Abstract
Steroids are primarily present in human fluids in their sulfated forms. Profiling of these compounds is important from both diagnostic and physiological points of view. Here, we present a novel method for the quantification of 11 intact steroid sulfates in human serum by LC-MS/MS. The compounds analyzed in our method, some of which are quantified for the first time in blood, include cholesterol sulfate, pregnenolone sulfate, 17-hydroxy-pregnenolone sulfate, 16-α-hydroxy-dehydroepiandrosterone sulfate, dehydroepiandrosterone sulfate, androstenediol sulfate, androsterone sulfate, epiandrosterone sulfate, testosterone sulfate, epitestosterone sulfate, and dihydrotestosterone sulfate. The assay was conceived to quantify sulfated steroids in a broad range of concentrations, requiring only 300 μl of serum. The method has been validated and its performance was studied at three quality controls, selected for each compound according to its physiological concentration. The assay showed good linearity (R2 > 0.99) and recovery for all the compounds, with limits of quantification ranging between 1 and 80 ng/ml. Averaged intra-day and between-day precisions (coefficient of variation) and accuracies (relative errors) were below 10%. The method has been successfully applied to study the sulfated steroidome in diseases such as steroid sulfatase deficiency, proving its diagnostic value. This is, to our best knowledge, the most comprehensive method available for the quantification of sulfated steroids in human blood.
Keywords: sulfated steroids, liquid chromatography-tandem mass spectrometry, recessive X-linked ichthyosis
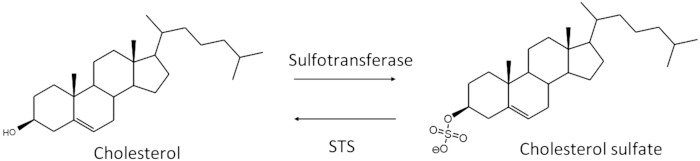
Human adrenal glands, and those of some primates, secrete high concentrations of conjugated steroids, particularly 5-androsten-3β-ol-17-one-3-sulfate [dehydroepiandrosterone sulfate (DHEAS)], which reaches different peripheral tissues through blood (1). Sulfonation increases the solubility of steroids in water-based biological fluids such as blood or urine. Sulfated steroids, also known in the literature as steroid sulfates or steroid sulfonates, represent the most abundant fraction of steroids in human blood. Because the physiological activity has been traditionally assigned to the unconjugated steroids, the classical dogma assumes that sulfated steroids are inactive forms of steroids which require sulfate cleavage to be active. In this context, human steroid sulfatase (STS) is the enzyme that desulfates the sulfated steroids to obtain free forms. The complementary reaction, sulfonation, is provided by sulfotransferase enzymes (Fig. 1). This cycle allows for the regulation and excretion of steroids.
Fig. 1.
Inter-conversion of free cholesterol and CS. Cholesterol is conjugated by the action of a sulfotransferase, which requires 3′-phosphoadenosine-5′-phosphosulfate (PAPS) as cosubstrate. CS can be desulfated by the action of STS. The lack of STS in humans dramatically increases the concentration of CS in blood.
The concept of steroid sulfates as inactive compounds is under revision. A specific transporter for sulfated steroids in the human testis was recently described (2). Moreover, it is well-known that humans can convert intact sulfated steroids directly into other sulfated steroids without cleaving the sulfate group (3). Interestingly, lack of STS in humans, a condition known as STS deficiency or recessive X-linked ichthyosis (RXLI), is compatible with life. Of note, the only compounds elevated in RXLI patients, independent of their age, are 5-cholesten-3β-ol-3-sulfate [cholesterol sulfate (CS)] and oxysterol sulfates (4). High levels of CS seem to be related to the skin scaling present in most of the RXLI males (5).
There is a direct relationship between blood concentrations of sulfated steroids, age, and sex. The sulfated steroid studied with more detail is DHEAS, which reaches its higher concentrations in blood at around 20–30 years of age in males and females and decreases markedly in the years thereafter (6, 7).
Factors like the broad range of concentrations of sulfated steroids within a sample, or between different patients, and the chemical differences among some of them, from polar steroids like 5-androsten-3β,16α-diol-17-one-3-sulfate [16-α-hydroxy-dehydroepiandrosterone sulfate (16OHDHEAS)] to more lipophilic molecules like CS, has traditionally limited the number of these compounds quantified in a single analysis.
The application of LC-MS to the steroidomics field allowed for the study of intact sulfated steroids without any modification, replacing immunoassays. These compounds exhibit good ionization properties, especially under ESI. Additionally, their specific fragmentation pattern permits the use of MS/MS for detection purposes. GC-MS can be used to quantify sulfated steroids too, but only after chemical or enzymatic cleavage of the sulfated group and a further derivatization step. An important limitation of GC-MS, when applied to the analysis of sulfated steroids, is that other conjugates, particularly steroid glucuronides, can contribute to the signal of sulfated steroids. Most commercial sulfatase enzymes have glucuronidase activity too, and chemical acidification cannot distinguish among sulfates and glucuronides.
In 1990, Shackleton et al. (8) reported the first method for the quantification of intact DHEAS in human blood by LC-MS. Yet then, they described several signals of sulfated steroids with a m/z of 369 in negative detection mode. The authors described how the analysis of a specific androgen sulfate is influenced by the presence of other structurally related compounds, and their baseline separation is therefore highly desirable. Additionally, internal standards (ISs) should be carefully selected.
Ever since then, several methods have been developed for the analysis of sulfated steroids by LC-MS. Most of these assays focus on the analysis of unconjugated steroids and include DHEAS in their panel. Recent examples can be found in (9, 10). Other methods were developed to analyze only one compound (11, 12) or a limited number of sulfated steroids (13, 14). In 2003, Liu, Griffiths, and Sjövall (15) provided a method to obtain a more detailed profiling of sulfated steroids in human plasma. Recent work has focused on extending the number of sulfated steroids analyzed simultaneously in a single LC-MS analysis (16, 17).
We have developed a method to quantify 11 sulfated steroids simultaneously, in order to achieve a better understanding of the sulfated steroidome in human blood. Some compounds were analyzed with our assay for the first time in human blood: 5-pregnen-3β,17α-diol-20-one-3-sulfate [17-hydroxy-pregnenolone sulfate (17OHPregS)], 16OHDHEAS, 4-androsten-17β-ol-3-one-17-sulfate [testosterone sulfate (TS)], 4-androsten-17α-ol-3-one-17-sulfate [epitestosterone sulfate (eTS)], and 5α-androstan-17β-ol-3-one-17-sulfate [dihydrotestosterone sulfate (DHTS)]. We applied it to the analysis of serum or plasma samples from several groups, including healthy volunteers, RXLI patients, the mothers of two RXLI patients, patients suffering of ichthyosis vulgaris, and a baby with cholestasis
MATERIALS AND METHODS
Chemicals and reference steroids
All reference steroids and the ISs were purchased from C/D/N Isotopes Inc. (Quebec, Canada), Sigma-Aldrich (Taufkirchen, Germany), Steraloids Inc. (Newport, RI), or from LGC Standards GmbH (Wesel, Germany). Reference steroids and ISs were CS, 5-pregnen-3β-ol-20-one-3-sulfate (PregS), 17OHPregS, DHEAS, TS, eTS, DHTS, 5α-androstan-3α-ol-17-one-3-sulfate [androsterone sulfate (AnS)], 5α-androstan-3β-ol-17-one-3-sulfate [epiandrosterone sulfate (epiAnS)], 5-androsten-3β,17β-diol-3-sulfate [androstenediol sulfate (AnDiolS)], 16OHDHEAS, d6DHEAS, d3TS, d3eTS, d3DHTS, d4PregS, and d7CS. The IS for 17OHPregS, d317OHPregS, was synthesized in-house from d317OHPreg (C/D/N Isotopes Inc.), as previously described (18), but without the addition of NaOH to avoid deuterium exchange.
Water (LC-MS grade) and ammonium hydroxide were purchased from Fluka (Taufkirchen, Germany). Methanol (MeOH), acetonitrile (ACN), and n-hexane, chloroform, and pyridine, were obtained from Merck (Darmstadt, Germany). Ammonium acetate and sulfur trioxide trimethylamine were from Sigma-Aldrich. SepPak C18 cartridges (360 mg) were from Waters Corporation (Milford, MA) and zinc sulfate heptahydrate was from Roth (Karlsruhe, Germany).
MS
MS/MS was applied to the detection and quantification of all the sulfated steroids. The experiments were performed with a triple quadrupole mass spectrometer (TSQ, Quantum Ultra; Thermo Fisher Scientific, Dreieich, Germany), operated in negative mode detection with a HESI probe.
Monitoring reactions, collision energies, and tube lens voltages are shown in Table 1. Mass spectrometer conditions were as reported before (16). Briefly, the capillary temperature and the vaporizer temperatures were set to 270 and 350°C, respectively. The sheath gas was 50 arbitrary units and the auxiliary gas was 20 arbitrary units. The voltage applied was −3,500 V. The collision gas pressure was 1.5 mTorr for all experiments.
TABLE 1.
Quantification transitions and parameters for the mass spectrometer for each sulfated steroid
| Compound | Precursor ion (m/z) | Product ion (m/z) | Collision energy (eV) | Tube lens voltage (V) |
| 16OHDHEAS | 383.1 | 96.9 | 32 | 135 |
| DHEAS, TS, eTS | 367.1 | 96.9 | 39 | 125 |
| TS qualifier | 367.1 | 177.0 | 38 | 125 |
| d6DHEAS | 373.1 | 97.9 | 35 | 170 |
| d3TS, d3eTS | 370 | 97.9 | 39 | 140 |
| d3TS qual | 370.0 | 180.0 | 49 | 140 |
| AnDiolS, DHTS, AnS, epiAnS | 369.1 | 96.9 | 46 | 153 |
| d3DHTS | 372.0 | 97.9 | 41 | 116 |
| PregS | 395.1 | 96.9 | 32 | 181 |
| d4PregS | 399.2 | 96.9 | 30 | 174 |
| 17OHPregS | 411.1 | 96.9 | 32 | 120 |
| d317OHPregS | 414.2 | 96.9 | 30 | 120 |
| CS | 465.2 | 96.9 | 37 | 192 |
| d7CS | 472.2 | 96.9 | 34 | 167 |
LC
Chromatographic separation of the analytes was achieved with a column Accucore Phenyl -X (100 × 2.1 mm, 2.6 μm) from Thermo Fisher Scientific, connected to a HPLC system (Agilent 1200SL, Waldbronn, Germany).
Solvents used for separation of sulfated steroids were buffer solution A with ammonium acetate [10 mM (pH 7) dissolved in 85% water and 15% ACN] and solution B with organic solvents composed of 70% MeOH and 30% ACN (v/v). The flows and solvent compositions applied can be found in Table 2. The method had a total duration of 11.8 min.
TABLE 2.
Flows and solvent composition for the LC method
| Step | Seconds | Flow (ml/min) | Solvent A (%) | Solvent B (%) |
| 0 | 0 | 0.3 | 80 | 20 |
| 1 | 390 | 0.3 | 66 | 34 |
| 2 | 200 | 0.3 | 1 | 99 |
| 3 | 60 | 0.4 | 1 | 99 |
| 4 | 60 | 0.3 | 80 | 20 |
Sample preparation
Sample preparation was modified from a previous protocol (4). Three hundred microliters of each serum or plasma sample were incubated with 50 μl of IS mix during 15 min. Each IS in the mix had a concentration of 1 μg/ml, with the exception of d7CS, which was 6 μg/ml. Proteins were precipitated with 1 ml of ACN-ZnSO4 [89 g/l, 4:1 (v/v)]. After protein precipitation, the samples were incubated again for 15 min and then centrifuged for 10 min at 14,500 g. The supernatant, free of proteins, was then mixed with 3 ml of water in a glass tube. For each sample, a SepPak C18 cartridge was conditioned with 2 ml of MeOH and 2 ml of water. Next, the sample, diluted in water, was loaded onto the cartridge and washed with 3 ml of water, 3 ml of hexane, and 4 ml of chloroform. Sulfated steroids were eluted from the cartridge with 4 ml of MeOH. After evaporation of the methanolic fraction with nitrogen at 40°C, the samples were reconstituted with 250 μl of a solution of 79.75% water, 10% MeOH, 10% ACN, and 0.25% ammonium hydroxide. Samples were centrifuged after reconstitution and then injected in the LC-MS/MS system (10 μl). Each sample was injected at least three times.
Evaluation of the method
The method was validated in accordance with the US Food and Drug Administration and the EU European Medicines Agency guidelines for bioanalytical evaluation.
Linearity, calibration curves, limits of detection, and limits of quantification.
Linearity, calibration curves, limits of detection (LODs), and limits of quantification (LOQs) were studied for each sulfated steroid. The calibration curves were obtained by spiking increasing concentrations of standard solutions prepared in a mixture of MeOH and water [1:1 (v/v)] into 300 μl of in-house prepared charcoal-stripped serum and IS, as described in the previous section. The parameters for CS were studied at the following levels: 20, 40, 80, 200, 800, 2,000, 4,000, 8,000, 20,000, 40,000, 80,000, 160,000, and 480,000 ng/ml. For the other sulfated steroids, the parameters were tested for a range which included the following points: 0.25, 0.5, 1, 2.5, 10, 25, 50, 100, 250, 500, 1,000, 2,000, and 6,000 ng/ml. The calibration curves were constructed plotting the area ratio (peak area of the analyte divided by the peak area of the IS) of each calibration point against its corresponding concentration in nanograms per milliliter. LOD was determined at a signal-to-noise ratio higher than 3. LOQ was the lowest concentration with a signal-to-noise ratio of 10 or more and acceptable precision and accuracy.
Quality controls.
Quality control samples (QCs) containing different concentrations of each analyte were prepared and analyzed. The selected values for each sulfated steroid were in accordance with the expected concentrations reported in the bibliography (11, 13, 14) and with the preliminary results found in real samples prior to method evaluation. QCs were always prepared in stripped human serum, because a real serum sample lacking all the sulfated steroids to analyze could not be obtained.
Recovery.
The recovery of the method evaluates the loss of sulfated steroids due to the use of C18 cartridges. It was calculated by dividing the area ratios obtained for the QCs for each analyte by the area ratio measured after spiking the same amounts of the QC and IS postextraction. The postextraction addition consisted of spiking the standards and ISs onto the methanolic fraction obtained from the workup of charcoal-treated serum, which was afterwards evaporated and reconstituted as described above.
Matrix effects.
Matrix effects were studied by plotting response ratios of spike experiments prepared in charcoal-treated serum after sample preparation and reconstitution (Y axis) against the same concentrations of standards prepared in the final aqueous reconstitution solution without sample workup (X axis). A line slope of one corresponds to absence of matrix effects. Slopes above one indicate that there is an ion enhancement, and slopes below one are found whenever there is ion suppression.
Precision and accuracy.
Intra-day precision [percent coefficient of variation (CV)] and accuracy [percent relative error (RE)] studies were performed by analyzing five spiked replicates of each QC (Q1, Q2, and Q3) in one batch during the same day. Between-day precision and accuracy were measured in five different batches during different days with the same concentration in validation samples. The concentrations were calculated by calibration curves.
Stability of the samples.
Each real sample chosen for the stability test (serum samples C9 and C10 in the supplementary table) was analyzed three times to evaluate the stability of the sulfated steroids during freeze-thaw cycles. The time difference between re-analyses was 1 month.
Analysis of the samples.
We analyzed 11 samples from patients with STS deficiency, and a sample from a baby with cholestasis. All STS-deficient patients showed the classical RXLI phenotype with scales on their skin. One of the RXLI patients was undergoing a long-term treatment with prednisolone (10 mg/day) and was especially selected because his steroid levels were expected to be low. Among the control samples, there were patients with ichthyosis vulgaris, a skin condition with a similar phenotype as RXLI, but in which steroid sulfates are normal. Other controls were healthy volunteers and the mothers of two RXLI patients.
Data analysis
Calibration curves, linearity, LODs, and LOQs were studied with the program Xcalibur 2.1, applying a 1/x or equal weighting regression. Box plots were generated with BoxPlotR (19).
RESULTS
MS
The ESI heated probe was tested with three different temperatures, 325°C (capillary temperature 250°C, sheath and auxiliary gas 30 arbitrary units, −4,000 V), 350°C (capillary temperature 270°C, sheath gas 50 arbitrary units and auxiliary gas 20 arbitrary units, −3,500 V), and 400°C (capillary temperature 300°C, sheath gas 60 arbitrary units and auxiliary gas 40 arbitrary units, −3,500 V). Tests were performed during the same day using standards spiked in stripped serum and a sample from a healthy volunteer after sample workup. The selected parameters (16) provided the best area ratios (peak area of the sulfated steroid divided by the peak area of the IS) for the compounds which were present in lower concentrations in blood (PregS, 17OHPregS, DHTS, TS, and eTS) and for CS. For the remaining androgen sulfates, the area ratios were slightly higher at 325°C. The differences were not important considering the high concentrations of DHEAS, AnDiolS, AnS, and epiAnS in blood.
Analytical performance
The complete set of data can be found in Table 3. The table presents the information obtained at three different QCs for each sulfated steroid (33 QCs), which are within the physiological range. Linearity (R2) was always higher than 0.99. Most of the sulfated steroids showed good linearity, even at higher concentrations (data not shown). Those concentrations are not reflected in Table 3 because they are far from physiological. Recovery was good for all the QCs, ranging between 85.5 and 111.6% with an average value of 97.9%. Most of the compounds showed no matrix effects in our assay. Nevertheless, three compounds exhibited important ionization problems due to the components of the matrix. Both PregS and 17OHPregS suffered ion suppression, which in the case of 17OHPregS was 40%. On the other hand, DHTS signal was enhanced by 20%. To minimize the problems associated with matrix effect, all the calibrations were prepared with a surrogate of the original matrix; charcoal-stripped serum. Regarding accuracy and precision, the method showed good precision and accuracy at all QCs (always below 20%), with the exception of the first QC for 17OHPregS (5 ng/ml). At this concentration, and especially for inter-day experiments, the precision expressed as percent CV was close to a 24%. Precision at the LOQ for 17OHPregS was 31.1%.
TABLE 3.
Performance parameters obtained for the assay
| CS | PregS | 17OHPREGS | 16OHDHEAS | DHEAS | AnDiolS | AnS | EpiAnS | DHTS | TS | eTS | |
| IS | d7CS | d4PregS | d317OHPregS | d6DHEAS | d6DHEAS | d6DHEAS | d3DHTS | d3DHTS | d3DHTS | d3TS | d3eTS |
| Linearity (ng/ml) | 80–480,000 | 1–500 | 1–250 | 2.5–2,000 | 25–6,000 | 2.5–500 | 10–2,000 | 10–2,000 | 1–500 | 1–500 | 1–500 |
| R2 | >0.99 | >0.99 | >0.99 | >0.99 | >0.99 | >0.99 | >0.99 | >0.99 | >0.99 | >0.99 | >0.99 |
| LOQ (ng/ml) | 80.0 | 1.0 | 1.0 | 2.5 | 25.0 | 2.5 | 10.0 | 10.0 | 1.0 | 1.0 | 1.0 |
| LOQ (%CV) | 17.1 | 10.7 | 31.1 | 7.4 | 9.4 | 20.6 | 18.2 | 11.2 | 7.3 | 19.6 | 20.2 |
| LOD (ng/ml) | 40.0 | 0.5 | 0.5 | 1.0 | 1.0 | 1.0 | 1.0 | 2.5 | 0.5 | 0.5 | 0.5 |
| Matrix effects | 0.99 | 0.80 | 0.59 | 1.01 | 1.06 | 0.99 | 0.95 | 0.94 | 1.22 | 0.99 | 0.99 |
| Intra-day precision (%CV) | |||||||||||
| Q1 | −11.5 | 6.9 | 17.9 | −3.3 | 2.3 | 3.4 | 4.7 | 11.9 | −11.7 | −15.4 | 17.1 |
| Q2 | 5.7 | 1.3 | −10.7 | 12.7 | 13.9 | 10.5 | −4.1 | 6.7 | −2.9 | 0.3 | −1.1 |
| Q3 | 2.4 | 13.5 | −1.8 | 8.4 | 5.9 | −3.3 | −3.8 | −4.3 | 6.3 | −0.4 | 0.4 |
| Intra-day accuracy (%RE) | |||||||||||
| Q1 | 2.3 | 5.2 | 16.1 | 10.8 | 6.5 | 8.3 | 10.5 | 8.7 | 11.1 | 16.9 | 16.0 |
| Q2 | 3.0 | 4.6 | 15.2 | 5.5 | 14.8 | 8.8 | 11.4 | 7.5 | 14.0 | 16.6 | 9.6 |
| Q3 | 2.2 | 6.9 | 5.8 | 15.2 | 5.2 | 13.9 | 14.5 | 5.5 | 8.4 | 9.7 | 5.3 |
| Between-day precision (%CV) | |||||||||||
| Q1 | 13.2 | 8.3 | 23.8 | 8.8 | 8.6 | 6.8 | 9.1 | 7.9 | 8.5 | 4.1 | 17.8 |
| Q2 | 8.6 | 7.9 | 6.4 | 10.4 | 8.8 | 2.1 | 6.6 | 5.9 | 1.4 | 7.6 | 3.4 |
| Q3 | 6.9 | 7.6 | 12.4 | 5.4 | 1.4 | 7.4 | 6.9 | 8.1 | 4.1 | 11.1 | 3.3 |
| Between-day (%RE) | |||||||||||
| Q1 | −4.4 | −0.5 | −7.4 | 7.1 | 6.3 | 2.9 | −4.3 | 0.4 | −16.3 | −16.1 | −6.3 |
| Q2 | −1.9 | 2.6 | −7.4 | 6.8 | 5.2 | 12.0 | −0.2 | 5.1 | −2.0 | −0.7 | −2.7 |
| Q3 | 2.3 | 4.6 | 2.6 | 6.2 | 6.6 | 3.2 | −4.4 | 4.7 | 11.5 | −5.5 | −1.5 |
| Recovery (%) | |||||||||||
| Q1 | 108.0 | 86.1 | 90.6 | 104.5 | 101.6 | 109.0 | 107.6 | 101.7 | 87.8 | 94.5 | 88.5 |
| Q2 | 89.1 | 98.7 | 107.4 | 100.4 | 94.7 | 102.7 | 92.9 | 109.5 | 85.5 | 91.4 | 87.8 |
| Q3 | 92.2 | 92.1 | 111.6 | 110.8 | 97.8 | 92.2 | 103.7 | 100.8 | 103.0 | 88.9 | 97.3 |
| QCs (ng/ml) | |||||||||||
| Q1 | 1600 | 5 | 5 | 40 | 160 | 40 | 40 | 40 | 5 | 5 | 5 |
| Q2 | 12800 | 40 | 40 | 160 | 400 | 160 | 400 | 160 | 40 | 40 | 40 |
| Q3 | 32000 | 160 | 160 | 400 | 1500 | 400 | 1500 | 400 | 160 | 160 | 160 |
Stability of the samples
Freeze-thaw cycles, tested with the same sample during three consecutive months (C9S1, C9S2, C9S3 and C10S1, C10S2, C10S3 in the supplementary table), did not change the measured concentrations of the sulfated steroids.
Study of plasma samples
Half of the blood extracted from control donors C9 and C10 was processed to obtain serum, and the other half as plasma. Plasma samples were measured following the same sample preparation as for those of serum, and their concentrations were calculated by using calibrations performed with stripped serum. The concentrations found in plasma (C9P and C10P, respectively, in the supplementary table) demonstrate that the sample preparation and the analytical method are effective to profile sulfated steroids in plasma too.
DISCUSSION
The method described in this work shows a reliable quantitative performance for the profiling of sulfated steroids in human serum. Most of the compounds analyzed with our assay met the standards of the FDA guidelines. The compounds with precision and accuracy below 15% for all QC levels were CS, PregS, 16OHDHEAS, DHEAS, AnDiolS, AnS, and EpiAnS. In the case of 17OHPregS, the results were higher than 15% just for precision and only at the lower level, which could be related to the important matrix effects found for this analyte. Still at Q1, precision remained below 25%. The precision and accuracy values for DHTS, TS, and eTS were always below 20%.
Previously reported methods achieved a good separation for most important androgen sulfates (8, 15), showing the complexity of the signal m/z 369 in human blood. Interestingly, compounds like DHTS or TS were never analyzed before by LC-MS in human blood, probably because they are expected to be present in low concentrations (20). Additionally, there are two LC-MS assays focused on the analysis of CS in serum (11, 12). Other LC-MS-based methods performed the analysis of androgen sulfates (13, 14, 17). Our method is the first to quantify CS in combination with androgen sulfates, PregS, and 17OHPregS in a single run.
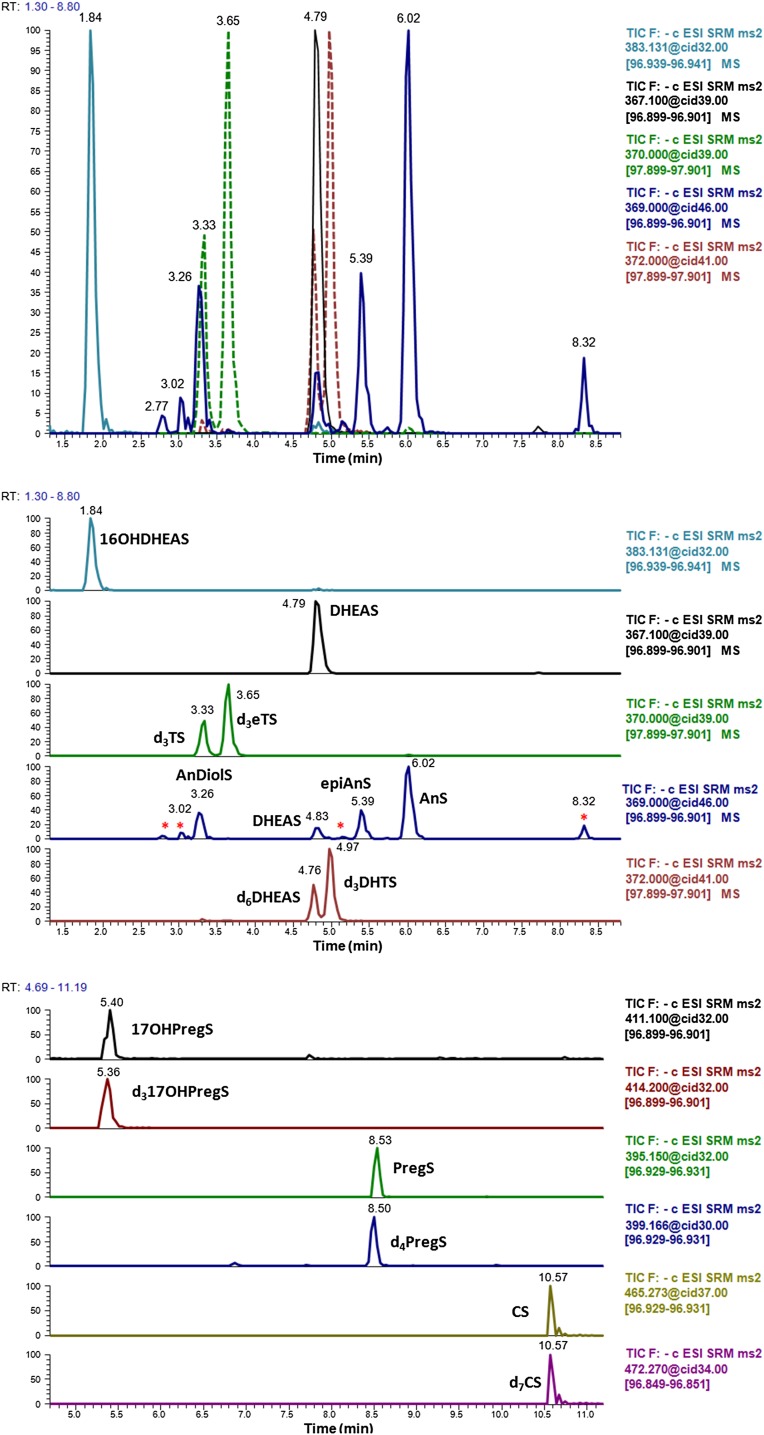
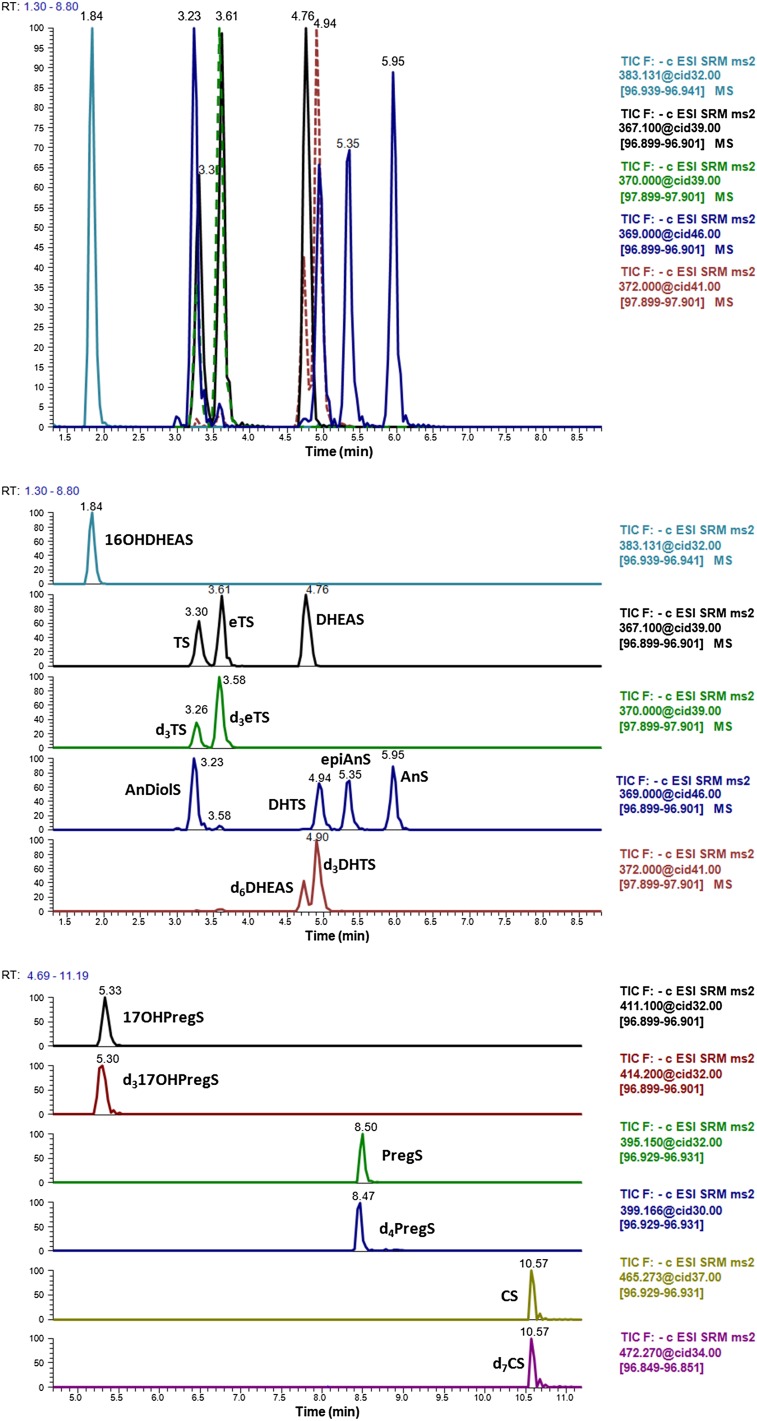
One of the goals of our work was to achieve a realistic profile of the androgen sulfates present in human blood within a runtime that permitted the analysis of a large number of samples. To achieve this, the method should provide a good separation of all isobaric compounds: DHTS, AnDiolS, AnS, and epiAnS (m/z 369); and TS, eTS, and DHEAS (m/z 367). Additionally, DHEAS should be always baseline resolved, because its high concentration might affect the m/z 369 signal as well. With the exception of 16OHDHEAS, all sulfated androgens exhibit a comparable polarity. A C18 reversed phase column was tested (Hypersil Gold column, 50 × 2.1 mm, 5 μm; Thermo Fisher Scientific), showing that resolution of all the mentioned compounds was not possible with such chromatography (data not shown). The introduction of ACN in the mobile phase and the selection of a different reversed-phase chromatography with phenyl groups allowed for effective separation of all the compounds of interest (Fig. 2). To ensure the analysis was always correct, we added deuterated ISs to qualitatively identify some of the sulfated steroids never studied before in blood by LC-MS/MS (d3eTS, d3TS, d3DHTS, and d317OHPregS). The chromatograms obtained for each analyte in the sample from a healthy volunteer (control C10) are depicted in Fig. 2A. This was chosen as a representative chromatogram for an adult male, where the sulfated steroids are in high concentrations. There it can be observed how the m/z 367 from DHEAS contributes to the transition m/z 369→97, because DHEAS is normally present in high concentrations in blood. Interestingly, a recent method analyzed DHEAS tracing transition m/z 369→97 instead of m/z 367→97 (17).
Fig. 2.
Chromatograms obtained from a healthy male and from standards spiked into charcoal-treated serum. A: Chromatograms of sulfated steroids in real serum from a healthy adult (sample C10 in the supplementary table). The first image shows a merged chromatogram for the androgen sulfates, which are labeled in the second image. Unidentified androgen sulfates are marked with an asterisk (*). In the third image, the chromatograms for 17OHPregS, PregS, and CS are depicted. B: Chromatograms of sulfated steroid standards (100 ng/ml each) spiked in stripped serum. Each image contains the same information as explained for (A).
Some of the expected sulfated steroids, TS, eTS and DHTS, were not found or they were below the LOQ of our method. This was confirmed by the retention time of each analyte and by the comparison with the IS. For TS, this absence was again corroborated by studying a unique transition of this compound; m/z 367→177 (Table 1). The ion m/z 177 is only detected in those steroids with a sulfate in position 17. Deuterium atoms in d3TS are located in carbons C16 and C17, which implies that the corresponding ion m/z 180 contains the sulfate group. In consonance, the m/z 177 matches with the formula C6H9O4S−, pointing out that it originates from the fragmentation of ring C (cleavage of bonds C12-C13 and C8-C14).
Of note, for the transition m/z 369→97, other unidentified peaks were always found with our method (Fig. 2A), in consonance with (11). For comparison, the retention times and peaks of commercial standards are shown in Fig. 2B.
Analysis of estrogen sulfates
Our assay showed a good separation of estrogen sulfates too, including estrone sulfate (E1S), estradiol sulfate (E2S), and estriol sulfate (E3S). These compounds appeared in our method at retention times 0.78 (E3S), 3.12 (E2S), and 4.06 (E1S), when they were spiked and measured after sample preparation. Estrogen sulfates were finally not included in the assay because of their poor performance. This could be attributed to the sample preparation and the physiological concentration of these compounds. The importance of sample workup for the analysis of estrogen sulfates was recently studied by Dury et al. (17). They published an LC-MS/MS method for the analysis of five sulfated steroids, including E1S. In their work, they tested the importance of several factors affecting the quantification of E1S, coming to the conclusion that protein precipitation with organic solvents during sample preparation modifies the concentration of estrogen sulfates, but does not affect the rest of compounds. This is probably due to the binding of their phenolic A-ring with proteins, and in order to include E1S in their assay they used solid phase extraction.
Measurement of sulfated steroids in real samples
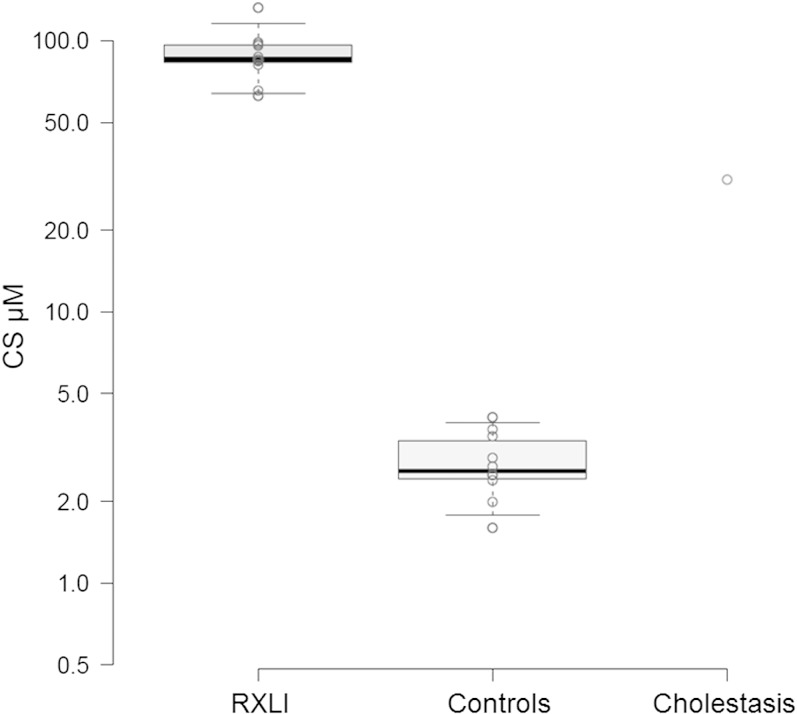
The method was applied to calculate the concentrations of the analytes in different serum samples. The values obtained are summarized in the supplementary table. As mentioned before, the compounds DHTS, TS, and eTS were not found in any of the samples. The sample from the RXLI patient treated with prednisolone (P2, supplementary table) showed an important decrease in the concentrations of all the sulfated steroids with the exception of CS. The CS level was similar to the rest of RXLI patients. This suggests that CS, unlike the rest of sulfated steroids, is not linked to the production of steroids by the adrenal glands.
The sample from the patient with cholestasis (P1) reflected a big increase in most of the sulfated steroids. The signal for AnDiolS could not be resolved because several interferences were found at the retention time of the peak. The rest of sulfated androgens were low or not found. This same sample was measured before to profile its levels of oxysterol sulfates, resulting in a high elevation of hydroxycholesterol sulfates (4).
Figure 3 shows a comparison of the levels of CS between the samples analyzed. The levels of CS were about 32 times higher in RXLI patients when compared with healthy donors, in good consonance with the method of Shackleton and Reid (11). The concentration of CS in the serum sample from the baby with cholestasis was three times lower than those of the RXLI patients.
Fig. 3.
Box plot comparing the different concentrations of CS in STS-deficient patients (RXLI), controls, and a cholestatic baby (data from the supplementary table). The Y axis, which represents the concentration of CS, is in logarithmic scale.
We studied different samples to test our assay. The analysis of serum from patients with STS deficiency has proven its utility for the diagnosis of this condition based on the levels of CS, even when compared with cholestasis. The profiling of sulfated steroids in plasma samples revealed that our method can be successfully applied to their quantification in that matrix as well.
Supplementary Material
Acknowledgments
The authors acknowledge the collaboration of all patients. The authors appreciate the expert assistance of Birgit Wardega and Kira Süßmuth.
Footnotes
Abbreviations:
- ACN
- acetonitrile
- AnDiolS
- 5-androsten-3β,17β-diol-3-sulfate (androstenediol sulfate)
- AnS
- 5α-androstan-3α-ol-17-one-3-sulfate (androsterone sulfate)
- CS
- 5-cholesten-3β-ol-3-sulfate (cholesterol sulfate)
- CV
- coefficient of variation
- DHEAS
- 5-androsten-3β-ol-17-one-3-sulfate (dehydroepiandrosterone sulfate)
- DHTS
- 5α-androstan-17β-ol-3-one-17-sulfate (dihydrotestosterone sulfate)
- epiAnS
- 5α-androstan-3β-ol-17-one-3-sulfate (epiandrosterone sulfate)
- E1S
- estrone sulfate
- E2S
- estradiol sulfate
- E3S
- estriol sulfate
- eTS
- 4-androsten-17α-ol-3-one-17-sulfate (epitestosterone sulfate)
- IS
- internal standard
- LOD
- limit of detection
- LOQ
- limit of quantification
- MeOH
- methanol
- 16OHDHEAS
- 5-androsten-3β,16α-diol-17-one-3-sulfate (16-α-hydroxy-dehydroepiandrosterone sulfate)
- 17OHPregS
- 5-pregnen-3β,17α-diol-20-one-3-sulfate (17-hydroxy-pregnenolone sulfate)
- PregS
- 5-pregnen-3β-ol-20-one-3-sulfate
- QC
- quality control sample
- RE
- relative error
- RXLI
- recessive X-linked ichthyosis
- STS
- steroid sulfatase
- TS
- 4-androsten-17β-ol-3-one-17-sulfate (testosterone sulfate)
This work was supported by the Selbsthilfe Ichthyose e. V. the Medical Faculty (OJ111409) of the University of Münster and of the German Research Foundation (DFG) within DFG Research Group 1369 “Sulfated Steroids in Reproduction” to subproject 7 (S.A.W., principal investigator).
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Labrie F., Luu-The V., Labrie C., Simard J. 2001. DHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinology. Front. Neuroendocrinol. 22: 185–212. [DOI] [PubMed] [Google Scholar]
- 2.Fietz D., Bakhaus K., Wapelhorst B., Grosser G., Günther S., Alber J., Döring B., Kliesch S., Weidner W., Galuska C. E., et al. 2013. Membrane transporters for sulfated steroids in the human testis–cellular localization, expression pattern and functional analysis. PLoS One. 8: e62638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts K. D., Bandi L., Calvin H. I., Drucker W. D., Lieberman S. 1964. Evidence that steroid sulfates serve as biosynthetic intermediates. IV. Conversion of cholesterol sulfate in vivo to urinary C19 and C21 steroidal sulfates. Biochemistry. 3: 1983–1988. [DOI] [PubMed] [Google Scholar]
- 4.Sánchez-Guijo A., Oji V., Hartmann M. F., Schuppe H-C., Traupe H., Wudy S. A. 2015. High levels of oxysterol sulfates in serum of patients with steroid sulfatase deficiency. J. Lipid Res. 56: 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elias P. M., Williams M. L., Choi E. H., Feingold K. R. 2014. Role of cholesterol sulfate in epidermal structure and function: Lessons from X-linked ichthyosis. Biochim. Biophys. Acta. 1841: 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orentreich N., Brind J. L., Vogelman J. H., Andres R., Baldwin H. 1992. Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J. Clin. Endocrinol. Metab. 75: 1002–1004. [DOI] [PubMed] [Google Scholar]
- 7.Sulcová J., Hill M., Hampl R., Stárka L. 1997. Age and sex related differences in serum levels of unconjugated dehydroepiandrosterone and its sulphate in normal subjects. J. Endocrinol. 154: 57–62. [DOI] [PubMed] [Google Scholar]
- 8.Shackleton C. H., Kletke C., Wudy S., Pratt J. H. 1990. Dehydroepiandrosterone sulfate quantification in serum using high-performance liquid chromatography/mass spectrometry and a deuterated internal standard: a technique suitable for routine use or as a reference method. Steroids. 55: 472–478. [DOI] [PubMed] [Google Scholar]
- 9.Søeborg T., Frederiksen H., Fruekilde P., Johannsen T. H., Juul A., Andersson A. M. 2013. Serum concentrations of DHEA, DHEAS, 17α-hydroxyprogesterone, δ4-androstenedione and testosterone in children determined by TurboFlow-LC-MS/MS. Clin. Chim. Acta. 419: 95–101. [DOI] [PubMed] [Google Scholar]
- 10.Peitzsch M., Dekkers T., Haase M., Sweep F. C. G. J., Quack I., Antoch G., Siegert G., Lenders J. W. M., Deinum J., Willenberg H. S., et al. 2015. An LC-MS/MS method for steroid profiling during adrenal venous sampling for investigation of primary aldosteronism. J. Steroid Biochem. Mol. Biol. 145: 75–84. [DOI] [PubMed] [Google Scholar]
- 11.Shackleton C. H. L., Reid S. 1989. Diagnosis of recessive X-linked ichthyosis: quantitative HPLC/mass spectrometric analysis of plasma for cholesterol sulfate. Clin. Chem. 35: 1906–1910. [PubMed] [Google Scholar]
- 12.Fong B. M. W., Tam S., Leung K. S. Y. 2013. Determination of plasma cholesterol sulfate by LC-APCI-MS/MS in the context of pediatric autism. Talanta. 116: 115–121. [DOI] [PubMed] [Google Scholar]
- 13.Mitamura K., Nagaoka Y., Shimada K., Honma S., Namiki M., Koh E., Mizokami A. 2003. Simultaneous determination of androstenediol 3-sulfate and dehydroepiandrosterone sulfate in human serum using isotope diluted liquid chromatography-electrospray ionization-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 796: 121–130. [DOI] [PubMed] [Google Scholar]
- 14.Mitamura K., Setaka M., Shimada K., Honma S., Namiki M., Koh E., Mizokami A. 2005. Determination of sulfates of androsterone and epiandrosterone in human serum using isotope diluted liquid chromatography-electrospray ionization-mass spectrometry. Biomed. Chromatogr. 19: 796–801. [DOI] [PubMed] [Google Scholar]
- 15.Liu S., Griffiths W. J., Sjövall J. 2003. Capillary liquid chromatography/electrospray mass spectrometry for analysis of steroid sulfates in biological samples. Anal. Chem. 75: 791–797. [DOI] [PubMed] [Google Scholar]
- 16.Galuska C. E., Hartmann M. F., Sánchez-Guijo A., Bakhaus K., Geyer J., Schuler G., Zimmer K. P., Wudy S. A. 2013. Profiling intact steroid sulfates and unconjugated steroids in biological fluids by liquid chromatography-tandem mass spectrometry (LC-MS-MS). Analyst. 138: 3792–3801. [DOI] [PubMed] [Google Scholar]
- 17.Dury A. Y., Ke Y., Gonthier R., Isabelle M., Simard J-N., Labrie F. 2015. Validated LC–MS/MS simultaneous assay of five sex steroid/neurosteroid-related sulfates in human serum. J. Steroid Biochem. Mol. Biol. 149: 1–10. [DOI] [PubMed] [Google Scholar]
- 18.Neunzig J., Sánchez-Guijo A., Mosa A., Hartmann M. F., Geyer J., Wudy S. A., Bernhardt R. 2014. A steroidogenic pathway for sulfonated steroids: the metabolism of pregnenolone sulfate. J. Steroid Biochem. Mol. Biol. 144: 324–333. [DOI] [PubMed] [Google Scholar]
- 19.Spitzer M., Wildenhain J., Rappsilber J., Tyers M. 2014. BoxPlotR: a web tool for generation of box plots. Nat. Methods. 11: 121–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saez J. M., Bertrand J., Migeon C. J. 1971. Metabolic clearance rate, urinary and plasma production rates of testosterone sulfate in man. Steroids. 17: 435–452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.