Key Points
A major part of CD8+ memory T cells expresses CD40L, the key molecule for T-cell–dependent help.
CD40L-expressing CD8+ T cells resemble functional CD4+ helper T cells.
Abstract
CD8+ T cells play an essential role in immunity against intracellular pathogens, with cytotoxicity being considered their major effector mechanism. However, we here demonstrate that a major part of central and effector memory CD8+ T cells expresses CD40L, one key molecule for CD4+ T-cell–mediated help. CD40L+ CD8+ T cells are detectable among human antigen-specific immune responses, including pathogens such as influenza and yellow fever virus. CD40L+ CD8+ T cells display potent helper functions in vitro and in vivo, such as activation of antigen-presenting cells, and exhibit a cytokine expression signature similar to CD4+ T cells and unrelated to cytotoxic CD8+ T cells. The broad occurrence of CD40L+ CD8+ T cells in cellular immunity implicates that helper functions are not only executed by major histocompatibility complex (MHC) class II–restricted CD4+ helper T cells but are also a common feature of MHC class I–restricted CD8+ T cell responses. Due to their versatile functional capacities, human CD40L+ CD8+ T cells are promising candidate cells for immune therapies, particularly when CD4+ T-cell help or pathogen-associated molecular pattern signals are limited.
Introduction
Immune responses can be subdivided into cellular and humoral components, with CD4+ helper T cells being essential for both arms of immunity and CD8+ cytotoxic T cells mounting cellular immunity against intracellular pathogens and malignant cells. During thymic selection, CD4+ and CD8+ T cells are restricted to recognize antigens presented via major histocompatibility complex class II (MHC-II) or class I (MHC-I), respectively. According to the prevailing concept of T-cell immunity, the primary function of CD4+ T cells is to activate antigen-presenting cells (APCs) presenting exogenous antigens via MHC-II, whereas CD8+ T cells act as cytotoxic cells after recognition of antigens via MHC-I. However, in recent years, it emerged that these central processes of adaptive immune responses are much more flexible and diverse than previously thought. CD4+ T cells have been described that have acquired cytotoxic function,1 and protective CD8+ T-cell–mediated immunity is not only based on cytotoxicity but also depends on the secretion of cytokines, including interferon γ (IFN-γ), interleukin (IL)-2, or tumor necrosis factor α (TNF-α).2 The mechanisms of how T-cell fates are modified may involve instruction by distinct subsets of APCs that are able to crosspresent exogenous antigens on MHC-I and/or present endogenous antigens on MHC-II.3,4 A key signaling event in adaptive immune responses is the transient expression of the costimulatory molecule CD40L on activated CD4+ T cells.5 The expression of CD40L by activated CD4+ T cells is known to be critical for licensing dendritic cells (DCs) to prime antigen-specific cytotoxic CD8+ T-cell responses.6-8 CD4+ T cells are thought to express the vast bulk of CD40L.9 However, CD8+ T cells can be induced to express CD40L in vitro,10-12 and recent in vivo studies comparing wild-type (WT) with CD40−/− mice depleted of CD4+ T cells provided functional evidence that CD8+ T cells are also competent to express this key helper T-cell molecule.13 However, a direct identification and characterization of CD8+ T cells with helper functions has not yet been attempted. Such CD8+ T cells could be essential for certain immune responses to achieve autonomy from classical CD4+ T-cell help, particularly in situations when MHC-II antigen-presentation and/or pathogen-associated molecular patterns are limited.
The study presented here identifies and characterizes CD8+ T cells with helper functions directly according to CD40L expression. These cells are present in various immune responses and form a major subset of CD8+ memory/effector T cells, including different subsets such as Th1- and Th2-type cells. CD40L+ CD8+ T cells comprise on average 25% of human memory CD8+ T cells and are characterized by a cytokine expression signature resembling conventional CD4+ helper T cells rather than CD8+ cytotoxic T cells. Furthermore CD40L-expressing CD8+ T cells exert helper functions in vitro and in vivo and represent a new substantial source of CD40L.
Materials and methods
Human cell preparation
This study was conducted in accordance with the Declaration of Helsinki. Human blood was obtained from healthy volunteers, including individuals who received the attenuated YFV-17D vaccination (Stamaril; Sanofi Pasteur) after providing informed consent. Peripheral blood mononuclear cells (PBMCs) were separated from heparinized whole blood using Ficoll-Hypaque (PAA) gradient and were cultured in RPMI 1640 medium (Gibco) supplemented with 100 U/mL penicillin, 0.1 mg/mL streptomycin, 0.3 mg/mL glutamine, and 10% inactivated human AB serum (PAA). All experiments followed protocols approved by the local authorities.
Mice and cell preparation
C57BL/6J mice were purchased from Charles River Laboratories. The following mice were obtained from The Jackson Laboratory and bred and housed under specific pathogen-free conditions at the institution’s animal facility (Charité) (stock number in parentheses): CD40L−/− (002428), OT-1xThy1.1 (003831), and OT-1xThy1.1xCD40L−/−.
Mice were challenged at 6 to 12 weeks of age and sacrificed at indicated time points. Single-cell suspensions were obtained from spleens or lymph nodes (LN) and were cultured in RPMI medium supplemented with 100 U/mL penicillin, 0.1 mg/mL streptomycin, 0.3 mg/mL glutamine, 10% inactivated FCS (PAA), and 50 μM β-mercaptoethanol (Gibco). All animal experiments were performed in accordance with German law and with permission from the local authorities.
Antibodies
The following antibodies (clones) conjugated to fluorescein isothiocyanate, phycoerythrin (PE), PerCP, APC, APC-Cy7, PE-Cy7, Alexa 488, eFlour 605, or PacBlue were purchased from the listed companies. BD: αhCD3 (SK7), αhCD4 (SK3), αhCD8 (SK1), αhCD69 (FN50), αhIL-2 (MQ1-17H12), αhIL-4 (MP4-25D2), αhIFN-γ (B27), αhCD28 (CD28.2), αhCD62L (Dreg56), αhCCR4 (1G1), αhCLA (HECA-452), αhCCR5 (2D7/CCR5), αhCXCR3 (1C6/CXCR3), αhIntegrinB7 (FIB504), αhTNF-α (Mab11), αhMIP-1β (D21-1351), αhCD83 (HB15e), αhCD107a (H4A3), αhGranzymeB (GB11), αhCD40L (TRAP1), αmCD4 (RM4-5), αmCD8 (53-6.7); R&D: αhCCR7 (150503); Immunotech: αCD127 (R34.34); self-conjugated: αhCD27 (2E4), αhCD3 (UCHT-1), αhCD8 (GN11/134.7), αhCD4 (TT1), αhCD19 (BU12); Miltenyi Biotec: αhCD40L (5c8), αmCD40 (FGK 45.5), αmCD11c (N418), αhCRTh2 (BM16); eBioscience: αhCD4 (OKT04), αhIL17 (eBio64Dec17), αhIFN-γ (4S.B3), αmCD4 (GK1.5), αmIFN-γ (XMG1.2), αmCD62L (MEL-14); Biolegend: αhCD40L (24-31), αhCD57 (HCD57), αhIFN-γ (B27), αhPerforin (dG9), αmCD3 (145-2C11), αmCD8 (53-6.7), αmCD44 (IM7), αmCD11b (M1/70), αmMHC-II (M5/114.15.2), αmCD80 (16-10A1), αmCD45.1 (A20). All conjugates were titrated to determine their optimal dilution. To avoid Fc-receptor binding, human cells were stained in the presence of 1 mg/mL Beriglobin (Sanofi-Aventis) and murine cells with 2 µg/mL αmFcγ-receptor (2.4G2).
Flow cytometry, cell sorting, and stimulation
Enrichment of human or mouse T cells was performed using the appropriate microbeads (Miltenyi Biotec). The purity was checked routinely using fluorescence-activated cell sorting (FACS) and was ≥95%. For isolation of specific populations, CD8+ T cells were stained for 10 minutes for differentiation markers and were subsequently sorted with an FACS Aria (BD). For the sorting and analysis of live cells, 400 nM DAPI (Molecular Probes) was added. Sorted cells (2 × 105 to 2 × 106 cells per mL) were stimulated polyclonally at 37°C for 6 hours with 10 ng/mL PMA and 1 µg/mL phorbol ester/ionomycin (P/I) (Sigma-Aldrich) in the presence of 1 µg/mL αCD40 (G28.5) for the assessment of extracellular CD40L expression or with 2 µg/mL brefeldin A (Sigma-Aldrich) for intracellular staining.14 For the analysis of human antigen-specific T cells, 5 × 106 PBMCs were stimulated for 6 hours at 37°C with 1 µg peptide per mL with the following peptide pools or single peptides: EBV-LMP1, EBV-LMP2, CMV-PP65, CMV-IE1, Flu-MP, Flu-NP, and YFV-NS4B214–222.15 Intracellular cytokine and CD40L staining was performed for 30 minutes at 4°C after fixation and permeabilization with FACS-Lysing and FACS-Perm2 Solution (BD) according to the manufacturer’s protocol. The cells were subsequently analyzed using an LSRII. For CD107a staining, αCD107a–Alexa 488 was added during stimulation. FACS data were analyzed with FlowJo (Tree Star).
In vitro APC cocultures and stability
For the APC cocultures, the CD8+ and CD4+ T-cell subsets were sorted with magnetic-activated cell sorting (MACS) and FACS and were stimulated with P/I before the cells were sorted for CD40L expression. The sorted fractions were irradiated with 30 Gy and 105 T cells were cocultured at 37°C in 96-well plates (Greiner) with 2 × 104 carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled B cells. B cells were purified using αhCD19-Microbeads (Miltenyi Biotec) and labeled using 5 µM CFDA (Invitrogen). On day 8, the supernatant was collected for immunoglobulin M and immunoglobulin G enzyme-linked immunosorbent assay (ELISA) and the CFSE dilution of CD3−CD19+ cells was analyzed by FACS. The ELISAs were performed as described previously.16
Similarly, the sorted T-cell subsets were cocultured at a ratio of 5:1 for 36 hours at 37°C with immature (CD83−) monocyte-derived DCs (moDCs). The moDCs were generated as previously described.17 Maturation was measured according to CD83 expression by moDCs. Both cocultures were performed with and without 10 µg/mL of blocking αhCD40L (5c8). Additionally, DCs were cultured with 10 µg/mL αhCD40. The supernatant concentrations of cytokines were measured on an LSRII with a bead array (eBioscience).
To test the capability to re-express CD40L, CD3+/CD8+/CD4−/CD45RA− memory T cells were sorted using MACS and FACS and were stimulated with P/I before the cells were sorted for CD40L expression. The sorted T cells were labeled using 5 µM CFDA and cultured with and without rhIL-2 and rhIL-12 (both 10 ng/mL; Miltenyi Biotec). On day 7, the cells were restimulated with P/I and stained for surface CD40L.
Microarray analysis
Isolated RNA was subjected to quality control with an Agilent 2100 bioanalyzer and quantification with a NanoDrop ND-1000 spectrophotometer as previously described.18 Complementary DNA was hybridized to Affymetrix HG U133 Plus2.0 GeneChips.
Gene array analysis was performed using R.19 CEL files were normalized utilizing the robust multiarray average algorithm and then implemented in Bioconductor.20 The expression of cytokines was extracted from the normalized data and without further filtering was subjected to hierarchical clustering using the Manhattan distance and Average linkage functions. The microarray data have been deposited in the Gene Expression Omnibus with the accession number GSE44126 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=xdqtxqkukgugots&acc=GSE4412).
In vivo DC activation
CD40L−/− mice were injected with 106 CD8+ T cells from OT-1xThy1.1 or OT-1xThy1.1xCD40L−/− enriched by MACS. The next day, mice were immunized with 100 µg ovalbumin (OVA)-peptide SIINFEKL subcutaneously (R. Volkmer, Charité). One group without T-cell transfer received 500 µg αmCD40 (FGK45). The serum and draining LN (lnn. subiliaci) were harvested 36 hours later. Serum IL-12 concentrations were determined with an ELISA (Invitrogen). Cell suspensions of the draining LN were measured on an LSRII.
Statistics
The nonparametric 2-tailed Mann-Whitney test, 2-tailed Wilcoxon signed-rank test, Friedman test, and Dunn’s post hoc test were used for direct comparisons. For multiple group analysis, analysis of variance with the Bonferroni multiple comparison posttest was selected (GraphPad Prism 5.02).
Results
Human CD40L+ CD8+ T cells represent memory cells
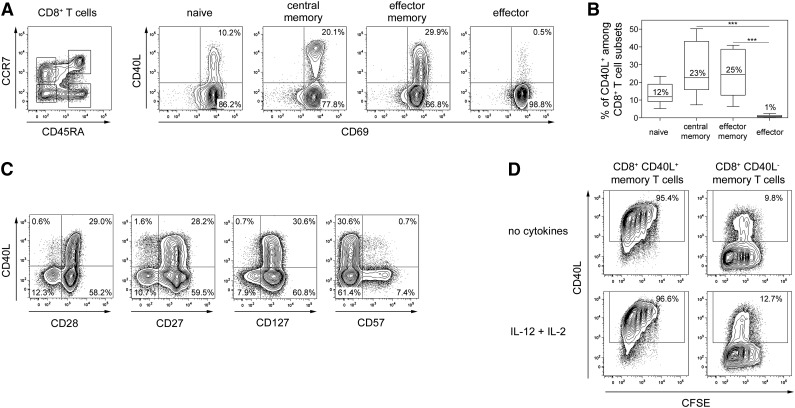
At first, we analyzed whether distinct subsets of human CD8+ T cells characterized by differential expression of CD45RA and CCR7 express CD40L after polyclonal stimulation.21-23 On average, 25% of both central memory (CCR7+ CD45RA−) and effector memory (CCR7− CD45RA−) CD8+ T cells expressed CD40L (Figure 1A-B) and central memory cells expressed higher levels of CD40L than effector memory CD8+ T cells comparable to the expression levels of CD4+ T helper cells (supplemental Figure 1). Effector (CCR7− CD45RA+) CD8+ T cells did not express CD40L, and about 10% of naïve (CCR7+ CD45RA+) CD8+ T cells expressed CD40L. Most CD40L+ CD8+ T cells represented memory T cells (CD27+ and CD28+ CD57−) and coexpressed the IL-7 receptor (CD127) (Figure 1C), a characteristic of long-living memory T cells.24 To elucidate the migratory potential of CD8+ T cells capable of expressing CD40L, we analyzed various cell-surface molecules involved in lymphocyte homing and recirculation. Memory CD8+ T cells expressing CCR4, CLA, CRTh2, or CD62L were significantly enriched in CD40L-expressing cells, suggesting secondary lymphoid organ and skin migratory potential. Few CD40L+ CD8+ T cells expressed CCR5, CXCR3, or integrin β7 (supplemental Figure 2), indicating a reduced inflammatory or gut-homing capability of CD40L+ CD8+ T cells. We further evaluated whether CD40L expression by human memory CD8+ T cells is a stable functional feature. Approximately 95% of purified CD40L+ CD8+ memory T cells re-expressed CD40L after 1 week of culturing and restimulation, while CD40L− CD8+ memory T cells barely acquired CD40L expression during in vitro culture (Figure 1D). Even upon adding IL-12 and IL-2, cytokines known to promote CD8+ T-cell differentiation, CD40L+ cells kept their capability to re-express CD40L, suggesting that in vivo–primed CD40L+ CD8+ T cells constitute a stable phenotype of CD8+ memory T cells.
Figure 1.
Phenotype of human CD40L+ CD8+ T cells. (A) The left dot-plot shows representative gates for ex vivo–sorted CD3+ CD8+ CD4− T-cell subsets based on CCR7 and CD45RA expression (CCR7+ CD45RA+ naive, CCR7+ CD45RA− central memory, CCR7- CD45RA− effector memory, or CCR7− CD45RA+ effector cells). The isolated cells were stimulated with P/I in the presence of αCD40 antibodies and were subsequently assessed for CD40L expression among activated CD69+ cells. (B) The frequencies of CD40L+ CD8+ T-cell subsets are shown as the median ± standard error of the mean (SEM) (n = 13); the Friedman test and Dunn’s post hoc test were used (***P < .001). (C) P/I-stimulated CD8+ T cells were analyzed for their capacity to coexpress CD40L with CD27, CD28, CD127, or CD57. (D) Ex vivo–sorted human memory CD8+CD45RA− T cells were stimulated with P/I and subsequently separated into CD40L+ and CD40L− fractions. Proliferation after 7 days in vitro culture with and without IL-2 and IL-12 was assessed by CFSE dilution and capability to re-express CD40L after 6-hour P/I stimulation. (C-D) One representative experiment out of 3 to 5 is shown.
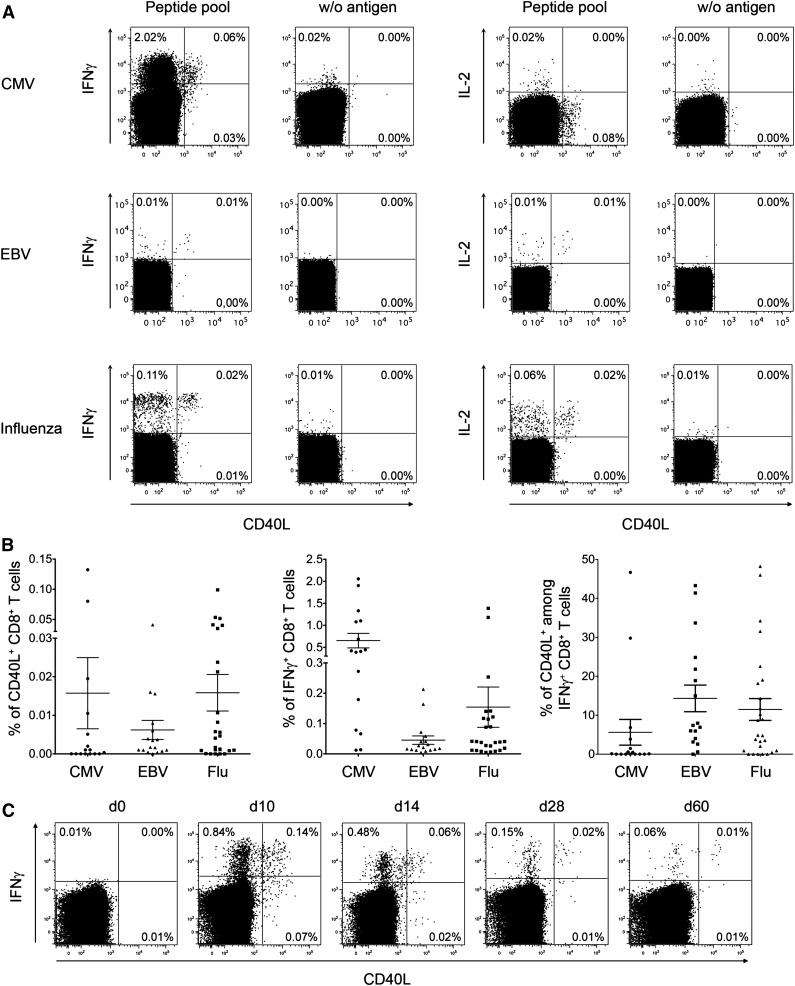
We then investigated whether common human CD8+ T cell responses, such as those specific for influenza (flu), cytomegalovirus (CMV), and Epstein-Barr virus (EBV), comprise CD40L-expressing cells. Pathogen-specific IFN-γ+ CD8+ T cells of CMV-, EBV-, and Flu-reactive donors included 6%, 14%, and 12% of CD40L+ cells, respectively (Figure 2A-B). Additionally, we analyzed in parallel the CD40L expression of virus-specific CD4+ T cells, although due to the amount of different proteins expressed by pathogens (eg, CMV = 160 proteins,25 EBV = 89 proteins26) and the big variation of epitope usage in different HLA types, it is difficult to compare CD40L expression frequencies on antigen-activated CD4+ and CD8+ T cells (supplemental Figure 3). In selected donors demonstrating both CD40L+ CD4+ and CD8+ T cells, we could show that CD8+ T cells express on average less CD40L than CD4+ T cells.
Figure 2.
CD40L+ CD8+ T cells in human antigen–specific responses. (A) Human PBMCs from healthy donors were stimulated with peptide pools from different virus antigens. The dot-plots show representative intracellular cytokine and CD40L staining of CD3+ CD8+ CD4−–gated lymphocytes. (B) The frequencies of CD40L+ and IFN-γ+ virus-specific CD8+ T cells and the corresponding ratio of CD40L+ cells among virus-specific IFN-γ+ CD8+ T cells are summarized for CMV (n = 16), EBV (n = 17), and flu (n = 26) (mean ± SEM). (C) At different time points after YFV vaccination, PBMCs from healthy donors were stimulated with the immunodominant peptide YFV-NS4B. The dot-plots show the intracellular IFN-γ and CD40L staining of CD3+ CD8+ CD4−–gated lymphocytes from 1 representative donor (n = 6).
Furthermore, CD8+ T-cell responses elicited during yellow fever virus (YFV) vaccination were analyzed in order to monitor a primary human immune response leading to almost lifelong immunity.27 In all donors, CD40L expression was detectable in up to 20% of YFV-specific IFN-γ+ CD8+ T cells independent of the time point after vaccination (Figure 2C-D).
CD40L+ CD8+ T cells share functional features of CD4+ T helper cells
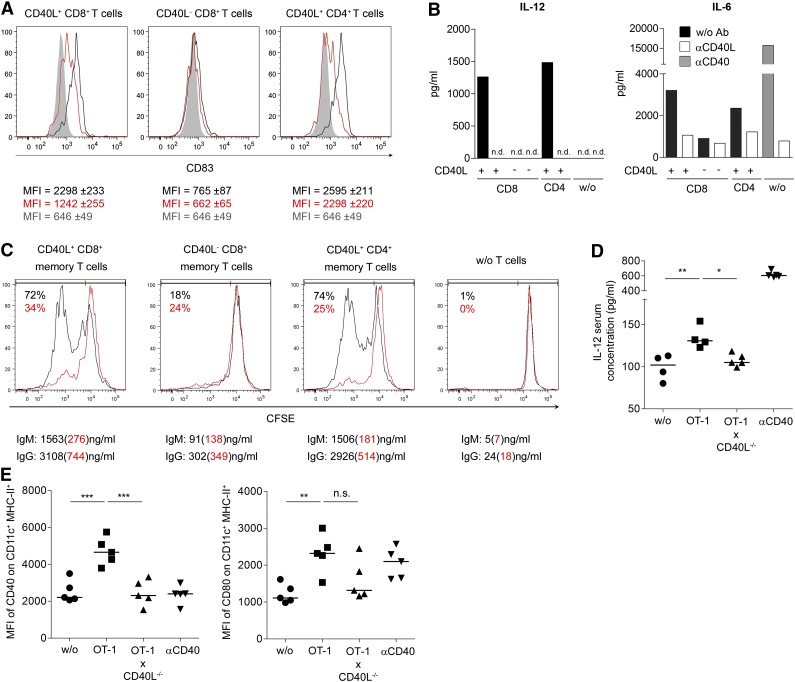
In order to evaluate functional features of CD40L-expressing CD8+ T cells, we next determined whether human CD40L+ CD8+ T cells induce DC maturation and B-cell activation similar to CD4+ helper T cells. Activated human CD40L+ CD8+ T cells induced maturation of moDCs, as defined by the induction of CD83 expression,17 and the secretion of cytokines such as IL-12 in a CD40L-dependent manner (Figure 3A-B and supplemental Figure 4). Activated CD40L− CD8+ memory T cells largely failed to induce DC maturation. In a similar approach, CD40L+ but not CD40L− CD8+ memory T cells also activated proliferation and antibody secretion of B cells (Figure 3C).
Figure 3.
CD40L+ CD8+ T cells functionally resemble CD4+ T helper cells. (A) CD8+ and CD4+ T cells were stimulated with P/I and CD40L+ and CD40L− cells were separated. Next, the T-cell subsets were cocultured for 24 hours with immature moDCs in the presence (red) or absence (black) of blocking αCD40L antibodies. Gray filled lines represent CD83 expression on DCs without T cells. The median ± SEM of the CD83 expression is written beneath the histograms in matching colors. (B) Supernatants of experiments described in panel A were collected to determine cytokine concentrations. MoDC controls without T cells were also cultured in the presence of αCD40 antibody or αCD40L antibody (gray or white bars, respectively). (C) As in panel A, the same sorted T-cell populations were cocultured with B cells in the presence (red) or absence (black) of blocking αCD40L antibodies. The proliferation of cocultured B cells was assessed after 8 days based on CFSE dilution, and the supernatant from the same cocultures was analyzed by ELISA for secretion of immunoglobulin M and immunoglobulin G. The percentages represent the frequencies of proliferated CFSElow B cells. (D-E) CD40L−/− mice received 106 OT-1 and OT-1xCD40L−/− CD8+ T cells (intravenously) and were challenged 1 day later with OVA-peptide (SIINFEKL) (subcutaneously). (D) The level of IL-12 in the blood serum was quantified by ELISA 36 hours postimmunization, and (E) the mean fluorescence intensity (MFI) of CD40 and CD80 from draining LN CD11c+ MHC-II+ cells was determined. (D-E) For statistical analysis, analysis of variance with the Bonferroni multiple comparison posttest was used (*P < .05; **P < .01; ***P < .001). (A-E) One representative experiment out of 2 or 3 is shown.
To test whether CD40L+ CD8+ T cells can induce APC activation as well in vivo, we measured IL-12 blood serum concentration and activation status of draining LN DCs in OVA-peptide immunized CD40L−/− mice that had received either WT OT-1 or CD40L−/− OT-1 CD8+ T cells before (Figure 3D-E). Thus, CD40L expression by other cell types is excluded. Significantly elevated IL-12 serum levels and upregulation of CD80 and CD40 on CD11c+ DCs from draining LN were detectable only in recipient mice transferred with CD40L-competent WT OT-1 CD8+ T cells, confirming that CD40L+ CD8+ T cells can exert potent helper functions also in vivo.
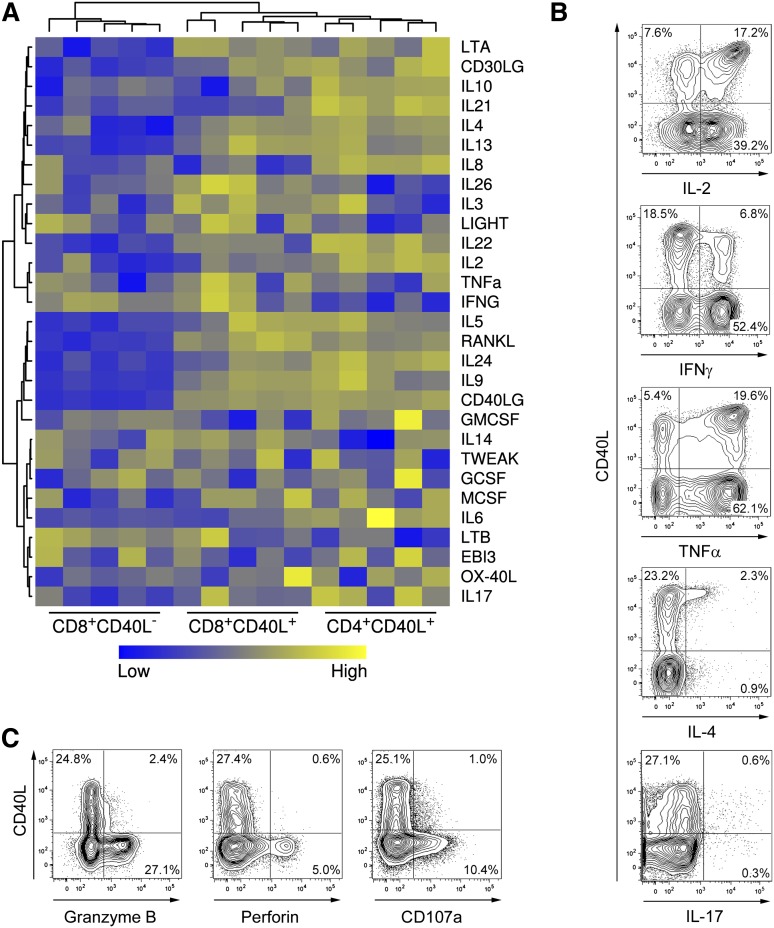
Another key functional feature of memory CD4+ helper T cells in addition to CD40L expression is the secretion of distinct cytokines depending on their polarization.28 In order to assess cytokine secretion potentials in detail, cytokine expression profiles of activated human CD40L+ and CD40L− memory CD8+ and CD40L+ CD4+ memory T cells were compared using microarray technology. Unsupervised clustering analysis revealed that the cytokine expression profile of CD40L+ CD8+ T cells is more closely related to the profile of CD40L+ CD4+ T cells than to the profile of CD40L− CD8+ T cells (Figure 4A and supplemental Figure 5). Based on these results, we performed direct cytometric analysis of CD40L and helper T cell cytokines in ex vivo activated human CD8+ T cells. Compared with CD40L− cells, CD40L+ CD8+ T cells were highly enriched in IL-2+ and IL-4+ cells, expressed comparable levels of TNF-α or IL-17, and expressed low levels of IFN-γ (Figure 4B), suggesting that differentially polarized CD8+ T-cell subsets with helper functions exist, similar to CD4+ T cells. Frequencies of polyfunctional T cells,29 characterized by ≥3 parameters such as IL-2, IFN-γ, TNF-α, MIP1β, and CD107a expression, were similar between CD40L+ and CD40L− CD8+ T cells (supplemental Figure 6). Notably, CD40L+ polyfunctional CD8+ T cells predominantly expressed IL-2, whereas CD107a was exclusively detected among CD40L− polyfunctional CD8+ T cells. In agreement with these results, the cytotoxic effector molecules granzyme B and perforin were predominantly expressed by CD40L− CD8+ T cells following polyclonal stimulation (Figure 4C). In summary, CD40L+ CD8+ T cells account for a substantial, distinct subset of memory T cells in the peripheral human T-cell pool characterized by a functional potential closely related to CD4+ helper T cells.
Figure 4.
Human CD40L+ CD8+ T cells express helper cytokines but not cytolytic molecules. (A) CD8+ and CD4+CD45RA− memory T-cell subsets were sorted after 6-hour P/I stimulation and messenger RNA expression of cytokine genes was analyzed with microarrays. (B) CD8+ T cells were stimulated with P/I and were analyzed for CD40L expression and coexpression of cytokines using an intracellular cytokine assay. (C) CD8+ T cells were stimulated and analyzed for CD40L and degranulation (CD107a+) as well as for coexpression of perforin and granzyme B. (B-C) One representative experiment out of 5 is shown.
Discussion
We here demonstrate that CD40L expression and other established helper T-cell characteristics are a feature of not only MHC-II–restricted CD4+ helper T cells but also of a novel and prominent subset of MHC-I–restricted CD8+ T cells. Assessment of CD40L ex vivo after stimulation has so far been hampered by the fact that CD40L is downregulated and degraded rapidly after interaction with CD40.30 Expression of CD40L was therefore difficult to detect, or its actual expression level may have been underestimated due to these limitations. Employing methods, previously established by us,14,31 we here show directly that CD40L expression is a feature of a distinct prominent subset of memory CD8+ T cells functionally resembling CD4+ helper T cells.
Putative helper properties of CD8+ T cells were first postulated in the early 2000s.32-34 Later, it was reported that lack of CD40L by CD8+ T cells leads to diminished T-cell responses in the absence of CD4+ helper T cells.35 Based on studies using blocking αCD40L antibody, CD8+ T cells have been suspected previously to express also the key helper T-cell molecule CD40L.36 Our data directly reveal that CD40L is expressed by a major CD8+ T-cell subset. CD40L+ CD8+ T cells display cytokine expression profiles similar to CD4+ helper T cells and induce maturation of APCs as well as IL-12 production in vitro and in vivo. The T-cell–induced serum IL-12 levels seem to be low but are after T-cell–dependent DC activation, where lower IL-12 levels can be expected compared with systemic administration of Toll-like receptor ligands like lipopolysaccharide. According to their helper T-cell–like functions, CD40L+ CD8+ T cells can be classified as CD8+ helper T cells.
The fact that rapid CD40L expression is a feature of resting memory CD8+ T cells supports the notion that CD40L+ CD8+ T cells may exert their helper functions not only in the initial effector phase of an immune response but also during a secondary challenge. One prominent function of CD40L during a secondary pathogen encounter could be to protect APC from cytotoxic T lymphocyte (CTL)-mediated elimination. While so far this function had been attributed mainly to CD4+ T cells,37 it has recently been suggested also for CD8+ memory T cells,38 particularly in interaction with crosspresenting DCs.
Analysis of the cytokine expression profiles of CD40L+ and CD40L− CD8+ T-cell subsets revealed that most Tc2 cells, defined by expression of IL-4, IL-5, or IL-13, as well as CD8+ T cells expressing other CD4+ helper T-cell–associated cytokines such as IL-9, IL-17, or IL-22, are components of the CD40L-expressing CD8+ T-cell population, suggesting the existence of diverse CD40L-expressing CD8+ T-cell subsets in analogy to CD4+ helper T-cell subsets. While mostly IFN-γ–producing CD40L+ CD8+ T cells were detected in human-virus–specific immune responses, the majority of human IL-2+ CD40L+ CD8+ T cells did not produce IFN-γ. This discrepancy could indicate that IL-2+ CD40L+ CD8+ T cells have different pathogen specificities or are primed under different conditions than IFN-γ–producing CD40L+ CD8+ T cells. Frequencies of CD40L expression among CMV-specific CD8+ T cells were lower than among EBV- and flu-specific CD8+ T cells, probably due to the special characteristics of CMV that drive antigen-specific CD8+ T cells to a terminally differentiated effector phenotype.39 This could support a notion that CD40L+ CD8+ T cells are decreased in chronic infection. In contrast, in the course of vaccination with YFV, which resembles an acute primary virus infection, CD40L expression by antigen-specific CD8+ T cells was detected in all vaccinated individuals at all analyzed time points.
In addition to a putative role in protecting APCs from lysis by effector CTLs, CD8+ T cells with helper properties could be essential to achieve certain autonomy from CD4+ T-cell help during the activation of cellular immunity. The prevailing concept comprises a sequential 3-cell interaction including specific CD4+ and CD8+ T cells and DCs, leaving open the question of how specific CD8+ T cells always encounter a DC after it has been licensed by specific CD4+ T cells.40 A 2-cell interaction would more adequately explain the rapid activation and expansion of T cells, particularly during a second pathogen encounter. The ubiquitous presence of CD8+ T cells capable of expressing CD40L may provide an answer.
In summary, the feasibility to directly assess CD8+ T cells with helper properties based on CD40L expression will permit the further analysis of their functions in intercellular activation and communication during immune reactions. Furthermore, due to their unique ability to conduct help in a MHC-I–restricted manner, CD40L+ CD8+ T cells represent a very attractive T-cell population for cellular therapies aimed against infection and malignant diseases.
Acknowledgments
The authors thank Jane Grogan, Ulf Klein, Meaghan Stolk, and Bob Jack for their critical reading of the manuscript and Karolina Grzeschik, Manuela Krüger, Beate Möwes, and the BCRT Flow Cytometry Laboratory for their expert technical help.
This work was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereiche SFB 633 (B10), SFB TR36 (A11), and DFG Th 806/5-1), a flexible funds grant from the BCRT/BMBF, and the BMBF research network STThera (01GU0802).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.F., R.S., and A.T. developed the main hypothesis; M.F., R.S., N.M., S.M., S.D., A.R.S., F.G., and G.H. planned studies, performed experiments and/or analyzed data; U.S. and K.J. analyzed the microarrays; M.A.R., M.R.B., and D.B. contributed reagents, intellectual input, and editorial assistance; M.F. and A.T. wrote the manuscript and obtained funding; A.T. supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marco Frentsch, Regenerative Immunology and Aging, Berlin-Brandenburg Center for Regenerative Therapies, CVK Charité University Medicine, Föhrerstr. 15, 13533 Berlin, Germany; e-mail: marco.frentsch@charite.de; and Andreas Thiel, Regenerative Immunology and Aging, Berlin-Brandenburg Center for Regenerative Therapies, CVK Charité University Medicine, Föhrerstr. 15, 13533 Berlin, Germany; e-mail: andreas.thiel@charite.de.
References
- 1.Appay V, Zaunders JJ, Papagno L, et al. Characterization of CD4(+) CTLs ex vivo. J Immunol. 2002;168(11):5954–5958. doi: 10.4049/jimmunol.168.11.5954. [DOI] [PubMed] [Google Scholar]
- 2.Messingham KA, Badovinac VP, Jabbari A, Harty JT. A role for IFN-gamma from antigen-specific CD8+ T cells in protective immunity to Listeria monocytogenes. J Immunol. 2007;179(4):2457–2466. doi: 10.4049/jimmunol.179.4.2457. [DOI] [PubMed] [Google Scholar]
- 3.Kurts C, Robinson BW, Knolle PA. Cross-priming in health and disease. Nat Rev Immunol. 2010;10(6):403–414. doi: 10.1038/nri2780. [DOI] [PubMed] [Google Scholar]
- 4.Strawbridge AB, Blum JS. Autophagy in MHC class II antigen processing. Curr Opin Immunol. 2007;19(1):87–92. doi: 10.1016/j.coi.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 6.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393(6684):474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 7.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393(6684):478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 8.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393(6684):480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 9.van Kooten C, Gaillard C, Galizzi JP, Hermann P, Fossiez F, Banchereau J, Blanchard D. B cells regulate expression of CD40 ligand on activated T cells by lowering the mRNA level and through the release of soluble CD40. Eur J Immunol. 1994;24(4):787–792. doi: 10.1002/eji.1830240402. [DOI] [PubMed] [Google Scholar]
- 10.Sad S, Krishnan L, Bleackley RC, Kägi D, Hengartner H, Mosmann TR. Cytotoxicity and weak CD40 ligand expression of CD8+ type 2 cytotoxic T cells restricts their potential B cell helper activity. Eur J Immunol. 1997;27(4):914–922. doi: 10.1002/eji.1830270417. [DOI] [PubMed] [Google Scholar]
- 11.Hermann P, Van-Kooten C, Gaillard C, Banchereau J, Blanchard D. CD40 ligand-positive CD8+ T cell clones allow B cell growth and differentiation. Eur J Immunol. 1995;25(10):2972–2977. doi: 10.1002/eji.1830251039. [DOI] [PubMed] [Google Scholar]
- 12.Xydia M, Ge Y, Quitsch U, Beckhove P. CD40L co-stimulation from CD8(+) to CD4(+) effector memory T cells supports CD4(+) expansion. Immunol Cell Biol. 2011; 89(6):670-680. [DOI] [PubMed]
- 13.Hernandez MG, Shen L, Rock KL. CD40 on APCs is needed for optimal programming, maintenance, and recall of CD8+ T cell memory even in the absence of CD4+ T cell help. J Immunol. 2008;180(7):4382–4390. doi: 10.4049/jimmunol.180.7.4382. [DOI] [PubMed] [Google Scholar]
- 14.Frentsch M, Arbach O, Kirchhoff D, et al. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med. 2005;11(10):1118–1124. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- 15.Akondy RS, Monson ND, Miller JD, et al. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J Immunol. 2009;183(12):7919–7930. doi: 10.4049/jimmunol.0803903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heine G, Anton K, Henz BM, Worm M. 1alpha,25-dihydroxyvitamin D3 inhibits anti-CD40 plus IL-4-mediated IgE production in vitro. Eur J Immunol. 2002;32(12):3395–3404. doi: 10.1002/1521-4141(200212)32:12<3395::AID-IMMU3395>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Zhou LJ, Tedder TF. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995;154(8):3821–3835. [PubMed] [Google Scholar]
- 18.Biesen R, Dähnrich C, Rosemann A, et al. Anti-dsDNA-NcX ELISA: dsDNA-loaded nucleosomes improve diagnosis and monitoring of disease activity in systemic lupus erythematosus. Arthritis Res Ther. 2011;13(1):R26. doi: 10.1186/ar3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. ISBN 3-900051-07-0.
- 20.Gentleman RC, Carey VJ, Bates DM, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5(10):R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 22.Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73(11):975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 23.van Lier RA, ten Berge IJ, Gamadia LE. Human CD8(+) T-cell differentiation in response to viruses. Nat Rev Immunol. 2003;3(12):931–939. doi: 10.1038/nri1254. [DOI] [PubMed] [Google Scholar]
- 24.Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci USA. 2004;101(15):5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davison AJ, Dolan A, Akter P, et al. The human cytomegalovirus genome revisited: comparison with the chimpanzee cytomegalovirus genome. J Gen Virol. 2003;84(Pt 1):17–28. doi: 10.1099/vir.0.18606-0. [DOI] [PubMed] [Google Scholar]
- 26.de Jesus O, Smith PR, Spender LC, Elgueta Karstegl C, Niller HH, Huang D, Farrell PJ. Updated Epstein-Barr virus (EBV) DNA sequence and analysis of a promoter for the BART (CST, BARF0) RNAs of EBV. J Gen Virol. 2003;84(Pt 6):1443–1450. doi: 10.1099/vir.0.19054-0. [DOI] [PubMed] [Google Scholar]
- 27.Monath TP. Yellow fever: an update. Lancet Infect Dis. 2001;1(1):11–20. doi: 10.1016/S1473-3099(01)00016-0. [DOI] [PubMed] [Google Scholar]
- 28.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112(5):1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107(12):4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yellin MJ, Sinning J, Covey LR, et al. T lymphocyte T cell-B cell-activating molecule/CD40-L molecules induce normal B cells or chronic lymphocytic leukemia B cells to express CD80 (B7/BB-1) and enhance their costimulatory activity. J Immunol. 1994;153(2):666–674. [PubMed] [Google Scholar]
- 31.Kirchhoff D, Frentsch M, Leclerk P, et al. Identification and isolation of murine antigen-reactive T cells according to CD154 expression. Eur J Immunol. 2007;37(9):2370–2377. doi: 10.1002/eji.200737322. [DOI] [PubMed] [Google Scholar]
- 32.Schüler T, Kammertoens T, Preiss S, Debs P, Noben-Trauth N, Blankenstein T. Generation of tumor-associated cytotoxic T lymphocytes requires interleukin 4 from CD8(+) T cells. J Exp Med. 2001;194(12):1767–1775. doi: 10.1084/jem.194.12.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang B, Norbury CC, Greenwood R, Bennink JR, Yewdell JW, Frelinger JA. Multiple paths for activation of naive CD8+ T cells: CD4-independent help. J Immunol. 2001;167(3):1283–1289. doi: 10.4049/jimmunol.167.3.1283. [DOI] [PubMed] [Google Scholar]
- 34.Mailliard RB, Egawa S, Cai Q, et al. Complementary dendritic cell-activating function of CD8+ and CD4+ T cells: helper role of CD8+ T cells in the development of T helper type 1 responses. J Exp Med. 2002;195(4):473–483. doi: 10.1084/jem.20011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernandez MG, Shen L, Rock KL. CD40-CD40 ligand interaction between dendritic cells and CD8+ T cells is needed to stimulate maximal T cell responses in the absence of CD4+ T cell help. J Immunol. 2007;178(5):2844–2852. doi: 10.4049/jimmunol.178.5.2844. [DOI] [PubMed] [Google Scholar]
- 36.Wong KL, Lew FC, MacAry PA, Kemeny DM. CD40L-expressing CD8 T cells prime CD8alpha(+) DC for IL-12p70 production. Eur J Immunol. 2008;38(8):2251–2262. doi: 10.1002/eji.200838199. [DOI] [PubMed] [Google Scholar]
- 37.Mueller SN, Jones CM, Stock AT, Suter M, Heath WR, Carbone FR. CD4+ T cells can protect APC from CTL-mediated elimination. J Immunol. 2006;176(12):7379–7384. doi: 10.4049/jimmunol.176.12.7379. [DOI] [PubMed] [Google Scholar]
- 38.Watchmaker PB, Urban JA, Berk E, et al. Memory CD8+ T cells protect dendritic cells from CTL killing. J Immunol. 2008;180(6):3857–3865. doi: 10.4049/jimmunol.180.6.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen G, Shankar P, Lange C, et al. CD8 T cells specific for human immunodeficiency virus, Epstein-Barr virus, and cytomegalovirus lack molecules for homing to lymphoid sites of infection. Blood. 2001;98(1):156–164. doi: 10.1182/blood.v98.1.156. [DOI] [PubMed] [Google Scholar]
- 40.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12(6):509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]