ml and 1.4 ± 0.9 pg/ml at 8 and 13 days after discontinuation of fish oil (Fig. 1C, left). PD1 concentrations in plasma after 4 g fish oil per day over 8 weeks of 3.1 ± 1.2 pg/ml were not different from baseline PD1 of 3.0 ± 1.5 pg/ml. Acute LPS had no discernable impact on PD1 concentrations in plasma, either in the inflammatory or resolution phases, as evident from 2.9 ± 1.0 pg/ml at 2 h post-LPS, followed by 2.5 ± 1.8 pg/ml, 2.8 ± 2.8 pg/ml, 2.7 ± 1.5 pg/ml, and 2.8 ± 0.4 pg/ml at 6, 12, 24, and 48–72 h post-LPS, respectively (Fig. 2D, top left). PDX, the isomer of PD1, was present in plasma samples from the evoked endotoxemia study in low picogram per milliliter concentrations, but without apparent modulation by either fish oil or LPS (supplementary Table 3, supplementary Fig. 3b). While maresin was consistently below the limit of detection before and after supplementation with high doses of fish oil, plasma samples before and after the administration of LPS showed mean concentrations between 4 and 6 pg/ml. In detail, 4.2 ± 1.4 pg/ml maresin as baseline before the initiation of fish oil was comparable to 4.3 ± 1.8 pg/ml after 8 weeks of supplementation. At 2 h post-LPS, maresin amounted to 6.3 ± 2.8 pg/ml, followed by 4.2 ± 3.5 pg/ml, 5.0 ± 1.6 pg/ml, 4.5 ± 3.0 pg/ml, and 5.8 ± 3.5 pg/ml at 6, 12, 24, and 48–72 h post-LPS administration, respectively (Fig. 2D, bottom). Rvd1, RvE1, maresin, and PD1 were not detected in urine from subjects supplemented with high doses of fish oil (chromatograms not shown).
Epoxides.
Plasma 17(18)-EpETE and 19(20)-EpDPE, two epoxides formed from EPA and DHA, respectively, were markedly modulated by the dietary intervention with high doses of fish oils. The 17(18)-EpETE increased from baseline values of 0.1 ± 0.1 ng/ml and 0.2 ± 0.1 ng/ml to 2.0 ± 0.8 ng/ml (1,820 ± 1,319%, Fig. 1G) and 1.7 ± 1.4 ng/ml (1,175 ± 793%, Fig. 1G) by days 12 and 24 of supplementation, falling to 0.6 ± 0.4 ng/ml and 0.4 ± 0.3 ng/ml 1 and 2 weeks post-intervention. Concentrations in a comparable range were observed for 19(20)-EpDPE: 0.3 ± 0.1 ng/ml as baseline, 1.5 ± 0.7 ng/ml (411 ± 256%, Fig. 1G) and 1.6 ± 1.2 ng/ml (355 ± 227%, Fig. 1G) at days 12 and 24 of supplementation, and 0.7 ± 0.4 ng/ml and 0.6 ± 0.3 ng/ml at 1 and 2 weeks after discontinuing fish oil. A similar modulation was seen for the EPA-derived epoxide species in plasma, 8(9)-EpETE, 11(12)-EpETE, and 14(15)-EpETE, as well as for the DHA-derived epoxide, 16(17)-EpDPE (supplementary Table 4). The ω-6 PUFA-derived epoxides in plasma, EETs, were not influenced by fish oil administration (Fig. 1, supplementary Table 4).
Supplementation with low doses of Lovaza fish oils increased the formation of 8(9)-EpETE by 419 ± 265% compared with pre-fish oil baseline, 11(12)-EpETE by 1,482 ± 1,239%, 14(15)-EpETE by 432 ± 288%, and 17(18)-EpETE by 311 ± 199% (absolute concentrations reported in supplementary Table 5). Similarly, 16(17)-EpDPE rose by 255 ± 190% and 19(20)-EpDPE by 1,375 ± 2,181%. Exposure to LPS did not induce directional changes in these epoxides. Neither fish oil nor LPS altered the plasma concentrations of EETs (supplementary Table 5).
iPs.
The ω-3 PUFA-derived iP, 5-epi-8,12-iso-iPF3α-VI, increased in urine from pretreatment values of 1.0 ± 0.3 ng/mg and 0.9 ± 0.4 ng/mg creatinine to 6.1 ± 1.9 ng/mg and 7.2 ± 2.2 ng/mg creatinine on days 12 and 24 of high dose supplementation, falling back to 2.8 ± 0.8 ng/mg and 1.5 ± 0.3 ng/mg creatinine at 1 and 2 weeks after discontinuing fish oil (Fig. 1E). Urinary 8,12-iso-iPF2α-VI, a ω-6 PUFA-derived iP, showed no modulation by the intervention; mean concentrations amounted to 3–4 ng/mg creatinine (Fig. 1F). The detection of ω-3 PUFA-derived iPs was established in healthy subjects on an ad libitum diet in a prior pilot study (unpublished observations). Urinary 5-epi-8,12-iso-iPF3α-VI concentrations amounted to 1.3 ± 0.4 ng/mg creatinine, while the well-established ω-6 PUFA-derived iPs, 8,12-iso-iPF2α-VI and the less abundant iPF2α-III, reached 7.5 ± 3.1 ng/mg and 0.7 ± 0.3 ng/mg creatinine, respectively, in a separate cohort of 21 healthy participants (age 28 ± 7.4 years of age; 12 male, 29% African American and 81% Caucasian).
Eight weeks of supplementation with 4 g/day Lovaza elevated urinary 5-epi-8,12-iso-iPF3α-VI from 0.7 ± 0.4 ng/mg to 3.5 ± 2.3 ng/mg creatinine. At 1 h post-LPS, 5-epi-8,12-iso-iPF3α-VI amounted to 3.3 ± 1.5 ng/mg creatinine, followed by 3.9 ± 1.0 ng/mg, 6.0 ± 5.3 ng/mg, and 2.8 ± 2.2 ng/mg creatinine at 2, 4, and 24 h post-LPS administration, respectively (Fig. 2I, left). Concentrations of the ω-6 PUFA-derived 8,12-iso-iPF2α-VI in urine showed no directional changes; 8.6 ± 4.5 ng/mg creatinine before and 7.5 ± 3.0 ng/mg creatinine after supplementation with fish oil, succeeded by 8.5 ± 4.9 ng/mg, 9.3 ± 3.6 ng/mg, 14.0 ± 10.4 ng/mg, and 6.0 ± 3.1 ng/mg creatinine at 1, 2, 4, and 24 h post-LPS administration, respectively (Fig. 2I, right).
Ratios of ω-3 and ω-6 PUFA in RBCs.
The ratios of ω-3 to ω-6 PUFA were assessed in erythrocytes to monitor the compliance with the high dose supplementation regimen. The relationship of EPA/AA rose by one order of magnitude from pretreatment 0.012 ± 0.004 and 0.011 ± 0.003 to 0.096 ± 0.031 and 0.113 ± 0.033 by days 12 and 24 of supplementation, falling to 0.073 ± 0.021 and 0.065 ± 0.017 at 1 and 2 weeks post-intervention. The ratio of DHA/AA in erythrocytes increased from 0.108 ± 0.025 and 0.103 ± 0.022 to 0.136 ± 0.024 and 0.158 ± 0.02 on days 12 and 24 on fish oil to 0.169 ± 0.021 for both 1 and 2 weeks post-fish oil (Fig. 1D).
In the endotoxemia study, ω-3 to ω-6 PUFAs were quantified as percentage of the total fatty acid pool in erythrocytes. Here, EPA increased from 0.5 ± 0.1% at baseline to 2.7 ± 0.6% after 8 weeks of 4 g/day Lovaza, while DHA rose form 6.0 ± 1.1% to 9.4 ± 1.7%. AA changed from 93.5 ± 1.0% at baseline to 87.0 ± 1.6% after taking fish oil (Fig. 2C)
Markers of inflammation
Administration of LPS elevated a panel of cytokines and acute phase proteins (Fig. 2E–K). The maximum increase in plasma concentrations of TNF-α occurred 2 h post-administration of LPS, from initially 0.8 ± 0.3 pg/ml prior to LPS to 12.1 ± 4.3 pg/ml. CRP rose from 0.6 ± 0.5 pg/ml to 10.5 ± 7.1 pg/ml at 24 h after LPS; MCP-1 from 142.0 ± 22.9 pg/ml to 1,919.6 ± 703.4 pg/ml at 4 h after LPS; IL-6 from 3.7 ± 2.5 pg/ml to 56.6 ± 34.8 pg/ml at 2 h after LPS; IL-10 from 0.9 ± 0.3 pg/ml to 15.1 ± 7.5 pg/ml at 4 h after LPS; SAA from 4.1 ± 2.4 pg/ml to 74.8 ± 25.7 pg/ml at 24 h after LPS; and IL-1-RA from 152.1 ± 55.8 pg/ml to 16,710 ± 9,061 pg/ml at 4 h post-LPS administration (Table 1).
TABLE 1.
Markers of inflammation in the human study Evoked Endotoxemia
| Marker | Unit | Pre-Fish Oil | After ∼8 Weeks of 4 g/day Fish Oil | ||||||
| 0 h | 1 h Post-LPS | 2 h Post-LPS | 4 h Post-LPS | 6 h Post-LPS | 12 h Post-LPS | 24 h Post-LPS | |||
| TNF-α | pg/ml | 1.1 ± 0.4 | 0.8 ± 0.3 | 4.9 ± 4.1 | 12.1 ± 4.3 | — | 3.1 ± 0.9 | 1.7 ± 0.5 | — |
| % | 0.0 | −15.5 ± 27.4 | 463.9 ± 533.4 | 1213 ± 670 | — | 214.9 ± 65.5 | 64.5 ± 18.8 | — | |
| CRP | mg/L | 0.5 ± 0.5 | 0.6 ± 0.5 | — | — | — | 1.2 ± 0.8 | 5.5 ± 1.5 | 10.5 ± 7.1 |
| % | 0.0 | 10.7 ± 45.6 | — | — | — | 214.5 ± 128.5 | 1,551.7 ± 771.7 | 3,336.4 ± 2,890.2 | |
| MCP-1 | pg/ml | 148.3 ± 26.9 | 142.0 ± 22.9 | 122.7 ± 30.4 | 684.2 ± 428.2 | 1,919.6 ± 703.4 | 373.7 ± 151.8 | 133.2 ± 25.2 | — |
| % | 0.0 | −3.0 ± 14.0 | 401.6 ± 425.2 | 1,160 ± 342.7 | 146.0 ± 83.7 | −16.7 ± 16.7 | −7.5 ± 24.0 | — | |
| IL-6 | pg/ml | 1.1 ± 0.5 | 3.7 ± 2.5 | 5.0 ± 2.9 | 56.6 ± 34.8 | 27.2 ± 11.6 | 11.1 ± 8.9 | 9.1 ± 8.7 | — |
| % | 0.0 | 121.9 ± 163.1 | 2,458.4 ± 1,885.3 | 2,318.8 ± 1,485.2 | 805.4 ± 795.2 | 217.1 ± 286.8 | 268.8 ± 331.0 | — | |
| IL-10 | pg/ml | 0.9 ± 0.4 | 0.9 ± 0.3 | 1.6 ± 1.3 | 9.1 ± 6.4 | 15.1 ± 7.5 | 4.5 ± 1.7 | 1.7 ± 0.9 | — |
| % | 0.0 | 6.5 ± 27.6 | 57.8 ± 93.0 | 892.4 ± 696.5 | 1,699.2 ± 1,079.2 | 413.7 ± 222.0 | 89.4 ± 109.8 | — | |
| SAA | mg/l | 4.3 ± 2.7 | 4.1 ± 2.4 | — | — | — | 16.4 ± 6.9 | 44.7 ± 16.7 | 74.8 ± 25.7 |
| % | 0.0 | 0.2 ± 24.0 | — | — | — | 326.0 ± 171.1 | 1,048.3 ± 332.6 | 1,817.2 ± 458.4 | |
| IL-1-RA | pg/ml | 150.3 ± 44.8 | 152.1 ± 55.8 | 150.2 ± 56.1 | 261.0 ± 118.3 | 16,710 ± 9,061 | 5,171 ± 2,212 | 880.1 ± 257.3 | — |
| % | 0.0 | 3.0 ± 35.7 | −0.37 ± 22.5 | 75.0 ± 58.8 | 12,076 ± 8,047 | 3,740 ± 2,198 | 527.0 ± 271.4 | — | |
DISCUSSION
Derivatives of fish oils have been implicated in a variety of biological activities (49), including the resolution of inflammation (50). Although the therapeutic efficacy of dietary supplementation with fish oils remains controversial (6, 7), observational studies have correlated diets rich in fish with desirable clinical outcomes (1, 51). Whether this relates to a substitution of dietary saturated fatty acids, a direct effect of fish oils, or confounding lifestyle factors associated with a diet rich in fish is unknown. When fish oils, such as EPA and DHA, substitute for dietary AA, there is a shift in formation from dienoic prostanoids (e.g., PGE2) to trienoic compounds (e.g., PGE3), which may differ in biological properties when applied in vitro (52). In recent years, there has been considerable interest in the transcellular formation of bioactive lipids, such as might result from substrate exchange between platelets, neutrophils, and endothelial cells (50). In particular, transcellular products formed from fish oils (resolvins, maresins, and protectins) have exhibited anti-inflammatory properties when administered in vitro or to animal models (53, 54). While such observations highlight the therapeutic potential of synthetic SPMs, their relevance as endogenous mediators of inflammation is more controversial. Interpretation of the literature is constrained by two limitations: i) the use of nonspecific immunoassays; and ii) the extrapolation of assays of cellular capacity to form SPMs, often with manipulation ex vivo, to infer actual rates of biosynthesis. Both limitations confounded estimates of prostanoid formation in the past; thus the capacity to form prostaglandins greatly exceeds actual biosynthetic rates (55), and immunoassays often grossly overestimated amounts of these compounds detected in biological fluids by more precise physicochemical methods (56).
Here we sought evidence for formation of bioactive lipids at baseline in healthy volunteers receiving either a high dose of marine lipids delivered as Lovaza fish oil (21 g/day), known to modulate blood pressure and platelet function (11, 12), or a dosing regimen (4 g/day) more commonly used in clinical trials designed to seek therapeutic efficacy (57). In the latter case, we further characterized the lipidomic response to LPS, both during the evoked inflammation and during the period of its resolution.
In both studies, supplementation with fish oils shifted the bioactive lipid profile in vivo. This was apparent in a supplementation-related increase in the ratio of EPA and DHA to AA in erythrocyte membranes and in the endogenous biosynthesis of ω-3-derived products of the epoxygenase enzymes and free radical-catalyzed formation of iPs. Dose-dependent changes in these compounds were observed, exemplified by the respective 3- and 10-fold increase from baseline for the EPA-derived epoxide, 18(19)-EpETE, at the two doses of fish oils. By contrast, SPMs were either undetectable or, when identified in trivial amounts, appeared largely unrelated to the periods or doses of fish oil supplementation. The inflammatory response to LPS administration was reflected by evoked cytokines; SPMs were not altered in response to LPS during either the inflammatory (2–4 h post-administration) or resolution phases (samples measured out to 48–72 h) in these healthy volunteers.
In the case of RvD1 and RvE1 in plasma, we utilized deuterated authentic derivatives as internal standards for their detection by MS and established lower limits of detection in plasma ranging from 3.4 to 4.3 pg/ml for RvD1 and from 7.8 to 10.4 pg/ml for RvE1. By contrast, PD1 levels were detectable, and doubled from ∼2 to 4 pg/ml in plasma during supplementation with 21 g/day Lovaza and fell after the period of supplementation (Fig. 1C). PD1 was also detectable on the lower dose of fish oils, but did not alter during the inflammatory or resolution phases post-LPS (Fig. 2D), suggesting that nonenzymatic formation of PD1 has to be considered (supplementary Table 8, supplementary Fig. 6b). Low concentrations of maresins were also detected, but apparently unaltered by either dietary supplementation or administration of LPS. For our analyses, we have no evidence that signals of the authentic endogenous SPMs might be suppressed by the respective deuterated internal standards (supplementary Table 9, supplementary Fig. 6c). SPMs were not detectable in urine samples from subjects with high dose supplementation of fish oils, however, some maresin and RvD1 could be present in its glucuronidated form (supplementary Tables 10–13, supplementary Fig. 6e, f).
Few studies have utilized MS to assess SPM formation in response to fish oil supplementation in humans (19) and none have assessed their formation during resolution of evoked inflammation in vivo. Average plasma levels of RvE1 of 0.521 nM (182.6 pg/ml) and of RvD1 of 0.0454 nM (17.1 pg/ml) were reported in a study of healthy volunteers on an ad libitum diet (58). By contrast, a randomized and placebo-controlled supplementation of 16 volunteers with 3 g/day fish oil over 10 weeks failed to detect RvD1 and RvE1 in plasma (59). However, labeled authentic standards were not applied in either study.
Although there is a paucity of data relating synthetic SPM administration with attained systemic exposure (60–64) and functional effects in mouse models (28), administration of 100 ng per mouse RvD1 resulted in plasma levels of immunoreactive RvD1 peaking at 850 pg/ml 3 h later and descending to 200 pg/ml over the ensuing 24 h, during which leukocyte infiltration in zymosan A-induced acute peritonitis was reduced. While no such data are available using physicochemical estimates of RvD biosynthesis, assumption of the analytical validity of the approach in this study infers an anti-inflammatory effect at concentrations greatly in excess of the limits of detection of our analysis.
In conclusion, we have failed to detect RvD1 or RvE1 in the plasma of volunteers administered fish oils or in response to an acute inflammatory stimulus after administration of LPS. While maresins and PD1 were detected at low concentrations, they were unaltered during evoked inflammation or its resolution. This was in marked contrast to the formation of epoxygenase and free radical-catalyzed products of fish oils. For now, the results of these studies question the relevance of endogenous SPMs to the putative anti-inflammatory effects of fish oils in humans.
Note added in proof
The author Xuanwen Li was inadvertently left out of the author list of the accepted version of this article. All other authors and the Journal’s accepting Associate Editor approved the addition after the article was in proof stage. Dr. Li will appear as an author in all forms of the article except in the originally accepted Paper in Press.
Supplementary Material
Acknowledgments
The authors are indebted to the participants volunteering for these clinical studies, the nursing, bionutritional, core lab, and supporting staff at the Clinical Translational Research Center of the University of Pennsylvania, as well as the support of the Investigational Drug Services. Deuterated internal standards for MS analysis of RvD1, RvE1, maresin, and PD1 were kindly provided by Drs. Bernd W. Spur and T. Peter Stein, Rowan University-School of Osteopathic Medicine, Stratford, NJ. Excellent clinical study coordination was provided by Ms. Lavenia Banas, RN, Ms. Marie Farley, and Ms. Kristina Alfaro. Excellent technical support was provided by Ms. Wenxuan Li and Ms. Helen Zou. For the Evoked Endotoxemia study, LovazaTM was provided to M.P.R. by GlaxoSmithKline.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- AcN
- acetonitrile
- bid
- twice a day
- CE
- collision energy
- CRP
- C-reactive protein
- EET
- epoxyeicosatrienoic acid
- ID#
- identification number
- IL
- interleukin
- iP
- isoprostane
- LPS
- lipopolysaccharide
- MCP-1
- monocyte chemoattractant protein-1
- MRM
- multiple reaction monitoring
- PD1
- protectin D1
- PDX
- protectin DX
- RA
- receptor antagonist
- RBC
- red blood cell
- Rv
- resolvin
- SAA
- serum amyloid A
- SPE
- solid phase extraction
- SPM
- specialized pro-resolving mediator
- tid
- three times a day
- UP
- ultra-performance
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Hu F. B., Bronner L., Willett W. C., Stampfer M. J., Rexrode K. M., Albert C. M., Hunter D., Manson J. E. 2002. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 287: 1815–1821. [DOI] [PubMed] [Google Scholar]
- 2.Amano T., Matsubara T., Uetani T., Kato M., Kato B., Yoshida T., Harada K., Kumagai S., Kunimura A., Shinbo Y., et al. 2011. Impact of omega-3 polyunsaturated fatty acids on coronary plaque instability: an integrated backscatter intravascular ultrasound study. Atherosclerosis. 218: 110–116. [DOI] [PubMed] [Google Scholar]
- 3.de Oliveira Otto M. C., Wu J. H., Baylin A., Vaidya D., Rich S. S., Tsai M. Y., Jacobs D. R., Jr, Mozaffarian D. 2013. Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2: e000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GISSI-Prevenzione Investigators. 1999. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 354: 447–455. [PubMed] [Google Scholar]
- 5.Yokoyama M., Origasa H., Matsuzaki M., Matsuzawa Y., Saito Y., Ishikawa Y., Oikawa S., Sasaki J., Hishida H., Itakura H., et al. 2007. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 369: 1090–1098. [DOI] [PubMed] [Google Scholar]
- 6.Hooper L., Thompson R. L., Harrison R. A., Summerbell C. D., Ness A. R., Moore H. J., Worthington H. V., Durrington P. N., Higgins J. P., Capps N. E., et al. 2006. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ. 332: 752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C., Harris W. S., Chung M., Lichtenstein A. H., Balk E. M., Kupelnick B., Jordan H. S., Lau J. 2006. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am. J. Clin. Nutr. 84: 5–17. [DOI] [PubMed] [Google Scholar]
- 8.Hu F. B., Manson J. E. 2012. Omega-3 fatty acids and secondary prevention of cardiovascular disease-is it just a fish tale?: comment on “Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease”. Arch. Intern. Med. 172: 694–696. [DOI] [PubMed] [Google Scholar]
- 9.Shearer G. C., Savinova O. V., Harris W. S. 2012. Fish oil–how does it reduce plasma triglycerides? Biochim. Biophys. Acta. 1821: 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris M. C., Sacks F., Rosner B. 1993. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation. 88: 523–533. [DOI] [PubMed] [Google Scholar]
- 11.Knapp H. R., FitzGerald G. A. 1989. The antihypertensive effects of fish oil. A controlled study of polyunsaturated fatty acid supplements in essential hypertension. N. Engl. J. Med. 320: 1037–1043. [DOI] [PubMed] [Google Scholar]
- 12.Knapp H. R., Reilly I. A., Alessandrini P., FitzGerald G. A. 1986. In vivo indexes of platelet and vascular function during fish-oil administration in patients with atherosclerosis. N. Engl. J. Med. 314: 937–942. [DOI] [PubMed] [Google Scholar]
- 13.De Caterina R. 2011. n-3 Fatty acids in cardiovascular disease. N. Engl. J. Med. 364: 2439–2450. [DOI] [PubMed] [Google Scholar]
- 14.Serhan C. N., Clish C. B., Brannon J., Colgan S. P., Chiang N., Gronert K. 2000. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 192: 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong S., Gronert K., Devchand P. R., Moussignac R. L., Serhan C. N. 2003. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J. Biol. Chem. 278: 14677–14687. [DOI] [PubMed] [Google Scholar]
- 16.Chiang N., Serhan C. N. 2006. Cell-cell interaction in the transcellular biosynthesis of novel omega-3-derived lipid mediators. Methods Mol. Biol. 341: 227–250. [DOI] [PubMed] [Google Scholar]
- 17.Oh S. F., Pillai P. S., Recchiuti A., Yang R., Serhan C. N. 2011. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J. Clin. Invest. 121: 569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao Z., Dong J., Wu W., Yang T., Wang T., Guo L., Chen L., Xu D., Wen F. 2012. Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARgamma/NF-kappaB pathway. Respir. Res. 13: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mas E., Croft K. D., Zahra P., Barden A., Mori T. A. 2012. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin. Chem. 58: 1476–1484. [DOI] [PubMed] [Google Scholar]
- 20.Colas R. A., Shinohara M., Dalli J., Chiang N., Serhan C. N. 2014. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am. J. Physiol. Cell Physiol. 307: C39–C54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho K. J., Spite M., Owens C. D., Lancero H., Kroemer A. H., Pande R., Creager M. A., Serhan C. N., Conte M. S. 2010. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am. J. Pathol. 177: 2116–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arita M., Bianchini F., Aliberti J., Sher A., Chiang N., Hong S., Yang R., Petasis N. A., Serhan C. N. 2005. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 201: 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clària J., Nguyen B. T., Madenci A. L., Ozaki C. K., Serhan C. N. 2013. Diversity of lipid mediators in human adipose tissue depots. Am. J. Physiol. Cell Physiol. 304: C1141–C1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer R., Konkel A., Mehling H., Blossey K., Gapelyuk A., Wessel N., von Schacky C., Dechend R., Muller D. N., Rothe M., et al. 2014. Dietary omega-3 fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway. J. Lipid Res 55: 1150–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giera M., Ioan-Facsinay A., Toes R., Gao F., Dalli J., Deelder A. M., Serhan C. N., Mayboroda O. A. 2012. Lipid and lipid mediator profiling of human synovial fluid in rheumatoid arthritis patients by means of LC-MS/MS. Biochim. Biophys. Acta. 1821: 1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalish B. T., Le H. D., Fitzgerald J. M., Wang S., Seamon K., Gura K. M., Gronert K., Puder M. 2013. Intravenous fish oil lipid emulsion promotes a shift toward anti-inflammatory proresolving lipid mediators. Am. J. Physiol. Gastrointest. Liver Physiol. 305: G818–G828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keelan J. A., Mas E., D’Vaz N., Dunstan J. A., Li S., Barden A. E., Mark P. J., Waddell B. J., Prescott S. L., Mori T. A. 2015. Effects of maternal n-3 fatty acid supplementation on placental cytokines, pro-resolving lipid mediators and their precursors. Reproduction. 149: 171–178. [DOI] [PubMed] [Google Scholar]
- 28.Recchiuti A., Codagnone M., Pierdomenico A. M., Rossi C., Mari V. C., Cianci E., Simiele F., Gatta V., Romano M. 2014. Immunoresolving actions of oral resolvin D1 include selective regulation of the transcription machinery in resolution-phase mouse macrophages. FASEB J. 28: 3090–3102. [DOI] [PubMed] [Google Scholar]
- 29.Prüss H., Rosche B., Sullivan A. B., Brommer B., Wengert O., Gronert K., Schwab J. M. 2013. Proresolution lipid mediators in multiple sclerosis - differential, disease severity-dependent synthesis - a clinical pilot trial. PLoS One. 8: e55859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasaki A., Fukuda H., Shiida N., Tanaka N., Furugen A., Ogura J., Shuto S., Mano N., Yamaguchi H. 2015. Determination of omega-6 and omega-3 PUFA metabolites in human urine samples using UPLC/MS/MS. Anal. Bioanal. Chem. 407: 1625–1639. [DOI] [PubMed] [Google Scholar]
- 31.Wang X., Zhu M., Hjorth E., Cortes-Toro V., Eyjolfsdottir H., Graff C., Nennesmo I., Palmblad J., Eriksdotter M., Sambamurti K., et al. 2015. Resolution of inflammation is altered in Alzheimer’s disease. Alzheimers Dement. 11: 40.e1-2–50.e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss G. A., Troxler H., Klinke G., Rogler D., Braegger C., Hersberger M. 2013. High levels of anti-inflammatory and pro-resolving lipid mediators lipoxins and resolvins and declining docosahexaenoic acid levels in human milk during the first month of lactation. Lipids Health Dis. 12: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arita M., Ohira T., Sun Y. P., Elangovan S., Chiang N., Serhan C. N. 2007. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 178: 3912–3917. [DOI] [PubMed] [Google Scholar]
- 34.Hasturk H., Kantarci A., Ohira T., Arita M., Ebrahimi N., Chiang N., Petasis N. A., Levy B. D., Serhan C. N., Van Dyke T. E. 2006. RvE1 protects from local inflammation and osteoclast- mediated bone destruction in periodontitis. FASEB J. 20: 401–403. [DOI] [PubMed] [Google Scholar]
- 35.Isobe Y., Arita M., Matsueda S., Iwamoto R., Fujihara T., Nakanishi H., Taguchi R., Masuda K., Sasaki K., Urabe D., et al. 2012. Identification and structure determination of a novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxy-eicosapentaenoic acid. J. Biol. Chem 287: 10525–10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu G., Fiala M., Mizwicki M. T., Sayre J., Magpantay L., Siani A., Mahanian M., Chattopadhyay M., La Cava A., Wiedau-Pazos M. 2012. Neuronal phagocytosis by inflammatory macrophages in ALS spinal cord: inhibition of inflammation by resolvin D1. Am. J. Neurodegener. Dis. 1: 60–74. [PMC free article] [PubMed] [Google Scholar]
- 37.Pedersen A. K., FitzGerald G. A. 1985. Cyclooxygenase inhibition, platelet function, and metabolite formation during chronic sulfinpyrazone dosing. Clin. Pharmacol. Ther. 37: 36–42. [DOI] [PubMed] [Google Scholar]
- 38.Ferguson J. F., Mulvey C. K., Patel P. N., Shah R. Y., Doveikis J., Zhang W., Tabita-Martinez J., Terembula K., Eiden M., Koulman A., et al. 2014. Omega-3 PUFA supplementation and the response to evoked endotoxemia in healthy volunteers. Mol. Nutr. Food Res. 58: 601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez A. R., Spur B. W. 2012. Total synthesis of resolvin D1, a potent anti-inflammatory lipid mediator. Tetrahedron Lett. 53: 6990–6994. [Google Scholar]
- 40.Rodriguez A. R., Spur B. W. 2012. Total synthesis of the macrophage derived anti-inflammatory lipid mediator Maresin 1. Tetrahedron Lett. 53: 4169–4172. [Google Scholar]
- 41.Balas L., Guichardant M., Durand T., Lagarde M. 2014. Confusion between protectin D1 (PD1) and its isomer protectin DX (PDX). An overview on the dihydroxy-docosatrienes described to date. Biochimie. 99: 1–7. [DOI] [PubMed] [Google Scholar]
- 42.Chang C. T., Patel P., Kang N., Lawson J. A., Song W. L., Powell W. S., FitzGerald G. A., Rokach J. 2008. Eicosapentaenoic-acid-derived isoprostanes: synthesis and discovery of two major isoprostanes. Bioorg. Med. Chem. Lett. 18: 5523–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacobo S. H., Chang C. T., Lee G. J., Lawson J. A., Powell W. S., Pratico D., FitzGerald G. A., Rokach J. 2006. Total synthesis of 8,12-iso-iPF3alpha-VI, an EPA-derived isoprostane: stereoselective introduction of the fifth asymmetric center. J. Org. Chem. 71: 1370–1379. [DOI] [PubMed] [Google Scholar]
- 44.Chang C. T., Patel P., Gore V., Song W. L., Lawson J. A., Powell W. S., FitzGerald G. A., Rokach J. 2009. A new approach to the synthesis of polyunsaturated deuterated isoprostanes: total synthesis of d4-5-epi-8,12-iso-iPF3alpha-VI and d4-8,12-iso-iPF3alpha-VI. Bioorg. Med. Chem. Lett. 19: 6755–6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim S., Powell W. S., Lawson J. A., Jacobo S. H., Pratico D., FitzGerald G. A., Maxey K., Rokach J. 2005. iPF2alpha-III-17,18,19,20-d4: total synthesis and metabolism. Bioorg. Med. Chem. Lett. 15: 1613–1617. [DOI] [PubMed] [Google Scholar]
- 46.Lawson J. A., Rokach J., FitzGerald G. A. 1999. Isoprostanes: formation, analysis and use as indices of lipid peroxidation in vivo. J. Biol. Chem. 274: 24441–24444. [DOI] [PubMed] [Google Scholar]
- 47.Song W. L., Lawson J. A., Wang M., Zou H., FitzGerald G. A. 2007. Noninvasive assessment of the role of cyclooxygenases in cardiovascular health: a detailed HPLC/MS/MS method. Methods Enzymol. 433: 51–72. [DOI] [PubMed] [Google Scholar]
- 48.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 49.Spite M., Claria J., Serhan C. N. 2014. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 19: 21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serhan C. N. 2014. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 510: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daviglus M. L., Stamler J., Orencia A. J., Dyer A. R., Liu K., Greenland P., Walsh M. K., Morris D., Shekelle R. B. 1997. Fish consumption and the 30-year risk of fatal myocardial infarction. N. Engl. J. Med. 336: 1046–1053. [DOI] [PubMed] [Google Scholar]
- 52.Wada M., DeLong C. J., Hong Y. H., Rieke C. J., Song I., Sidhu R. S., Yuan C., Warnock M., Schmaier A. H., Yokoyama C., et al. 2007. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J. Biol. Chem. 282: 22254–22266. [DOI] [PubMed] [Google Scholar]
- 53.Miyahara T., Runge S., Chatterjee A., Chen M., Mottola G., Fitzgerald J. M., Serhan C. N., Conte M. S. 2013. D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB J. 27: 2220–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwab J. M., Chiang N., Arita M., Serhan C. N. 2007. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 447: 869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Challis J. R., Tulchinsky D. 1974. A comparison between the concentration of prostaglandin F in human plasma and serum. Prostaglandins. 5: 27–31. [DOI] [PubMed] [Google Scholar]
- 56.Ricciotti E., FitzGerald G. A. 2011. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 31: 986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moertl D., Hammer A., Steiner S., Hutuleac R., Vonbank K., Berger R. 2011. Dose-dependent effects of omega-3-polyunsaturated fatty acids on systolic left ventricular function, endothelial function, and markers of inflammation in chronic heart failure of nonischemic origin: a double-blind, placebo-controlled, 3-arm study. Am. Heart J. 161: 915.e1–915.e9. [DOI] [PubMed] [Google Scholar]
- 58.Psychogios N., Hau D. D., Peng J., Guo A. C., Mandal R., Bouatra S., Sinelnikov I., Krishnamurthy R., Eisner R., Gautam B., et al. 2011. The human serum metabolome. PLoS One. 6: e16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dawczynski C., Massey K. A., Ness C., Kiehntopf M., Stepanow S., Platzer M., Grun M., Nicolaou A., Jahreis G. 2013. Randomized placebo-controlled intervention with n-3 LC-PUFA-supplemented yoghurt: effects on circulating eicosanoids and cardiovascular risk factors. Clin. Nutr. 32: 686–696. [DOI] [PubMed] [Google Scholar]
- 60.Keyes K. T., Ye Y., Lin Y., Zhang C., Perez-Polo J. R., Gjorstrup P., Birnbaum Y. 2010. Resolvin E1 protects the rat heart against reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 299: H153–H164. [DOI] [PubMed] [Google Scholar]
- 61.Seki H., Fukunaga K., Arita M., Arai H., Nakanishi H., Taguchi R., Miyasho T., Takamiya R., Asano K., Ishizaka A., et al. 2010. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J. Immunol. 184: 836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Settimio R., Clara D. F., Franca F., Francesca S., Michele D. 2012. Resolvin D1 reduces the immunoinflammatory response of the rat eye following uveitis. Mediators Inflamm. 2012: 318621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tjonahen E., Oh S. F., Siegelman J., Elangovan S., Percarpio K. B., Hong S., Arita M., Serhan C. N. 2006. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem. Biol. 13: 1193–1202. [DOI] [PubMed] [Google Scholar]
- 64.Wang Q., Zheng X., Cheng Y., Zhang Y. L., Wen H. X., Tao Z., Li H., Hao Y., Gao Y., Yang L. M., et al. 2014. Resolvin D1 stimulates alveolar fluid clearance through alveolar epithelial sodium channel, Na,K-ATPase via ALX/cAMP/PI3K pathway in lipopolysaccharide-induced acute lung injury. J. Immunol. 192: 3765–3777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.